Dissolving Microneedle Patches for Transdermal Insulin Delivery in Diabetic Mice: Potential for Clinical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dissolving Gelatin/CMC MN Patches
2.3. Fabrication of Drug-Loaded MN Patches
2.4. Ex Vivo Penetration Tests in Mouse Skin
2.5. Optical Coherence Tomography and Imaging of Transdermal Delivery
2.6. In Vivo Transdermal Delivery via Insulin-Loaded MNs
2.7. Transdermal Insulin Delivery via MN Patches
2.8. Tissue-Marking Dye Staining and OCT Measurement of Human Cadaveric Skin
3. Results and Discussion
3.1. Preparation and Characterization of Gelatin/CMC MN Patches
3.2. Insertion Capability of Gelatin/CMC MN Patches in Mice Skin
3.3. Optical Coherence Tomography and Confocal Microscopic Demonstrations of Penetration Depth and Position
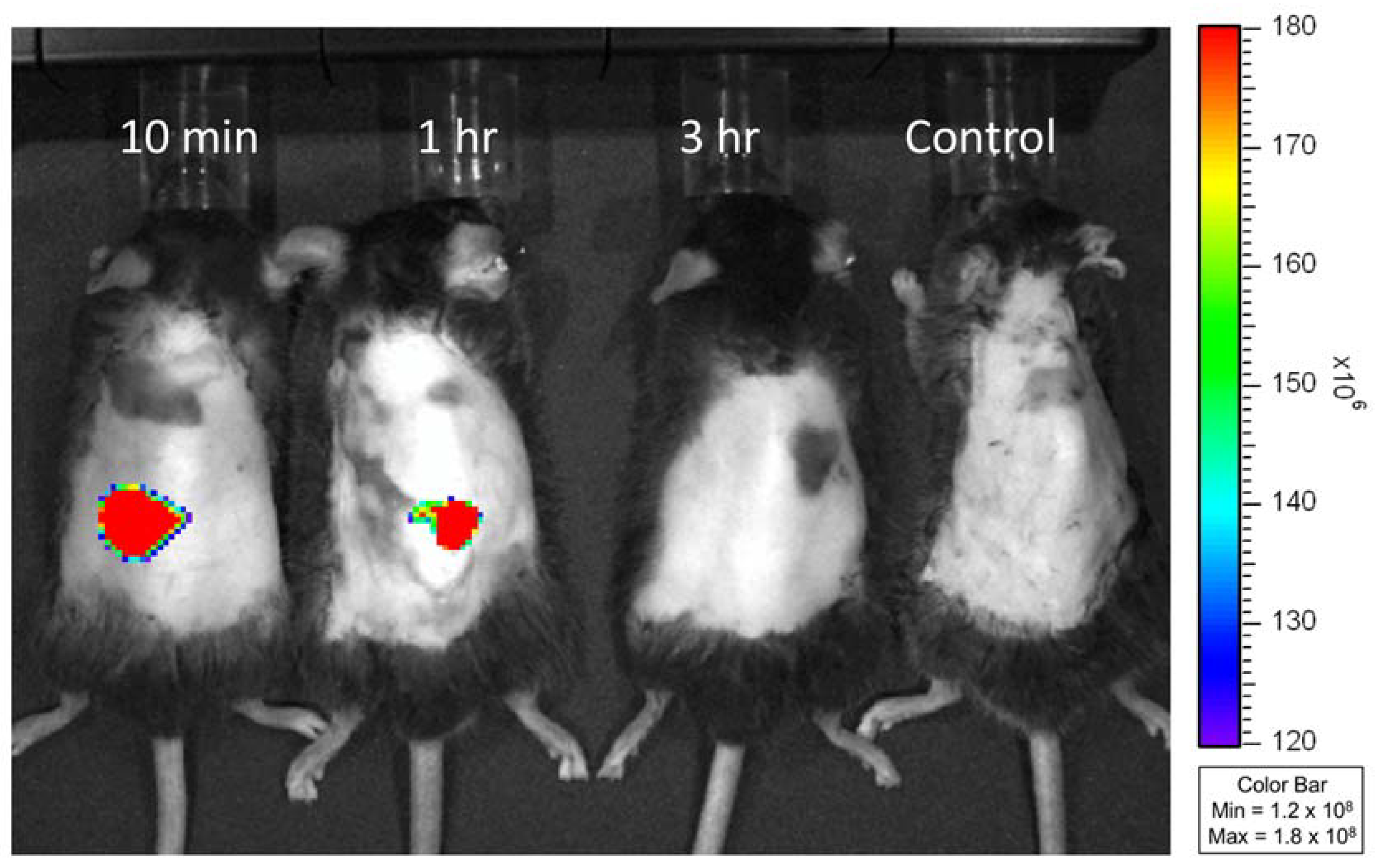
3.4. In Vivo Study of Transdermal Delivery of FITC-Insulin from MNs
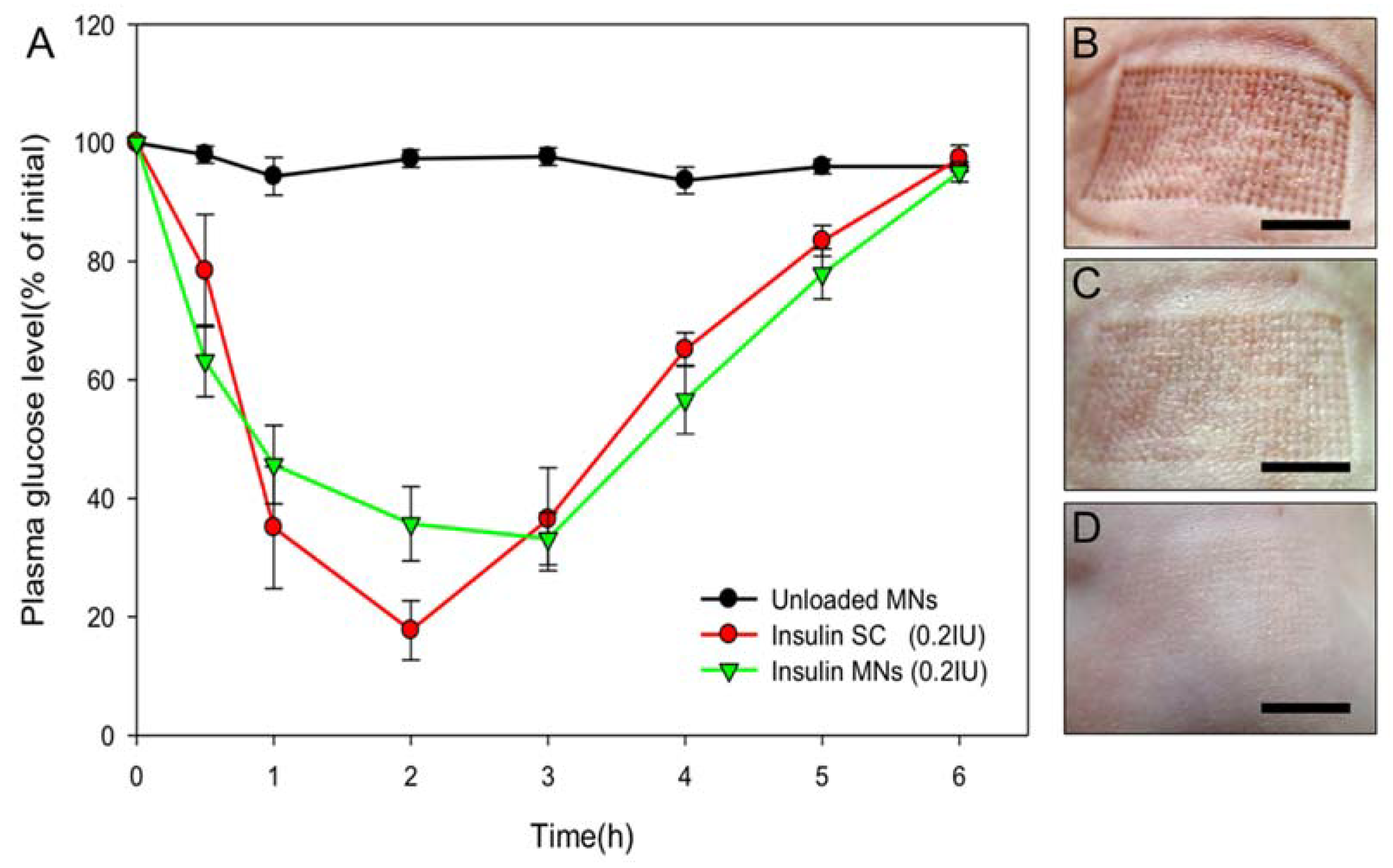
3.5. Transdermal Delivery of Insulin to Diabetic Mice
3.6. Human Cadaveric Skin Penetration Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ita, K.B. Chemical penetration enhancers for transdermal drug delivery—Success and challenges. Curr. Drug Deliv. 2015, 12, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pascual, C.; Lieu, C.; Oh, S.; Wang, J.; Zou, B.; Xie, J.; Li, Z.; Xie, J.; Yeomans, D.C.; et al. Analgesic microneedle patch for neuropathic pain therapy. ACS Nano 2017, 11, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettis, R.J.; Harvey, A.J. Microneedle delivery: Clinical studies and emerging medical applications. Ther. Deliv. 2012, 3, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Milewski, M.; Brogden, N.K.; Stinchcomb, A.L. Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin. Expert Opin. Drug Deliv. 2010, 7, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Burton, S.A.; Ng, C.Y.; Simmers, R.; Moeckly, C.; Brandwein, D.; Gilbert, T.; Johnson, N.; Brown, K.; Alston, T.; Prochnow, G.; et al. Rapid intradermal delivery of liquid formulations using a hollow microstructured array. Pharm. Res. 2011, 28, 31–40. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.C.; Lin, W.M.; Shu, J.C.; Tsai, S.W.; Chen, C.H.; Tsai, M.T. Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice. J. Biomed. Mater. Res. Part A 2017, 105, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; He, J.S.; Tsai, M.T.; Lin, K.C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J. Mater. Chem. B 2015, 3, 276–285. [Google Scholar] [CrossRef]
- Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticle Vaccine Formulation in Microneedles for Transdermal Immunization. 2018. Available online: https://europepmc.org/abstract/ppr/ppr49728 (accessed on 4 September 2018).
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, J.; Qiu, Y.; Zhang, S.; Xu, B.; Gao, Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int. J. Pharm. 2013, 447, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, J.D.; Lee, C.Y.; Her, S.; Jung, H. A high-capacity, hybrid electro-microneedle for in-situ cutaneous gene transfer. Biomaterials 2011, 32, 7705–7710. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Ise, A.; Morita, H.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm. Res. 2011, 28, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hirono, M.; Fukushima, K.; Sugioka, N.; Takada, K. Two-layered dissolving microneedles formulated with intermediate-acting insulin. Int. J. Pharm. 2012, 436, 387–393. [Google Scholar] [CrossRef] [PubMed]
- González-Vázquez, P.; Larrañeta, E.; McCrudden, M.T.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.-N.; Quan, Y.-S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Coulman, S.A.; Birchall, J.C.; Alex, A.; Pearton, M.; Hofer, B.; O’Mahony, C.; Drexler, W.; Povazay, B. In vivo, in situ imaging of microneedle insertion into the skin of human volunteers using optical coherence tomography. Pharm. Res. 2011, 28, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.-H.; Chen, M.-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Koya, D. Rodent models of diabetic nephropathy: Their utility and limitations. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.; Banga, A.K. Formation and closure of microchannels in skin following microporation. Pharm. Res. 2011, 28, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, O.; Sun, Y.; Liu, J.C.; Chien, Y.W. Facilitated transdermal transport of insulin. J. Pharm. Sci. 1987, 76, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wermeling, D.P.; Banks, S.L.; Huclson, D.A.; Gill, H.S.; Glupta, J.; Prausnitz, M.R.; Stinchcom, A.L. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc. Natl. Acad. Sci. USA 2008, 105, 2058–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Ward, J.; Quondamatteo, F.; Dockery, P.; Kelly, J.L. Skin thickness of the anterior, anteromedial, and anterolateral thigh: A cadaveric study for split-skin graft donor sites. Arch. Plast. Surg. 2014, 41, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Ripolin, A.; Quinn, J.; Larrañeta, E.; Vicente-Perez, E.M.; Barry, J.; Donnelly, R.F. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 2017, 521, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ha, R.Y.; Nojima, K.; Adams, W.P.; Brown, S.A. Analysis of facial skin thickness: Defining the relative thickness index. Plast. Reconstr. Surg. 2005, 115, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Shuster, S.; Black, M.M.; McVitie, E. The influence of age and sex on skin thickness, skin collagen and density. Br. J. Dermatol. 1975, 93, 639–643. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Shyu, V.B.-H.; Chen, C.-T. Dissolving Microneedle Patches for Transdermal Insulin Delivery in Diabetic Mice: Potential for Clinical Applications. Materials 2018, 11, 1625. https://doi.org/10.3390/ma11091625
Chen C-H, Shyu VB-H, Chen C-T. Dissolving Microneedle Patches for Transdermal Insulin Delivery in Diabetic Mice: Potential for Clinical Applications. Materials. 2018; 11(9):1625. https://doi.org/10.3390/ma11091625
Chicago/Turabian StyleChen, Chih-Hao, Victor Bong-Hang Shyu, and Chien-Tzung Chen. 2018. "Dissolving Microneedle Patches for Transdermal Insulin Delivery in Diabetic Mice: Potential for Clinical Applications" Materials 11, no. 9: 1625. https://doi.org/10.3390/ma11091625




