The Aerosol Deposition Method: A Modified Aerosol Generation Unit to Improve Coating Quality
Abstract
:1. Introduction
2. Materials and Methods
3. Results
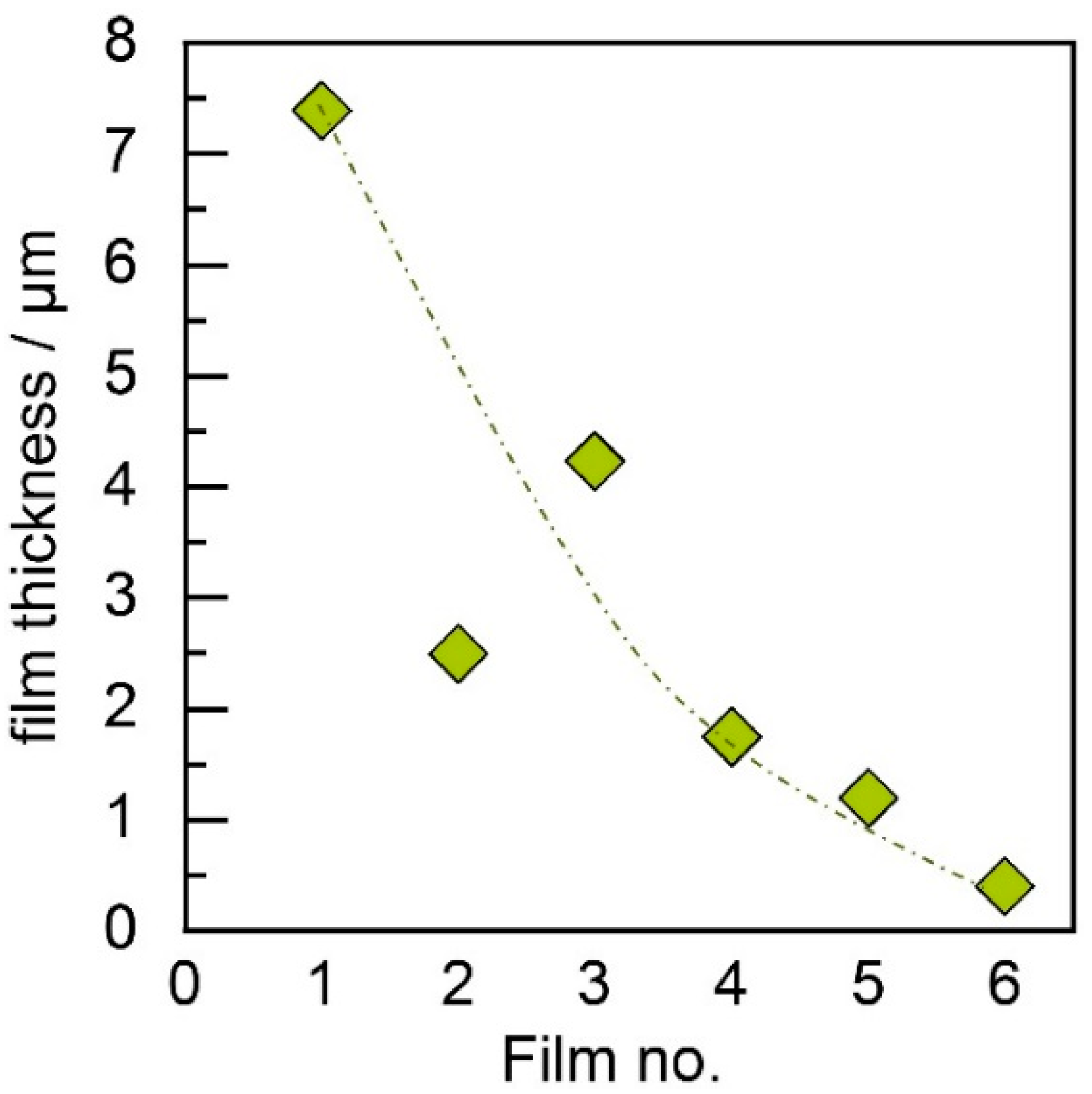
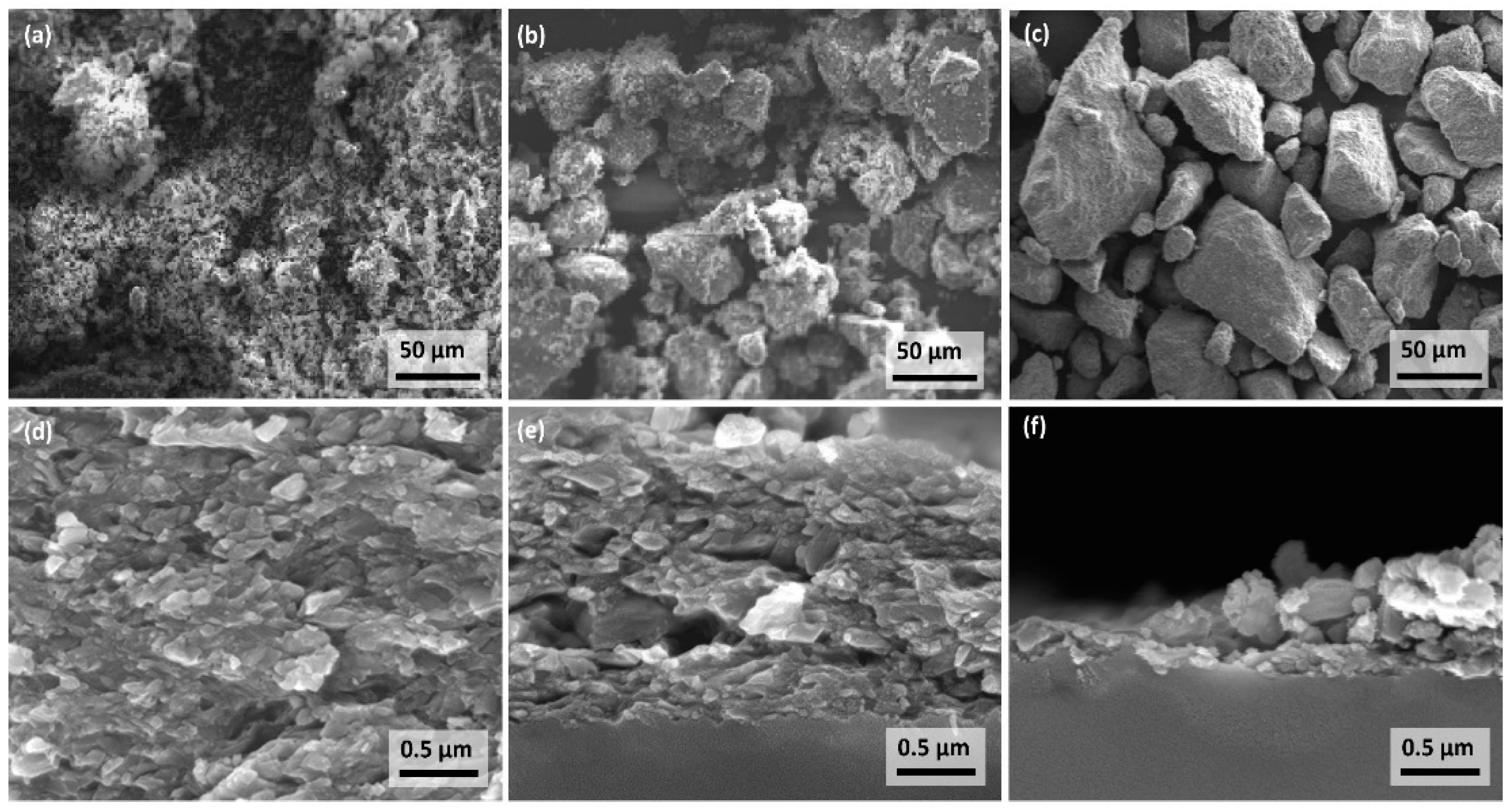
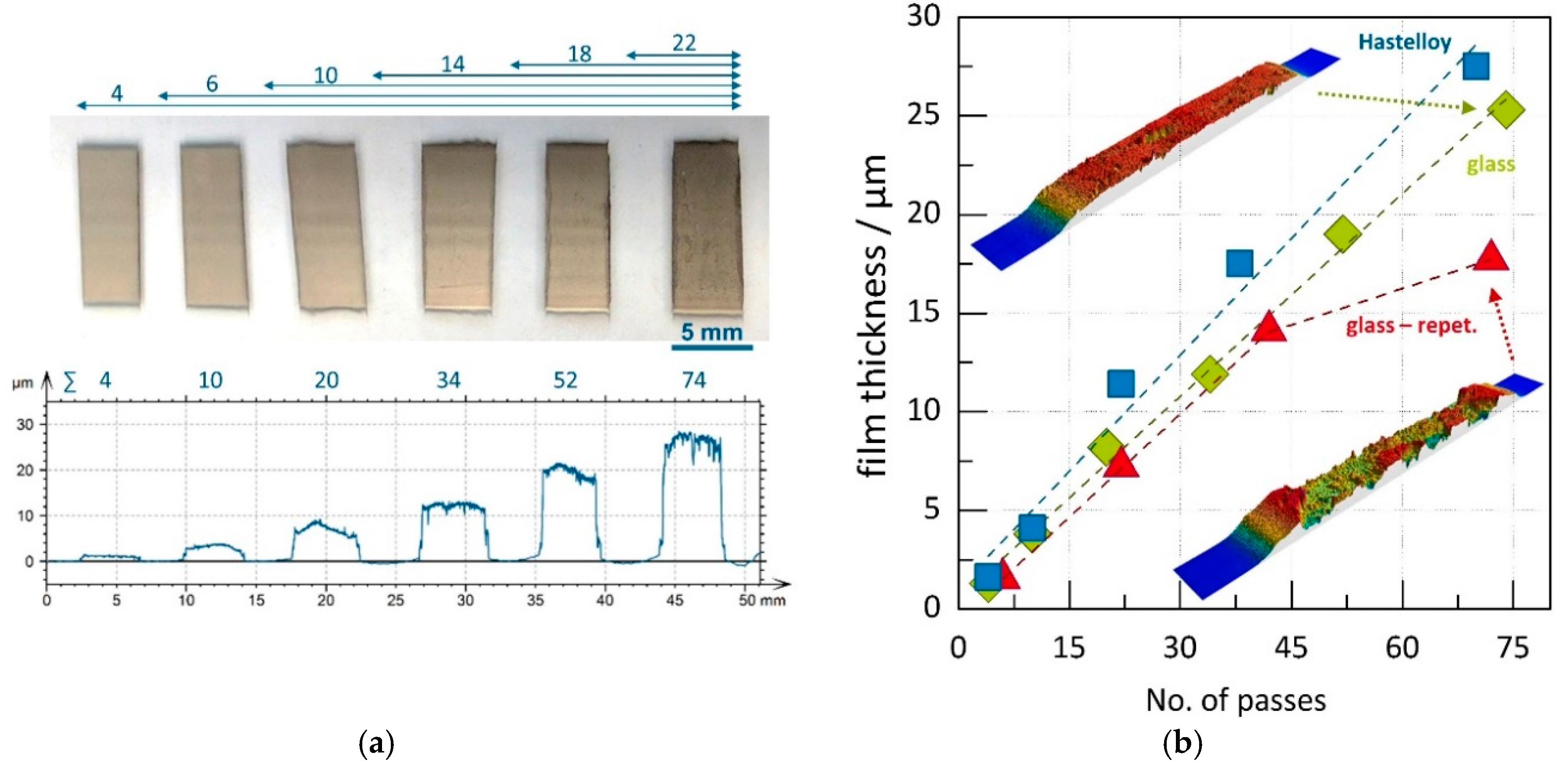
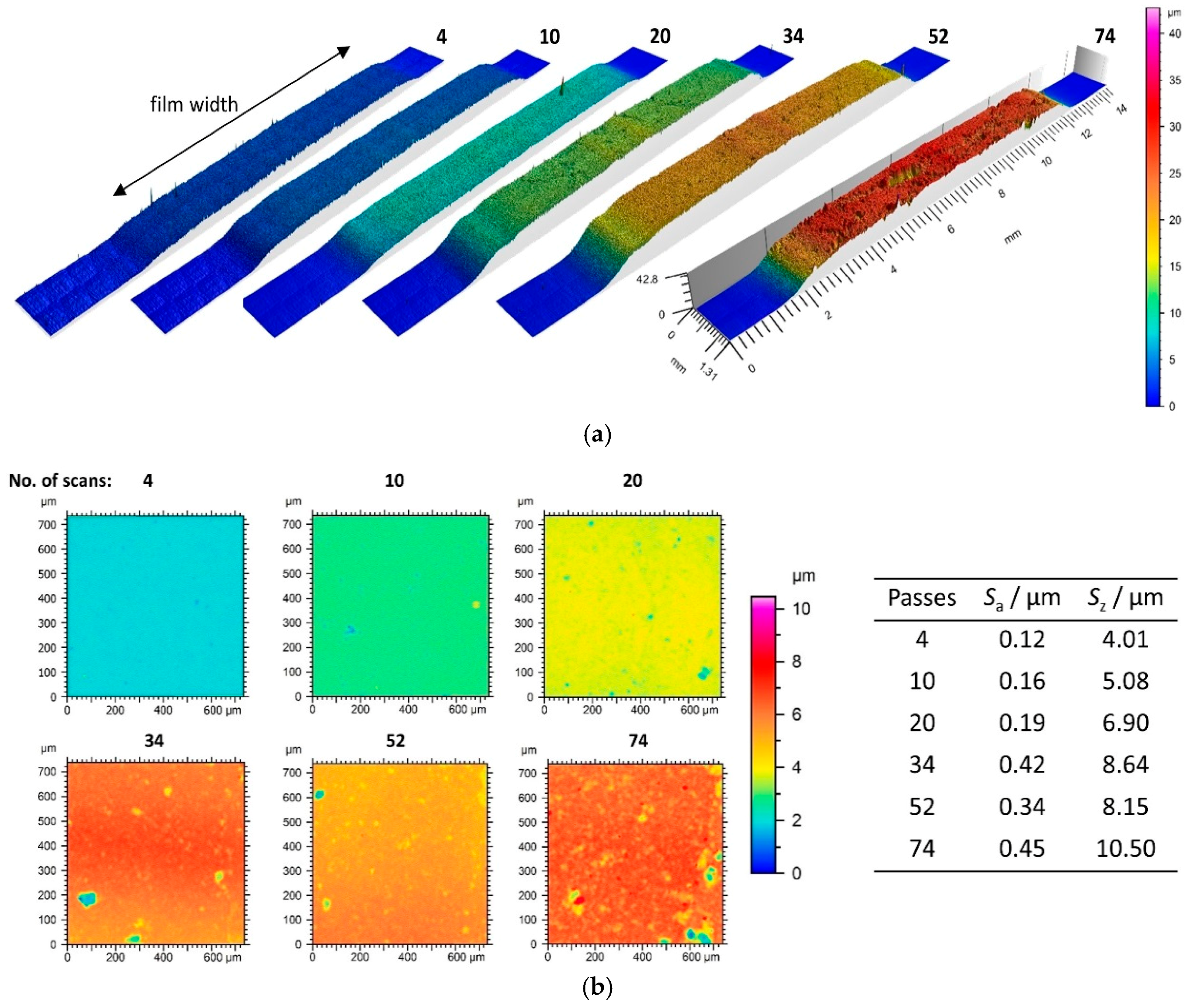
3.1. Film Thickness Evolvement for the Different Generator Principles
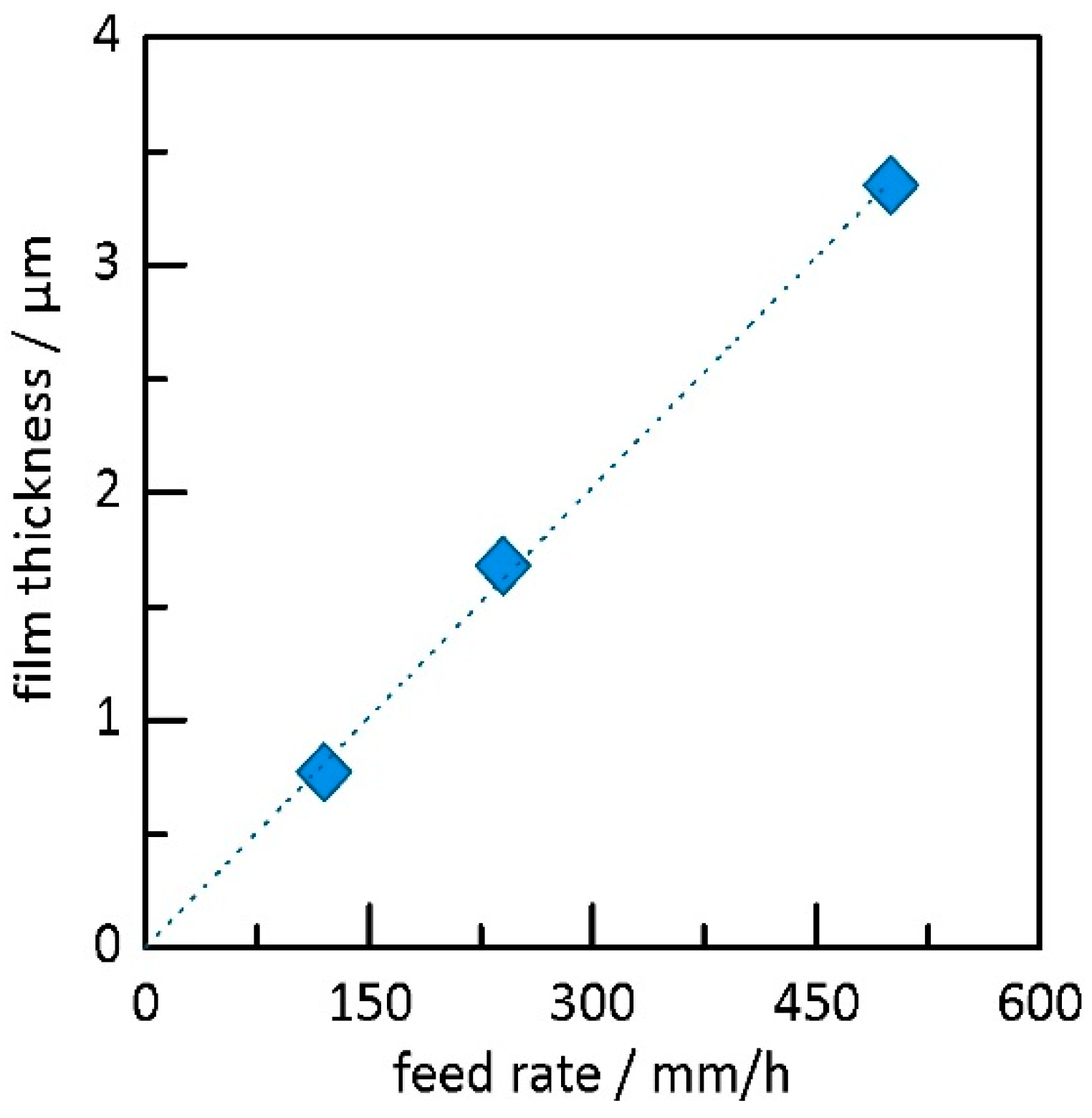
3.2. Deposition Experiments with Aerosol Brush Generator
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mihara, K.; Hoshina, T.; Takeda, H.; Tsurumi, T. Controlling factors of film-thickness in improved aerosol deposition method. J. Ceram. Soc. Jpn. 2009, 117, 868–872. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Park, D.-S.; Hahn, B.-D.; Choi, J.-J.; Ryu, J.; Choi, S.-Y.; Kim, J.; Yoon, W.-H.; Park, C. Effect of raw powder particle size on microstructure and light transmittance of α-alumina films deposited by granule spray in vacuum. Ceram. Int. 2015, 42, 3584–3590. [Google Scholar] [CrossRef]
- Chun, D.-M.; Choi, J.-O.; Lee, C.S.; Ahn, S.-H. Effect of stand-off distance for cold gas spraying of fine ceramic particles (<5 μm) under low vacuum and room temperature using nano-particle deposition system (NPDS). Surf. Coat. Technol. 2012, 206, 2125–2132. [Google Scholar] [CrossRef]
- Fuchita, E.; Tokizaki, E.; Ozawa, E.; Sakka, Y. Formation of zirconia films by aerosol gas deposition method using zirconia powder produced by break-down method. J. Ceram. Soc. Jpn. 2010, 118, 948–951. [Google Scholar] [CrossRef] [Green Version]
- Panzer, F.; Hanft, D.; Gujar, T.P.; Kahle, F.-J.; Thelakkat, M.; Köhler, A.; Moos, R. Compact Layers of Hybrid Halide Perovskites Fabricated via the Aerosol Deposition Process—Uncoupling Material Synthesis and Layer Formation. Materials 2016, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.D.; Kub, F.J.; Eddy, C.R., Jr. ZnS/Diamond composite coatings for infrared transmission applications formed by the aerosol deposition method. Proc. SPIE 2013, 8708, 87080T. [Google Scholar] [CrossRef]
- Fujihara, T.; Tsukamoto, M.; Abe, N.; Miyake, S.; Ohji, T.; Akedo, J. Hydroxyapatite film formed by particle beam irradiation. Vacuum 2004, 73, 629–633. [Google Scholar] [CrossRef]
- Akedo, J. Room Temperature Impact Consolidation (RTIC) of Fine Ceramic Powder by Aerosol Deposition Method and Applications to Microdevices. J. Therm. Spray Technol. 2008, 17, 181–198. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Schubert, M.; Stöcker, T.; Fuierer, P.; Moos, R. An overview of the aerosol deposition method: Process fundamentals and new trends in materials applications. J. Ceram. Sci. Technol. 2015, 6, 147–182. [Google Scholar] [CrossRef]
- Exner, J.; Fuierer, P.; Moos, R. Aerosol Codeposition of Ceramics: Mixtures of Bi2O3-TiO2 and Bi2O3-V2O5. J. Am. Ceram. Soc. 2014, 98, 717–723. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Moos, R. Thick-films of garnet-type lithium ion conductor prepared by the Aerosol Deposition Method: The role of morphology and annealing treatment on the ionic conductivity. J. Power Sources 2017, 361, 61–69. [Google Scholar] [CrossRef]
- Schubert, M.; Exner, J.; Moos, R. Influence of carrier gas composition on the stress of Al2O3 coatings prepared by the aerosol deposition method. Materials 2014, 7, 5633–5642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagster, D.F.; Tomi, D. The stresses within a sphere in simple flow fields. Chem. Eng. Sci. 1974, 29, 1773–1783. [Google Scholar] [CrossRef]
- Kousaka, Y.; Okuyama, K.; Shimizu, A.; Yoshida, T. Dispersion mechanism of aggregate particles in air. J. Chem. Eng. Jpn. 1979, 12, 152–159. [Google Scholar] [CrossRef]
- Calvert, G.; Ghadiri, M.; Tweedie, R. Aerodynamic dispersion of cohesive powders: A review of understanding and technology. Adv. Powder Technol. 2009, 20, 4–16. [Google Scholar] [CrossRef]
- Miyoshi, T. Preparation of full-dense Pb(Zr,Ti)O3 ceramics by aerosol deposition. J. Am. Ceram. Soc. 2008, 91, 2098–2104. [Google Scholar] [CrossRef]
- Lee, D.-W.; Kim, H.-J.; Nam, S.-M. Effects of starting powder on the growth of Al2O3 Films on Cu Substrates using the aerosol deposition method. J. Korean Phys. Soc. 2010, 57, 1115–1121. [Google Scholar] [CrossRef]
- Park, Y.; Park, D.-S.; Johnson, S.D.; Yoon, W.-H.; Hahn, B.-D.; Choi, J.-J.; Ryu, J.; Kim, J.-W.; Park, C. Effect of gas flow rates and nozzle throat width on deposition of α-alumina films of granule spray in vacuum. J. Eur. Ceram. Soc. 2017, 37, 2667–2672. [Google Scholar] [CrossRef]
- Kim, Y.; Ahn, C.-W.; Choi, J.-J.; Ryu, J.; Kim, J.-W.; Yoon, W.-H.; Park, D.-S.; Yoon, S.-Y.; Ma, B.; Hahn, B.-D. Next generation ceramic substrate fabricated at room temperature. Sci. Rep. 2017, 7, 6637. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Yamashita, T. Review of the superconducting properties of MgB2. Supercond. Sci. Technol. 2001, 14, R115–R146. [Google Scholar] [CrossRef]
- Sinha, B.B.; Chung, K.C. Fabrication and Properties of MgB2 Coated Superconducting Tapes. J. Supercond. Novel Magn. 2013, 26, 1507–1511. [Google Scholar] [CrossRef]
- Kauffmann-Weiss, S.; Hässler, W.; Guenther, E.; Scheiter, J.; Denneler, S.; Glosse, P.; Berthold, T.; Oomen, M.; Arndt, T.; Stöcker, T.; et al. Superconducting properties of thick films on Hastelloy prepared by the aerosol deposition method with Ex Situ MgB2 Powder. IEEE Trans. Appl. Supercond. 2017, 27, 1–4. [Google Scholar] [CrossRef]
- Sinha, B.B.; Chung, K.C.; Jang, S.H.; Park, D.S.; Hahn, B.-D. Fabrication of magnesium diboride thin films by aerosol deposition. Prog. Supercond. 2011, 13, 122–126. [Google Scholar]
- Zhao, F.; Xing, X.; Xiao, C.; Hu, R.; Xue, L.; Gao, H.; Xiao, L.; An, T. Study on thermodynamics and kinetics for the reaction of magnesium diboride and water by microcalorimetry. Am. J. Anal. Chem. 2011, 2, 270–275. [Google Scholar] [CrossRef]
- Palas GmbH. RBG 1000—Product Lines—Palas. Available online: https://www.palas.de/en/product/rbg1000 (accessed on 10 April 2018).
- Johnson, S.D.; Schwer, D.; Park, D.-S.; Park, Y.-S.; Gorzkowski, E.P. Deposition efficiency of barium hexaferrite by aerosol deposition. Surf. Coat. Technol. 2017. [Google Scholar] [CrossRef]
- Johnson, S.D.; Park, D.S. An Empirical Growth Dynamics Model for Aerosol Deposited Films. In Proceedings of the 12th Pacific Rim Conference on Ceramic and Glass Technology, Waikoloa, HI, USA, 21–26 May 2017. [Google Scholar]
- Chun, D.-M.; Ahn, S.-H. Deposition mechanism of dry sprayed ceramic particles at room temperature using a nano-particle deposition system. Acta Mater. 2011, 59, 2693–2703. [Google Scholar] [CrossRef]
- Piechowiak, M.A.; Henon, J.; Durand-Panteix, O.; Etchegoyen, G.; Coudert, V.; Marchet, P.; Rossignol, F. Growth of dense Ti3SiC2 MAX phase films elaborated at room temperature by aerosol deposition method. J. Eur. Ceram. Soc. 2014, 34, 1063–1072. [Google Scholar] [CrossRef]
- Belloy, E.; Thurre, S.; Walckiers, E.; Sayah, A.; Gijs, M.A.M. The introduction of powder blasting for sensor and microsystem applications. Sens. Actuators A 2000, 84, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Akedo, J.; Lebedev, M. Powder preparation in aerosol deposition method for lead Zirconate Titanate thick films. Jpn. J. Appl. Phys. 2002, 41, 6980–6984. [Google Scholar] [CrossRef]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. Ser. A 1909, 82, 172–175. [Google Scholar] [CrossRef]
- Lebedev, M.; Akedo, J. Patterning properties of PZT thick films made by aerosol deposition. Ferroelectrics 2002, 270, 117–122. [Google Scholar] [CrossRef]
| Deposition Parameters | Fluidized Bed Generator | Brush Generator |
|---|---|---|
| Piston diameter/mm | - | 20 |
| Brush rotation/rpm | - | 1200 |
| Carrier gas | N2 5.0 purity | |
| Flow rate/nL/min | 6 | 20 |
| Scan speed/mm/s | 1.5 | 1 |
| Piston speed/Feed rate/mm/h | - | 120–500 |
| Nozzle orifice/mm² | 5 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanft, D.; Glosse, P.; Denneler, S.; Berthold, T.; Oomen, M.; Kauffmann-Weiss, S.; Weis, F.; Häßler, W.; Holzapfel, B.; Moos, R. The Aerosol Deposition Method: A Modified Aerosol Generation Unit to Improve Coating Quality. Materials 2018, 11, 1572. https://doi.org/10.3390/ma11091572
Hanft D, Glosse P, Denneler S, Berthold T, Oomen M, Kauffmann-Weiss S, Weis F, Häßler W, Holzapfel B, Moos R. The Aerosol Deposition Method: A Modified Aerosol Generation Unit to Improve Coating Quality. Materials. 2018; 11(9):1572. https://doi.org/10.3390/ma11091572
Chicago/Turabian StyleHanft, Dominik, Philipp Glosse, Stefan Denneler, Thomas Berthold, Marijn Oomen, Sandra Kauffmann-Weiss, Frederik Weis, Wolfgang Häßler, Bernhard Holzapfel, and Ralf Moos. 2018. "The Aerosol Deposition Method: A Modified Aerosol Generation Unit to Improve Coating Quality" Materials 11, no. 9: 1572. https://doi.org/10.3390/ma11091572






