Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
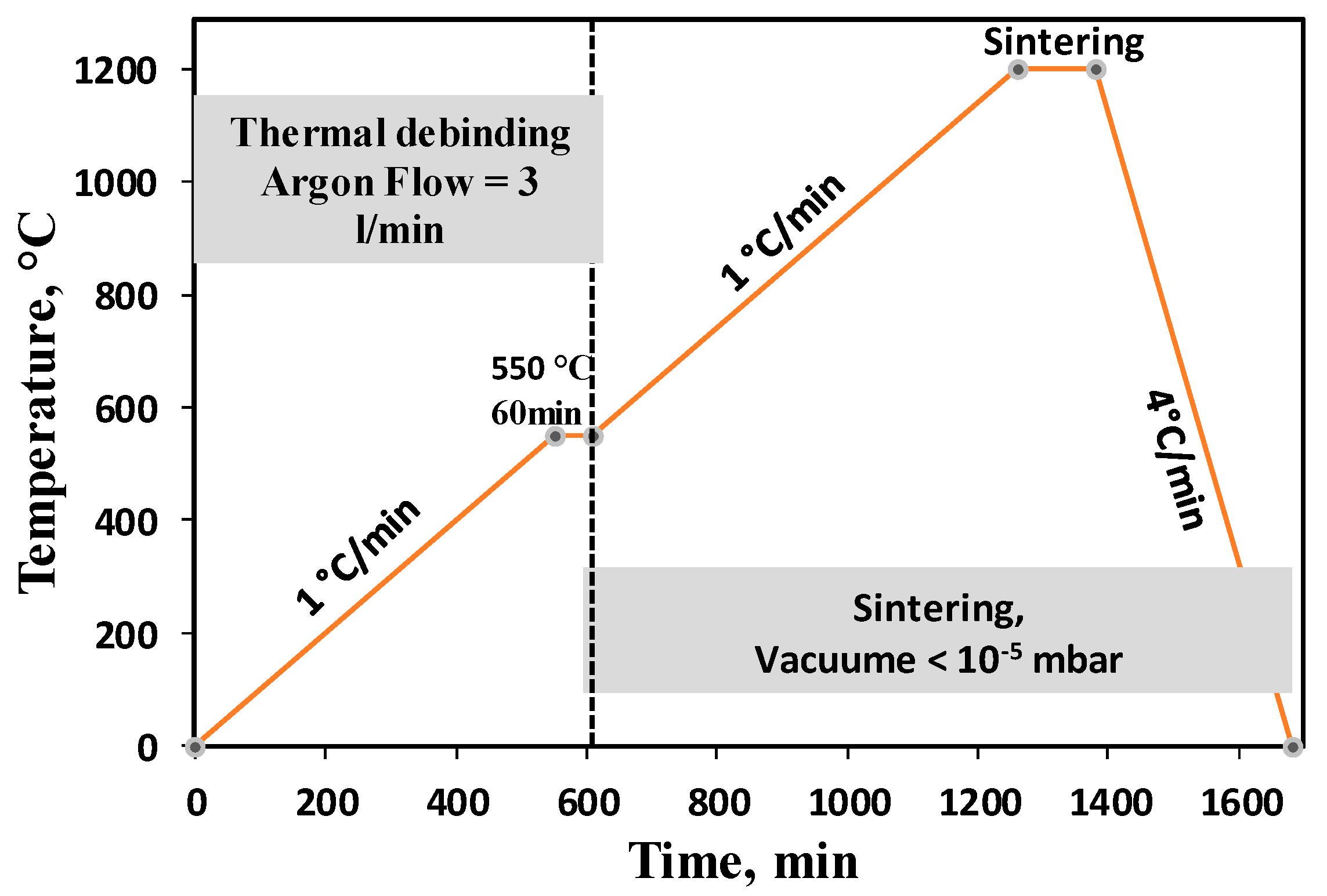
2.2. Injection Moulding, Debinding and Sintering
2.3. Materials Characterisation
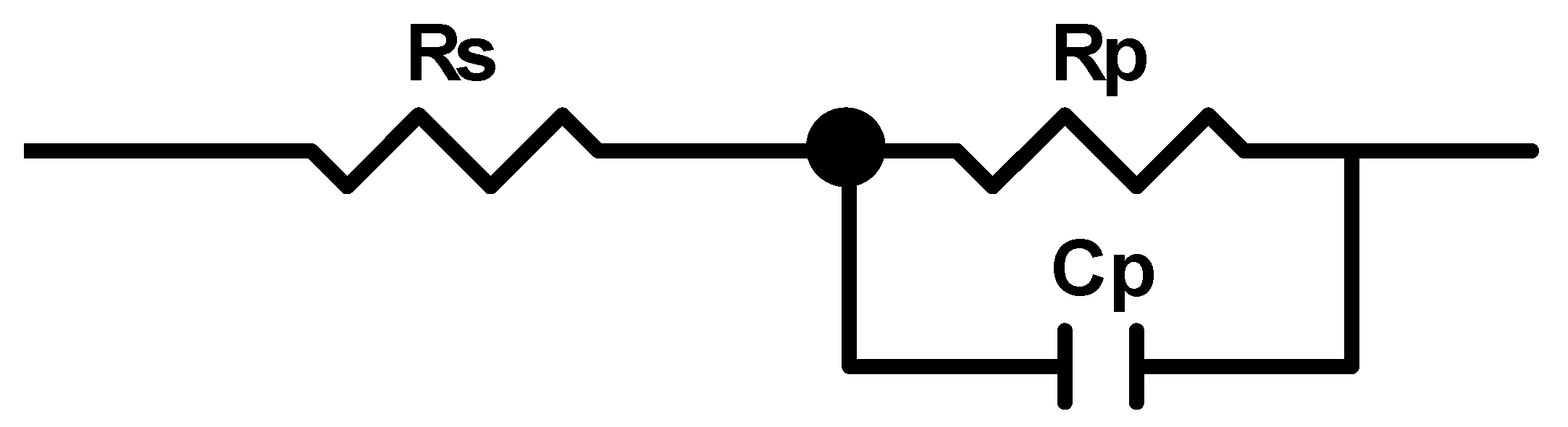
2.4. Corrosion Testing
3. Results and Discussion
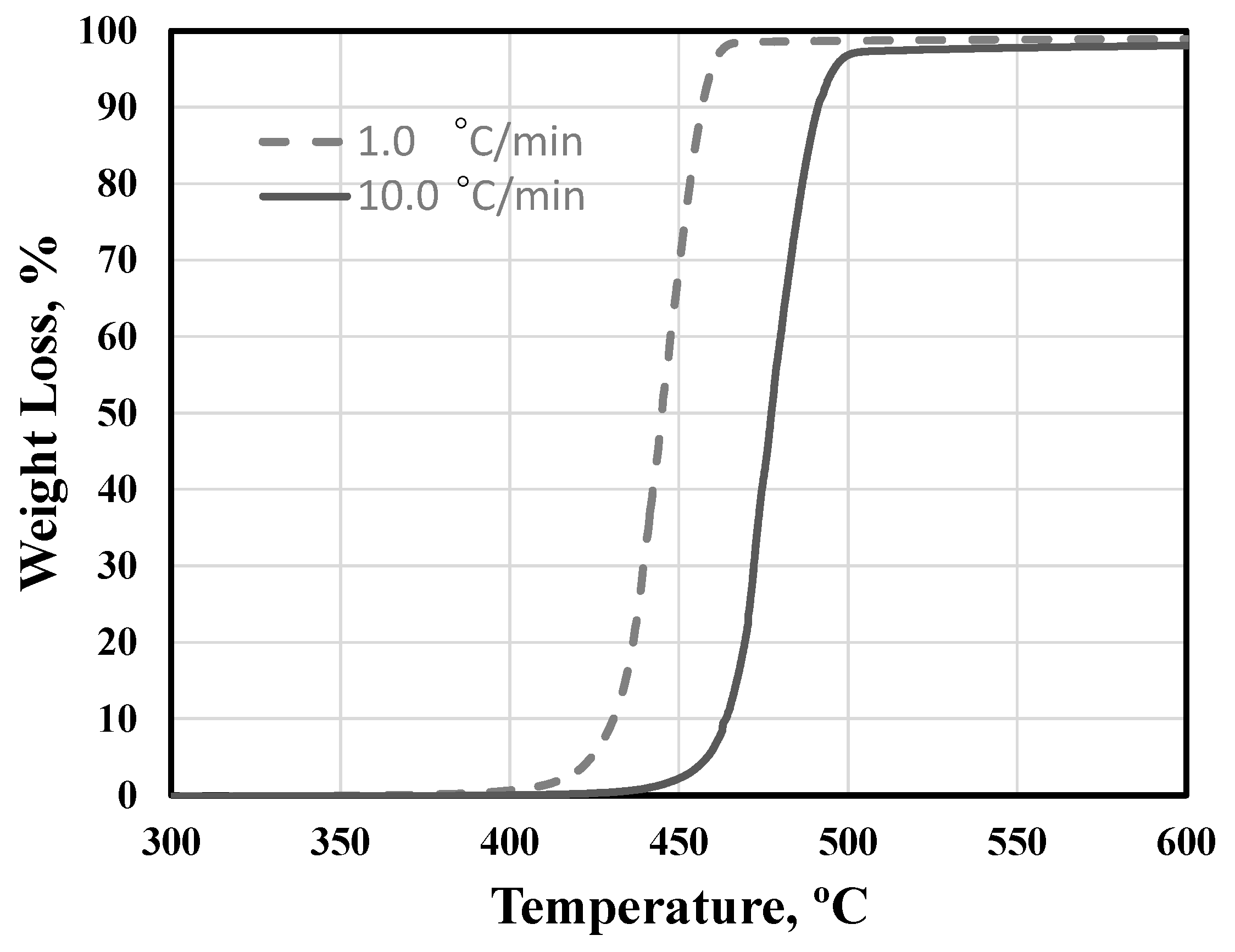
3.1. Binder Assessment and De-Binding
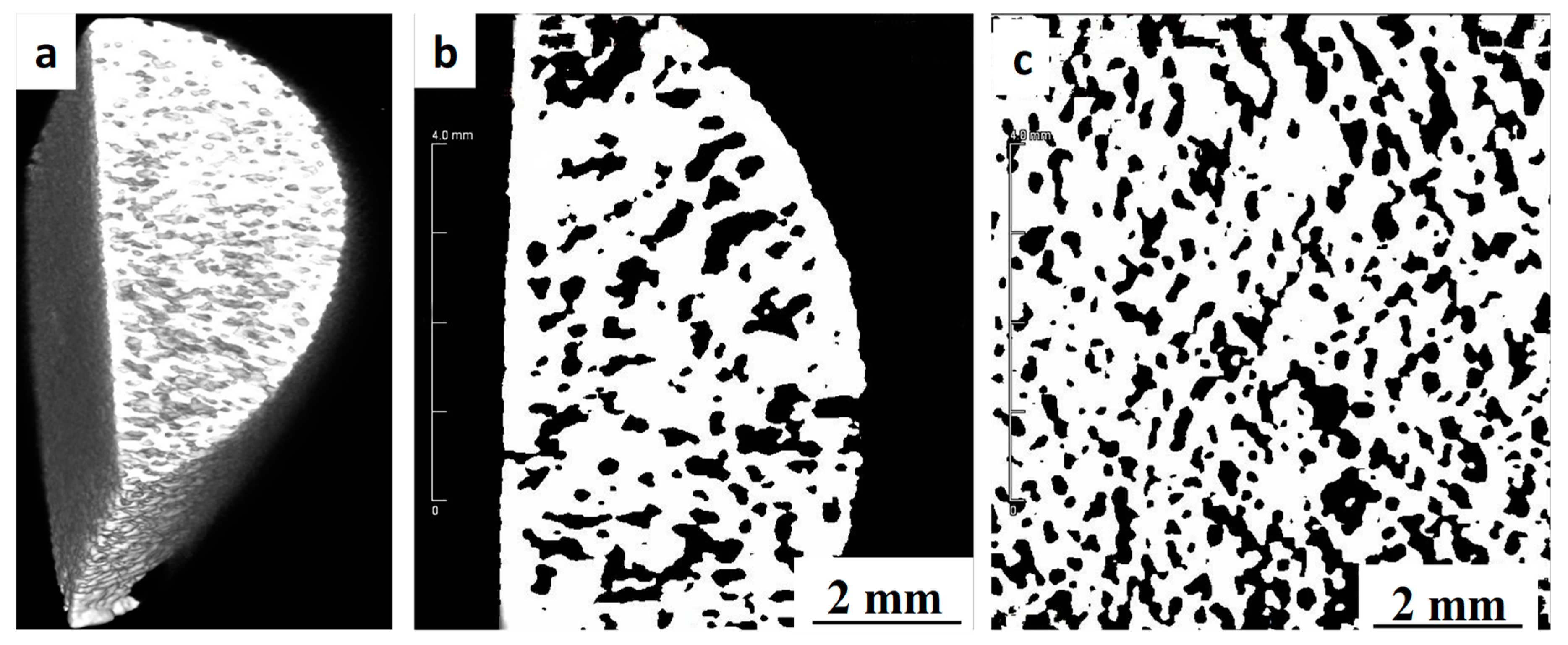
3.2. Shrinkage, Porosity and Pore Size Distribution
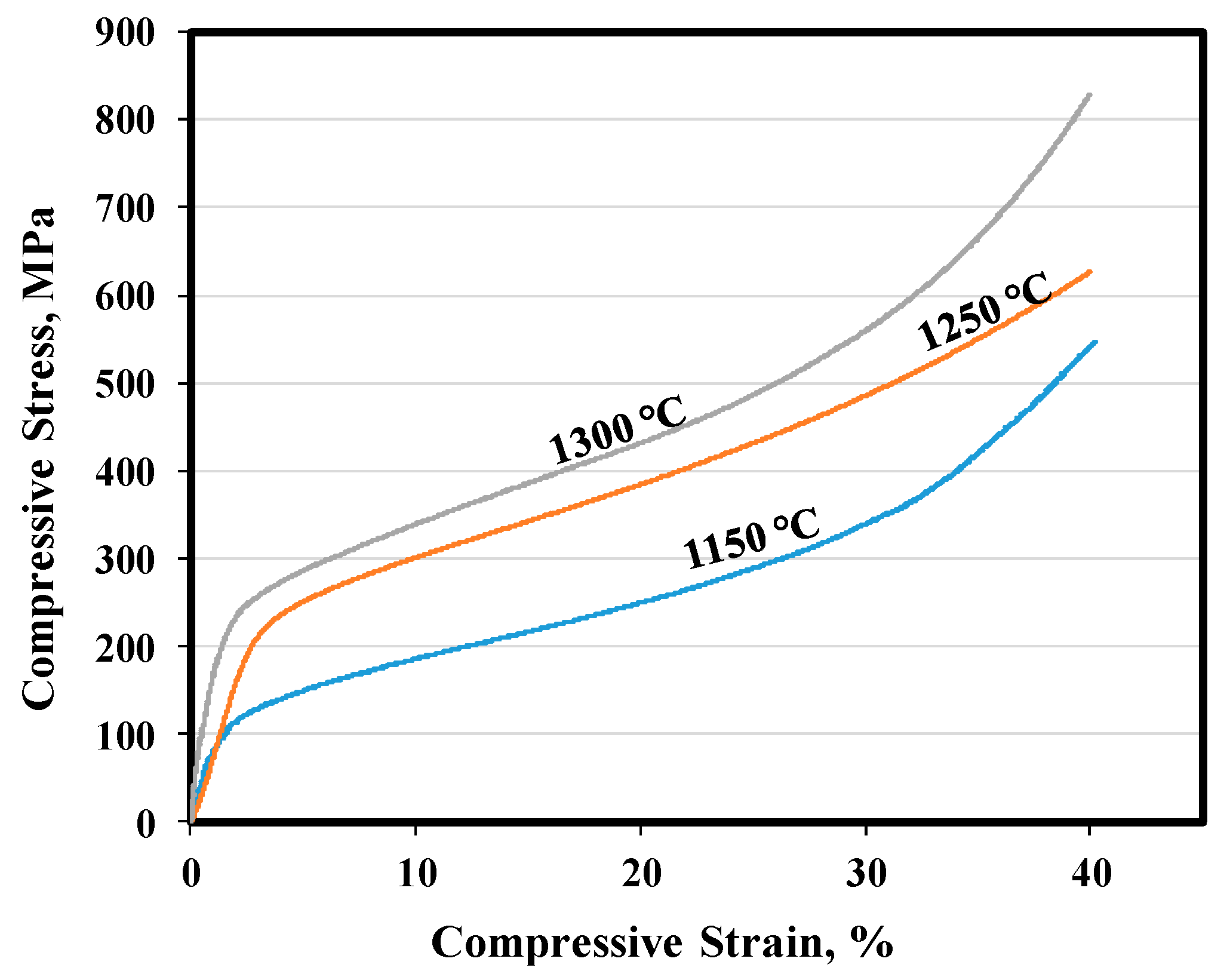
3.3. Mechanical Properties
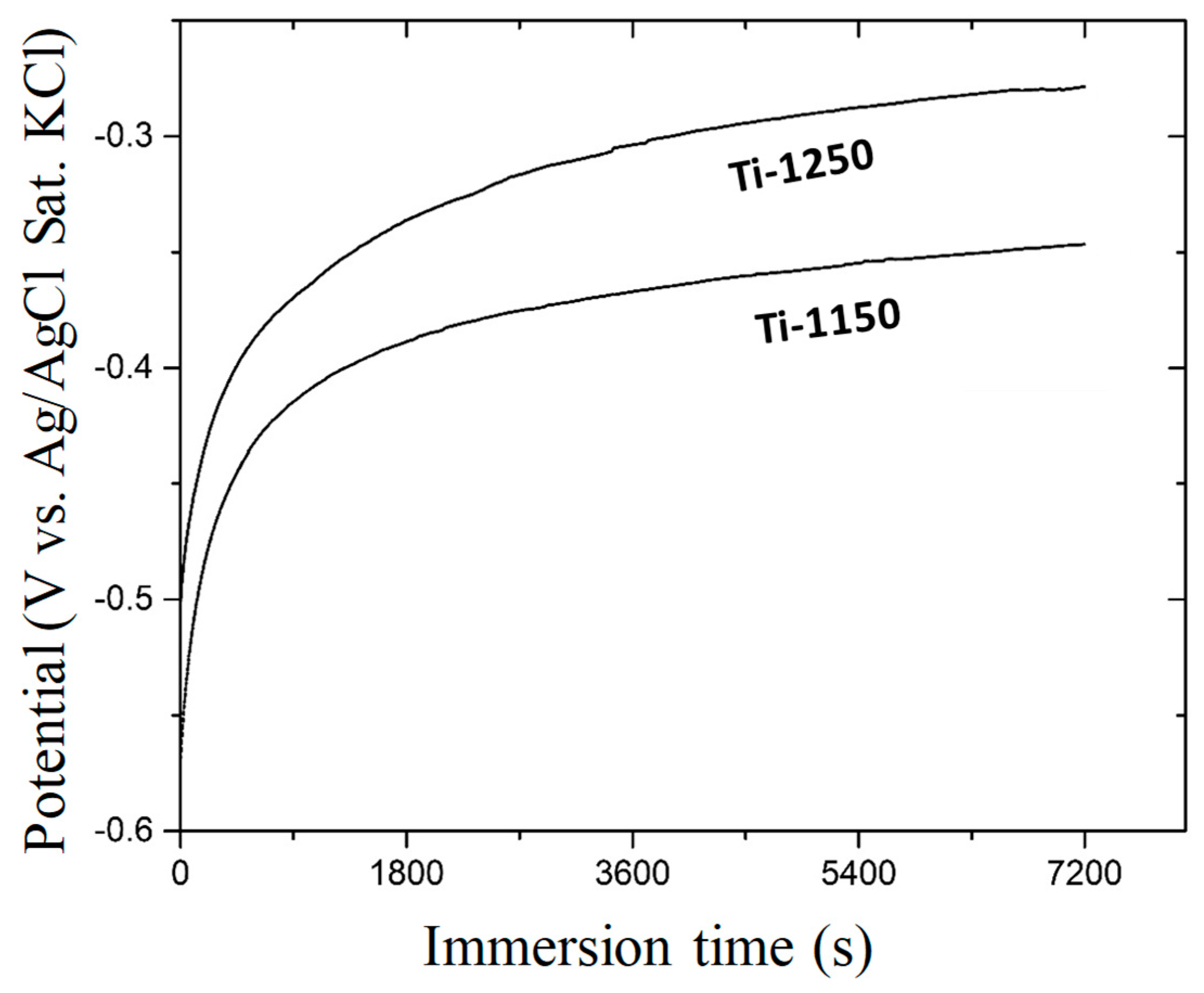
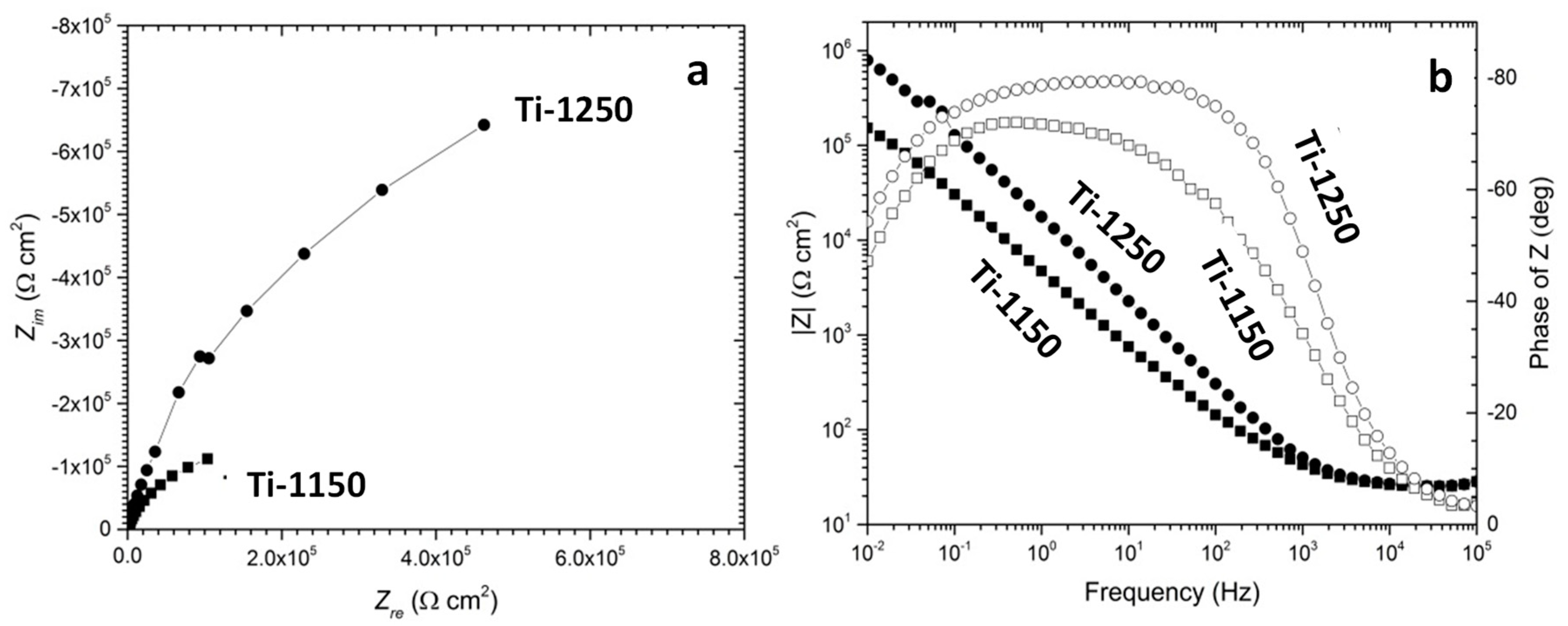
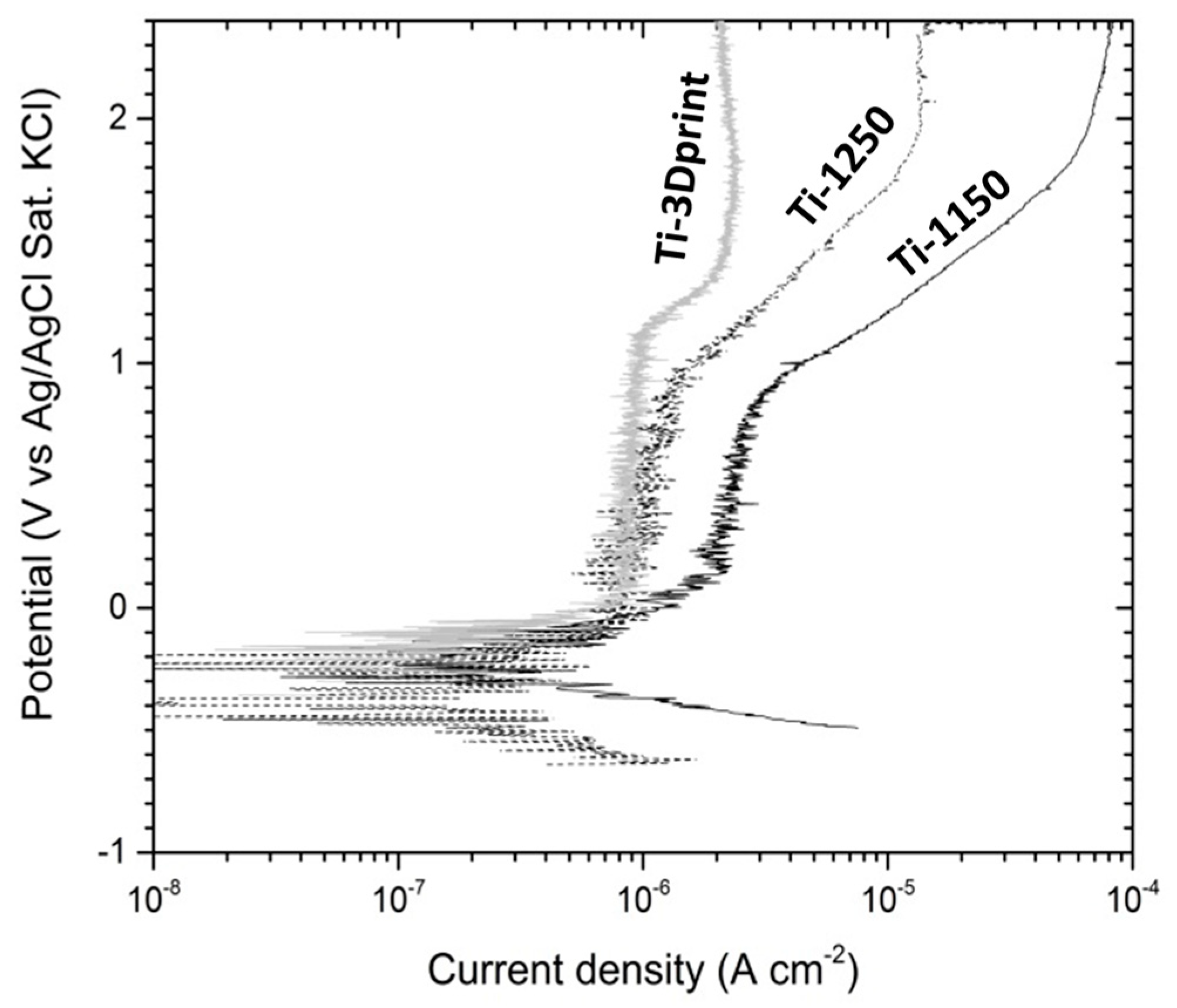
3.4. Evaluation of Corrosion Behaviours in Hank’s Solution
4. Conclusions
- (1)
- MIM is able to manufacture porous biomedical titanium scaffolds with controlled shrinkage, density, porosity and a highly interconnected pore structure;
- (2)
- Uniform shrinkage of around 12.0% was observed in all dimensions of the scaffold samples after sintering at 1250 °C or 1300 °C;
- (3)
- Samples sintered at 1250 °C for 120 min achieved mechanical properties that are very close to those of human cortical bone;
- (4)
- The corrosion resistance of scaffold titanium samples sintered at 1250 °C and 1150 °C in Hank’s solution changed with porosity. The higher the porosity, the lower the corrosion resistance;
- (5)
- Overall, sintering at 1250 °C for 120 min can be chosen as a desired sintering condition in terms of the resulting porosity level (40%), mechanical properties, dimensional control and corrosion resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lütjering, G.; Williams, J.C. Titanium, Engineering Materials and Processes, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 2007; p. 442. [Google Scholar]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Dippenaar, R.J. Development of β-phase morphologies during low temperature isothermal heat treatment of a Ti-5Al-5Mo-5V-3Cr alloy. Mater. Sci. Eng. A 2011, 528, 1833–1839. [Google Scholar] [CrossRef]
- Elias, C.; Lima, J.H.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.; Asokamani, R.; Gogia, A. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Inter. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chang, K.; Ebel, T.; Qian, M.; Willumeit, R.; Yan, M.; Pyczak, F. Microstructure and mechanical behavior of metal injection molded Ti–Nb binary alloys as biomedical material. J. Mech. Prop. Biomater. 2013, 28, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Xu, W.; Brandt, M.; Tang, H.P. Additive manufacturing and postprocessing of Ti-6Al-4V for superior mechanical properties. MRS Bull. 2016, 41, 775–783. [Google Scholar] [CrossRef]
- Chen, Y.; Kent, D.; Bermingham, M.; Dehghan-Manshadi, A.; Dargusch, M. Manufacturing of biocompatible porous titanium scaffolds using a novel spherical sugar pellet space holder. Mater. Lett. 2017, 195, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Torres-Sanchez, C.; Al Mushref, F.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chang, L.; Liu, H.; Li, Y.; Yang, H.; Ruan, J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb-Ti-Ta alloys as biomedical material. Mater. Sci. Eng. C 2017, 71, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Frith, J.E.; Dehghan-Manshadi, A.; Attar, H.; Kent, D.; Soro, N.D.M.; Bermingham, M.J.; Dargusch, M.S. Mechanical Properties and Biocompatibility of Porous Titanium Scaffolds for Bone Tissue Engineering. J. Mech. Prop. Biomater. 2017, 75, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.M.; Tseng, L.-F.; Iannolo, M.T.; Oest, M.E.; Henderson, J.H. Self-deploying shape memory polymer scaffolds for grafting and stabilizing complex bone defects: A mouse femoral segmental defect study. Biomaterials 2016, 76, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ebel, T. Advances in the Metal Injection Moulding of Titanium at Euro PM 2014. PIM Int. 2015, 9, 51–61. [Google Scholar]
- German, R.M. Progress in Titanium Metal Powder Injection Molding. Materials 2013, 6, 3641–3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, M. Metal Injection Moulding (MIM) of titanium and titanium hydride reviewed at PM Titanium 2013. PIM Int. 2013, 8, 67–74. [Google Scholar]
- Dehghan-Manshadi, A.; Bermingham, M.; Dargusch, M.; StJohn, D.; Qian, M. Metal Injection Moulding of Titanium and Titanium Alloys: Challenges and Recent Development. Powder Technol. 2017, 319, 289–301. [Google Scholar] [CrossRef]
- Ergul, E.; Gulsoy, H.O.; Gunay, V. Effect of sintering parameters on mechanical properties of injection moulded Ti-6Al-4V alloys. Powder Metall. 2009, 52, 65–71. [Google Scholar] [CrossRef]
- Hamidi, M.; Harun, W.; Samykano, M.; Ghani, S.; Ghazalli, Z.; Ahmad, F.; Sulong, A. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Bidaux, J.; Closuit, C.; Rodriguez-Arbaizar, M.; Zufferey, D.; Carreño-Morelli, E. Processing of a low modulus Ti-Nb biomaterial by Metal Injection Molding (MIM). PIM Int. 2012, 6, 72–75. [Google Scholar]
- Nelles, H.; Bram, M.; Buchkremer, H.P.; Stöver, D. Method for the Production of Near Net-Shaped Metallic and/or Ceramic Parts. U.S. Patent 7351371, 1 April 2008. [Google Scholar]
- Carreño-Morelli, E.; Rodríguez-Arbaizar, M.; Amherd, A.; Bidaux, J.E. Porous titanium processed by powder injection moulding of titanium hydride and space holders. Powder Metall. 2014, 57, 93–97. [Google Scholar] [CrossRef]
- Carreño-Morelli, E.; Amherd, A.; Rodriguez-Arbaizar, M.; Zufferey, D.; Várez, A.; Bidaux, J.-E. Porous titanium by powder injection moulding of titanium hydride and PMMA space holders. Eur. Cells Mater. 2013, 26, 16. [Google Scholar]
- Chen, L.-J.; Li, T.; Li, Y.-M.; He, H.; Hu, Y.-H. Porous titanium implants fabricated by metal injection molding. Trans. Nonferr. Met. Soc. China 2009, 19, 1174–1179. [Google Scholar] [CrossRef]
- Daudt, N.D.F.; Bram, M.; Barbosa, A.P.C.; Laptev, A.M.; Alves, C., Jr. Manufacturing of highly porous titanium by metal injection molding in combination with plasma treatment. J. Mater. Proc. Technol. 2017, 239, 202–209. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, L.; Fan, Y.; Li, Y. Fabrication of high porous NiTi shape memory alloy by metal injection molding. J. Mater. Proc. Technol. 2008, 206, 395–399. [Google Scholar]
- Laptev, A.M.; Daudt, N.A.F.; Guillon, O.; Bram, M. Increased Shape Stability and Porosity of Highly Porous Injection-Molded Titanium Parts. Adv. Eng. Mater. 2015, 17, 1579–1587. [Google Scholar] [CrossRef]
- Tuncer, N.; Bram, M.; Laptev, A.; Beck, T.; Moser, A.; Buchkremer, H.P. Study of metal injection molding of highly porous titanium by physical modeling and direct experiments. J. Mater. Proc. Technol. 2014, 214, 1352–1360. [Google Scholar] [CrossRef]
- Demangel, C.; Auzène, D.; Vayssade, M.; Duval, J.-L.; Vigneron, P.; Nagel, M.-D.; Puippe, J.-C. Cytocompatibility of titanium metal injection molding with various anodic oxidation post-treatments. Mater. Sci. Eng. C 2012, 32, 1919–1925. [Google Scholar] [CrossRef]
- Santos, P.F.; Niinomi, M.; Liu, H.; Cho, K.; Nakai, M.; Itoh, Y.; Narushima, T.; Ikeda, M. Fabrication of low-cost beta-type Ti-Mn alloys for biomedical applications by metal injection molding process and their mechanical properties. J. Mech. Prop. Biomater. 2016, 59, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.P.C.; Bram, M.; Stöver, D.; Buchkremer, H.P. Realization of a Titanium Spinal Implant with a Gradient in Porosity by 2-Component-Metal Injection Moulding. Adv. Eng. Mater. 2013, 15, 510–521. [Google Scholar] [CrossRef]
- Deing, A.; Luthringer, B.; Laipple, D.; Ebel, T.; Willumeit, R. A porous TiAl6V4 implant material for medical application. Int. J. Biomater. 2014, 2014, 904230. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Manshadi, A.; Qian, M.; Dargusch, M.; Chen, Y.; StJohn, D. Optimisation of Processing Parameters for Metal Injection Moulding of Titanium Using Non-Spherical Titanium Powders. J. Manuf. Process 2018, 31, 416–423. [Google Scholar] [CrossRef]
- Wiria, F.E.; Shyan, J.Y.M.; Lim, P.N.; Wen, F.G.C.; Yeo, J.F.; Cao, T. Printing of titanium implant prototype. Mater. Des. 2010, 31, S101–S105. [Google Scholar] [CrossRef]
- Molly, L. Bone density and primary stability in implant therapy. Clin. Oral Implants Res. 2006, 17, 124–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. A 2001, 58, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.T.; Burstein, A.H. The mechanical properties of cortical bone. JBJS 1974, 56, 1001–1022. [Google Scholar] [CrossRef]
- Sidambe, A.T.; Figueroa, I.A.; Hamilton, H.G.; Todd, I. Taguchi optimization of MIM titanium sintering. Int. J. Powder Metall. 2011, 47, 21–28. [Google Scholar]
- Takekawa, J.; Sakurai, N. Effect of the Processing Conditions on Density, Strength and Microstructure of Ti-12Mo Alloy Fabricated by PIM Process. J. Jpn. Soc. Powder Metall. 1999, 46, 877–881. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Alves, A.C.; Sendao, I.; Ariza, E.; Toptan, F.; Ponthiaux, P.; Pinto, A.M.P. Corrosion behaviour of porous Ti intended for biomedical applications. J. Porous Mater. 2016, 23, 1261–1268. [Google Scholar] [CrossRef]


| Components | g/L |
|---|---|
| CaCl2 | 0.1396 |
| MgSO4 (anhydrous) | 0.09767 |
| KCl | 0.4 |
| KH2PO4 (anhydrous) | 0.06 |
| NaCl | 8.0 |
| Na2HPO4 (anhydrous) | 0.04788 |
| D-Glucose | 1.0 |
| Sintering Temperature (°C) | Radial Shrinkage (%) | Longitudinal Shrinkage (%) | Density (g/cm3) | Overall Porosity (%) | Open Porosity (%) | Pore Interconnectivity (%) |
|---|---|---|---|---|---|---|
| 1150 | 9.54 ± 0.65 | 9.81 ± 0.47 | 2.60 ± 0.05 | 42.5 | 40.6 | 95.5 |
| 1250 | 12.38 ± 0.77 | 12.62 ± 0.71 | 2.86 ± 0.05 | 36.5 | 33.4 | 91.5 |
| 1300 | 13.04 ± 0.69 | 13.21 ± 0.65 | 2.96 ± 0.03 | 34.4 | 33.8 | 98.2 |
| Sintering Temperature (°C) | σ0.2 (MPa) | σ40 (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|
| 1150 | 123 | 553 | 8.40 |
| 1250 | 220 | 630 | 7.82 |
| 1300 | 230 | 831 | 21.69 |
| Human cortical bone [38] | 104–121 | - | 4–30 |
| Sample | Ecorr (V) | Icorr (µA cm−2) | Bc (mV) | Eb (V) | Etp (V) |
|---|---|---|---|---|---|
| Ti-1150 | −0.297 ± 0.009 | 0.32 ± 0.06 | −164 ± 22 | 0.806 ± 0.032 | 1.545 ± 0.338 |
| Ti-1250 | −0.510 ± 0.021 | 0.19 ± 0.02 | −109 ± 1 | 0.874 ± 0.071 | 1.893 ± 0.014 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehghan-Manshadi, A.; Chen, Y.; Shi, Z.; Bermingham, M.; StJohn, D.; Dargusch, M.; Qian, M. Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications. Materials 2018, 11, 1573. https://doi.org/10.3390/ma11091573
Dehghan-Manshadi A, Chen Y, Shi Z, Bermingham M, StJohn D, Dargusch M, Qian M. Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications. Materials. 2018; 11(9):1573. https://doi.org/10.3390/ma11091573
Chicago/Turabian StyleDehghan-Manshadi, Ali, Yunhui Chen, Zhiming Shi, Michael Bermingham, David StJohn, Matthew Dargusch, and Ma Qian. 2018. "Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications" Materials 11, no. 9: 1573. https://doi.org/10.3390/ma11091573
APA StyleDehghan-Manshadi, A., Chen, Y., Shi, Z., Bermingham, M., StJohn, D., Dargusch, M., & Qian, M. (2018). Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications. Materials, 11(9), 1573. https://doi.org/10.3390/ma11091573





