Antimicrobial Features of Organic Functionalized Graphene-Oxide with Selected Amines
Abstract
:1. Introduction
2. Materials and Methods
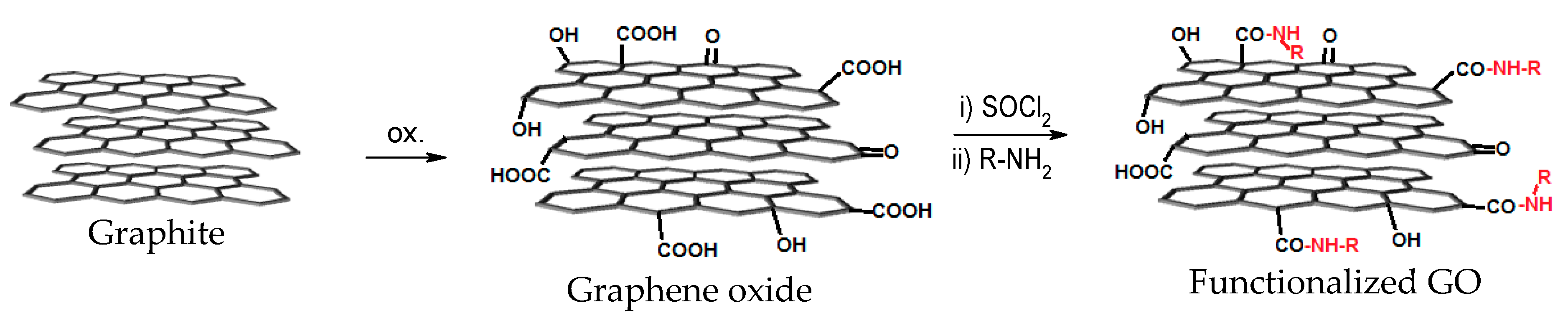
2.1. Graphene Oxide Synthesis and Functionalization
2.2. In Vitro Antimicrobial Activity Assay
3. Results and Discussion
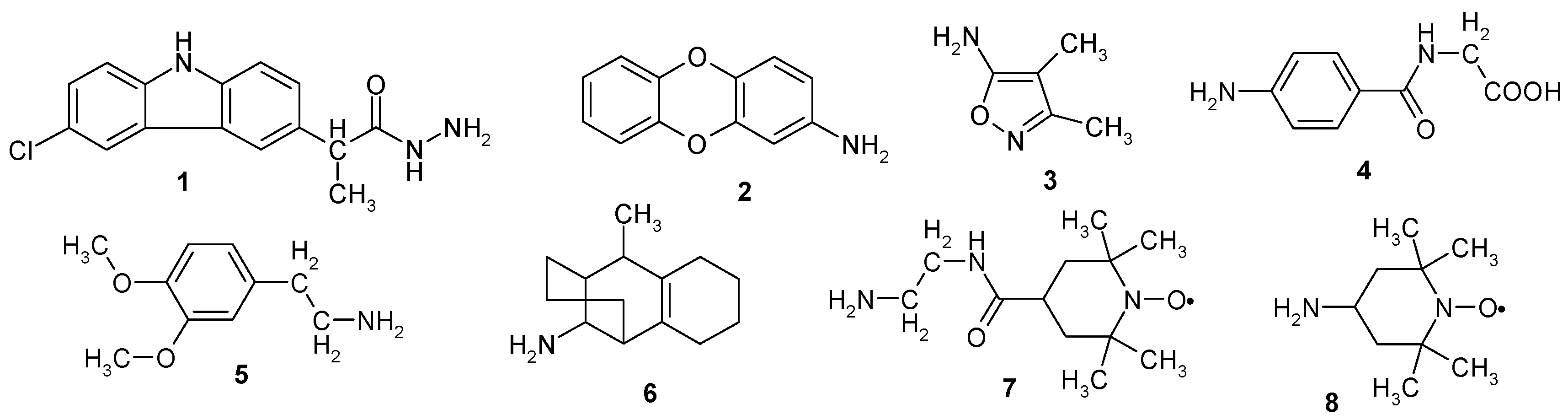
3.1. Synthesis
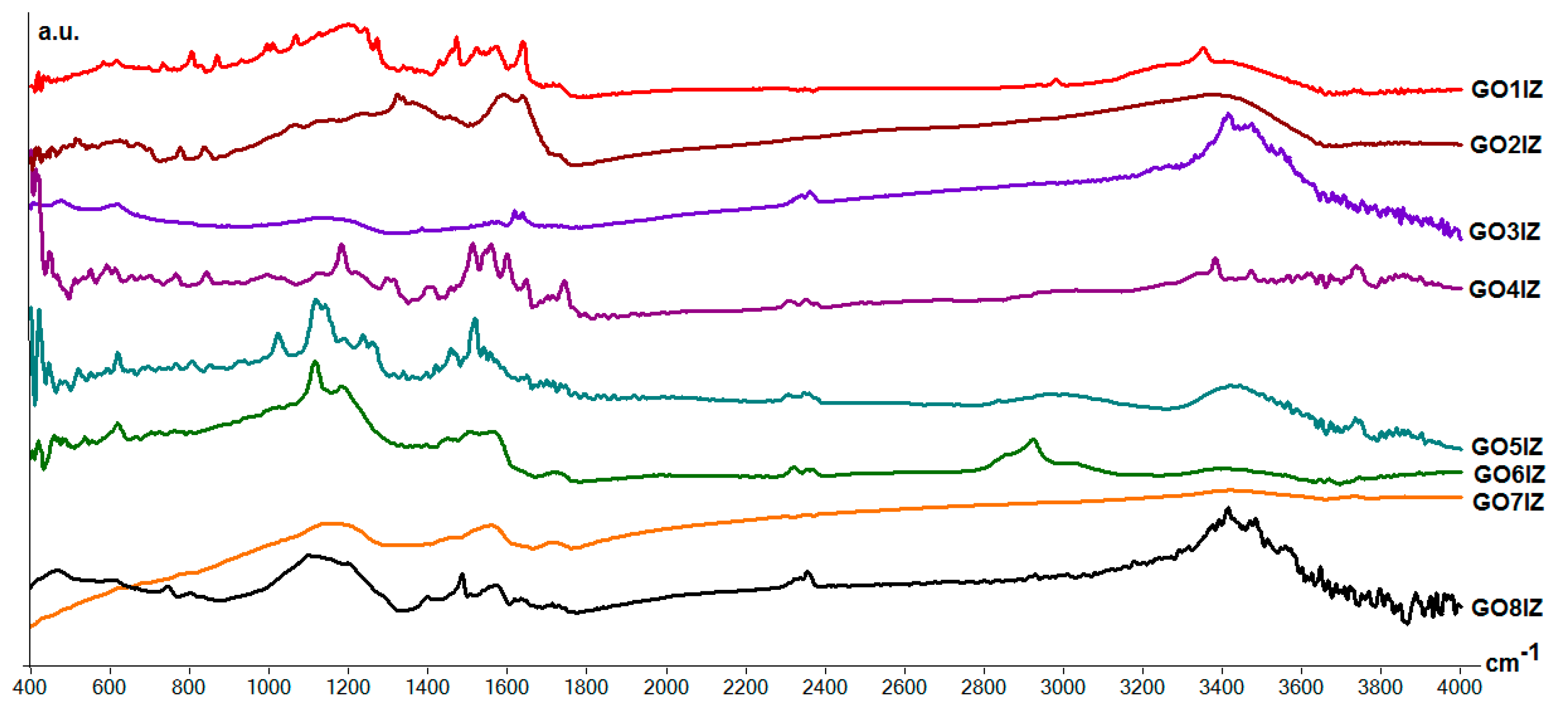
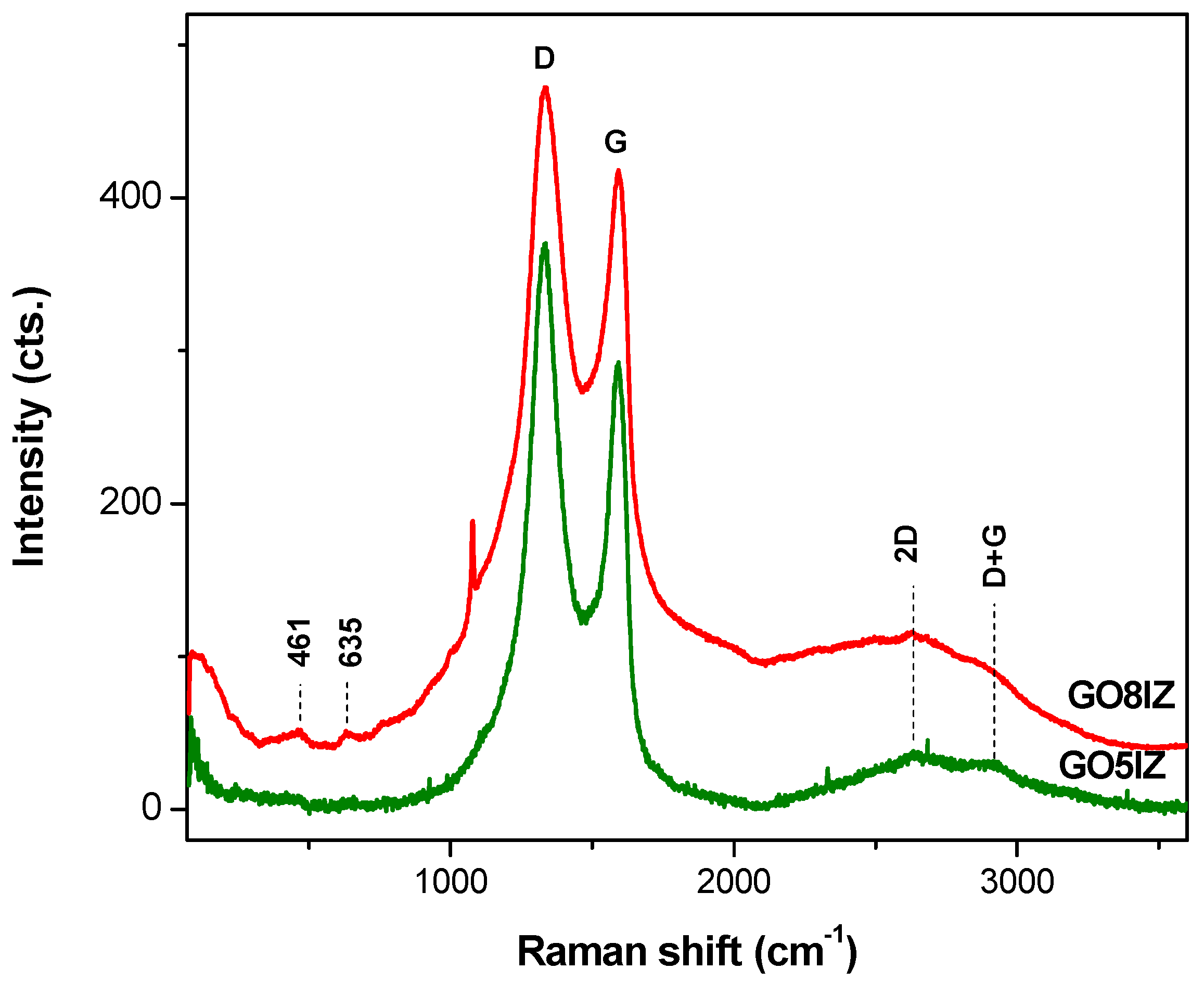
3.2. Structural Analysis
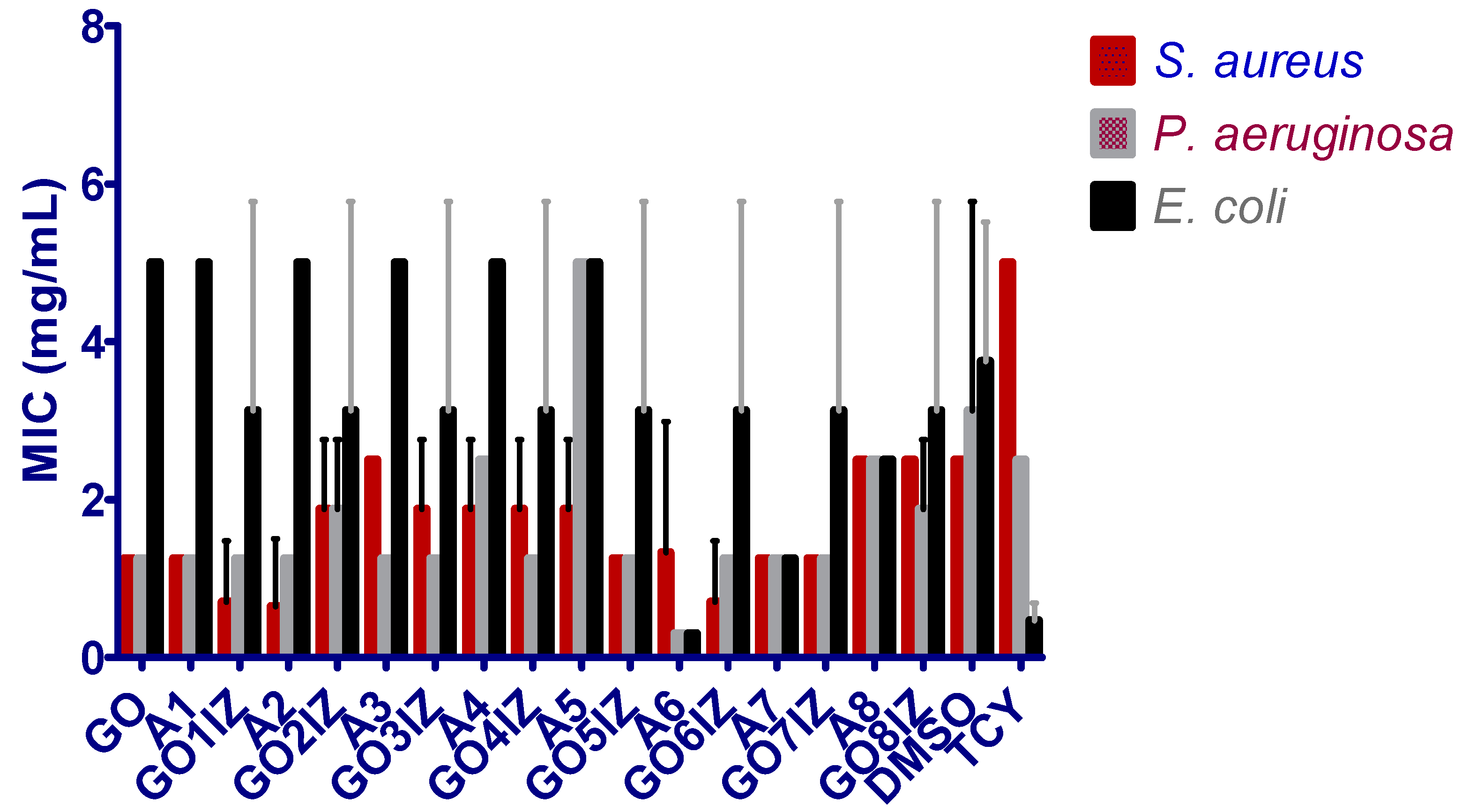
3.3. Biological Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Eigler, S.; Hirsch, A. Chemistry with Graphene and Graphene Oxide—Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Shakir, A.J.; Culita, D.C.; Moreno, J.C.; Musuc, A.; Carp, O.; Ionita, G.; Ionita, P. Covalently grafted TEMPO on graphene oxide: A composite material for selective oxidations of alcohols. Carbon 2016, 105, 607–614. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kahrstrom, C.T. Entering a post-antibiotic era? Nat. Rev. Microbiol. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.; Mekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Markovic, M.G. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Barret, G.C. Chemistry and Biochemistry of the Amino Acids; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology and Applications; Elsevier: New York, NY, USA, 2015. [Google Scholar]
- Rehman, M.; Madni, A.; Webster, T.J. The era of biofunctional biomaterials in orthopedics: What does the future hold? Expert Rev. Med. Devices 2018, 15, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of grapheme oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Balotescu, M.C.; Limban, C.; Missir, A.V.; Chirita, I.C.; Nitulescu, G.M. The synthesis and biological activities of some new 2-(4-methoxy-phenoxymethyl) benzoic acid thioureides. Rev. Chim. 2007, 58, 1064–1068. [Google Scholar]
- Chifiriuc, M.C.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S. Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wu, Y.J. Heterocycles and medicine: A survey of the heterocyclic drugs approved by the U.S. FDA from 2000 to present. Prog. Heterocycl. Chem. 2012, 24, 1–53. [Google Scholar]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prudhomme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphenesheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Carroll, N.J. Dietary modulation of oxidative stress in Alzheimer’s disease. Int. J. Molec. Sci. 2017, 18, 1583. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, H.; JOek, J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 2017, 16, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, A.A.K.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A new Raman metric for the characterisation of graphene oxide and its derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecera, P.; Chacon-Torres, J.C.; Pichler, T.; Reich, S.; Soni, H.R.; Gorling, A.; Edelthalhammer, K.; Peterlik, H.; Hauke, F.; Hirsch, A. Precise determination of graphene functionalization by in situ Raman spectroscopy. Nat. Comm. 2017, 8, 15192. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, K.A.; Vaidya, M.; Liauwa, C.M.; Brownson, D.A.C.; Ramalingam, P.; Kamieniak, J.; Rowley-Neale, S.J.; Tetlow, L.A.; Brown, D.; Banks, C.E. Antimicrobial activity of graphene oxide-metal hybrids. Int. Biodeterior. Biodegrad. 2017, 123, 182–190. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.J. Antibacterial activity of graphene oxide nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117. [Google Scholar] [CrossRef]
- Hou, W.C.; Lee, P.L.; Chou, Y.C.; Wang, Y.S. Antibacterial property of graphene oxide: The role of phototransformation. Environ. Sci. Nano 2017, 4, 647–657. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarafu, I.; Turcu, I.; Culiță, D.C.; Petrescu, S.; Popa, M.; Chifiriuc, M.C.; Limban, C.; Telehoiu, A.; Ioniță, P. Antimicrobial Features of Organic Functionalized Graphene-Oxide with Selected Amines. Materials 2018, 11, 1704. https://doi.org/10.3390/ma11091704
Zarafu I, Turcu I, Culiță DC, Petrescu S, Popa M, Chifiriuc MC, Limban C, Telehoiu A, Ioniță P. Antimicrobial Features of Organic Functionalized Graphene-Oxide with Selected Amines. Materials. 2018; 11(9):1704. https://doi.org/10.3390/ma11091704
Chicago/Turabian StyleZarafu, Irina, Ioana Turcu, Daniela C. Culiță, Simona Petrescu, Marcela Popa, Mariana C. Chifiriuc, Carmen Limban, Alexandra Telehoiu, and Petre Ioniță. 2018. "Antimicrobial Features of Organic Functionalized Graphene-Oxide with Selected Amines" Materials 11, no. 9: 1704. https://doi.org/10.3390/ma11091704




