Three-Dimensional Copper Foil-Powder Sintering Current Collector for a Silicon-Based Anode Lithium-Ion Battery
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussion
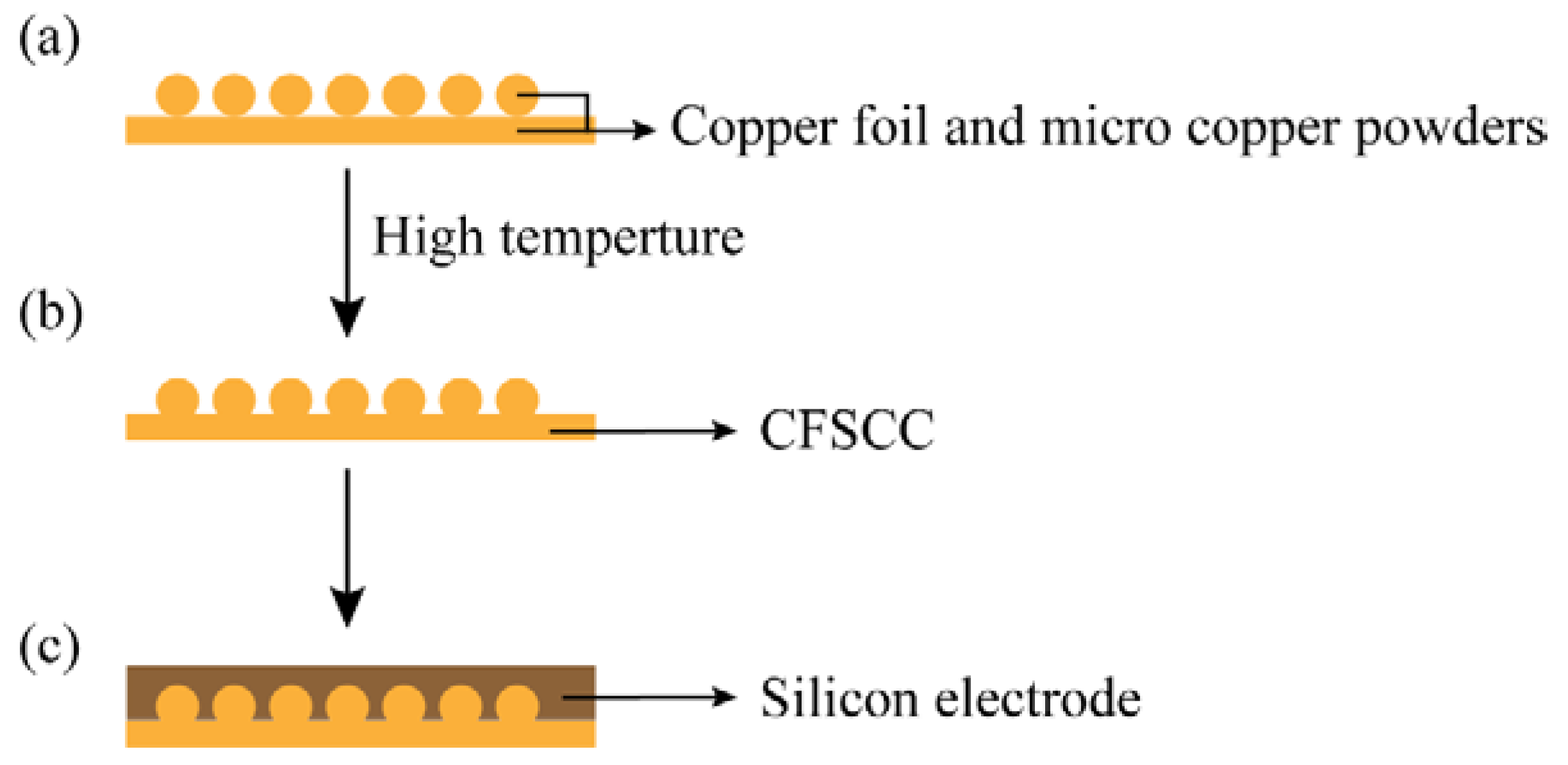
3.1. Foil-Powder Sintering
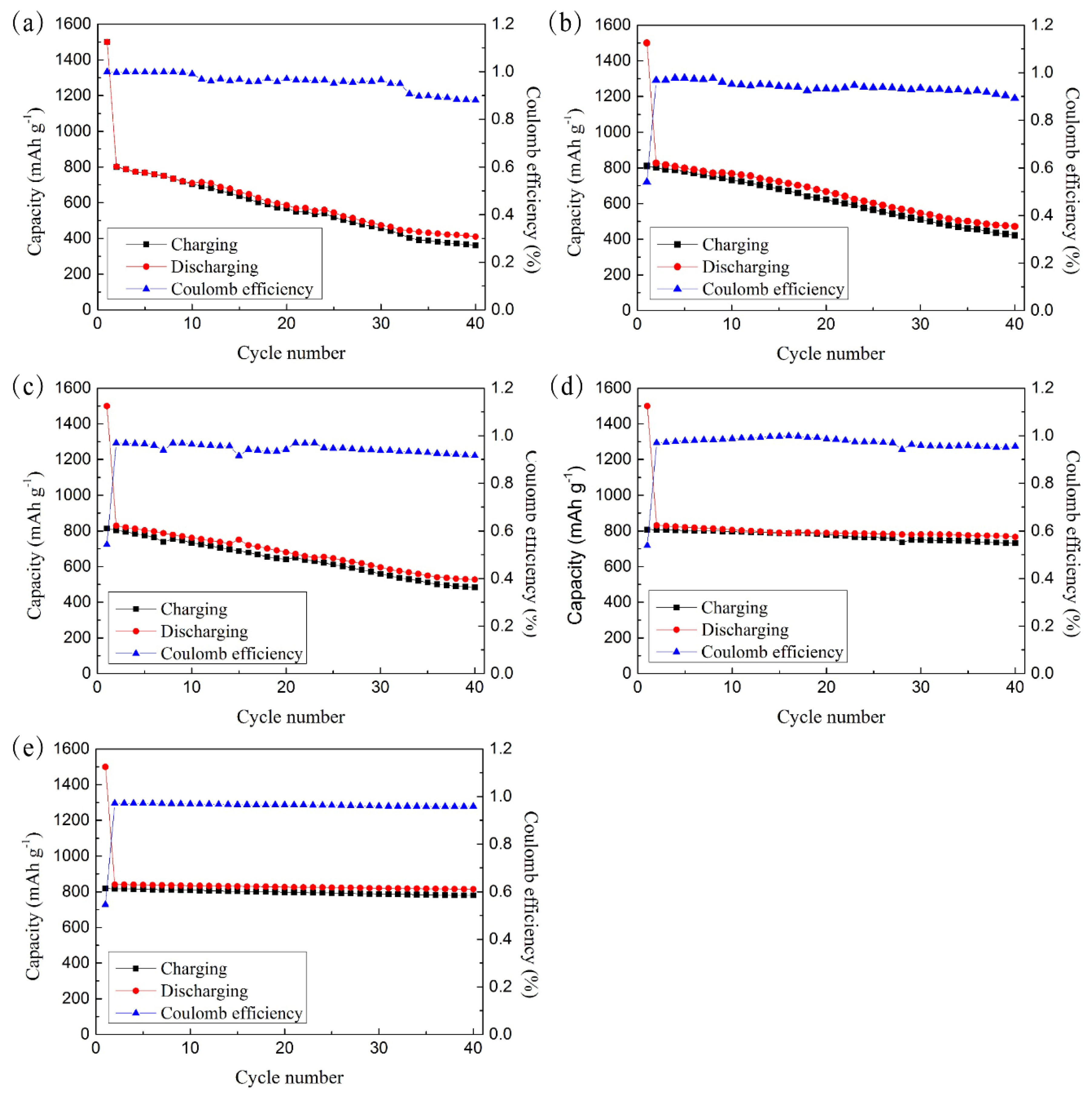
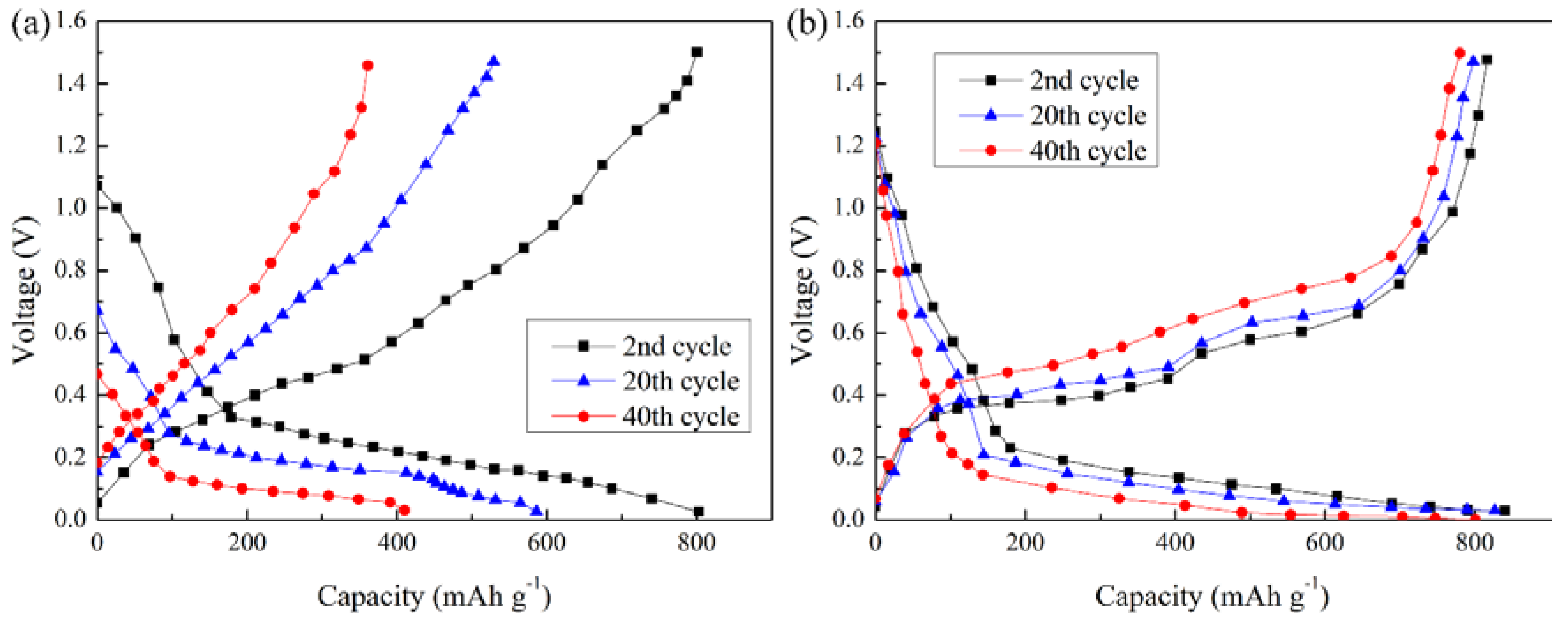
3.2. Electrochemical Performance
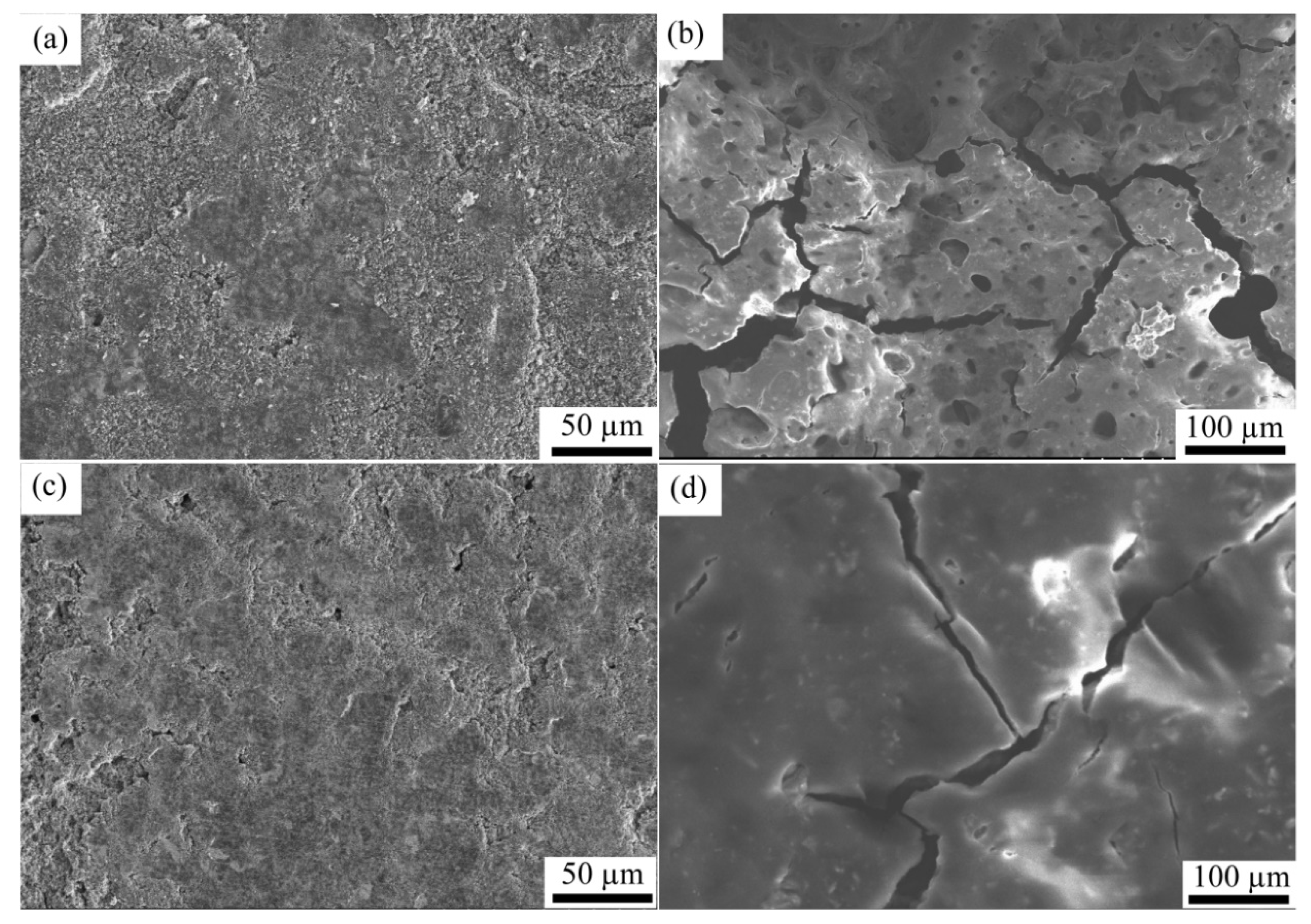
3.3. Surface Morphology Analysis
4. Conclusions
- (1)
- This sintering method can be easily applied to large-scale production.
- (2)
- The CFSCC is technically suitable for Si-based paste electrodes, which are inexpensive, and the paste-like electrode is commonly used in industrial production.
- (3)
- Through the electrochemical test and SEM images, the CFSCC shows the ability to constrain the Si volume change and had better electric performance. The CFSCC with a powder size of 75–100 μm performed best in this study. Compared with the flat current collector, the capacitance of the electrodes with CFSCC remained as high as 92.2% of its second cycle after 40 cycles, whereas that of electrodes with the flat current collector only remained at 51.1%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Wu, H.; Hu, L.; Rowell, M.W.; Kong, D.; Cha, J.J.; McDonough, J.R.; Zhu, J.; Yang, Y.; McGehee, M.D.; Cui, Y. Electrospun metal nanofiber webs as high-performance transparent electrode. Nano Lett. 2010, 10, 4242–4248. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terranova, M.L.; Orlanducci, S.; Tamburri, E.; Guglielmotti, V.; Rossi, M. Si/C hybrid nanostructures for Li-ion anodes: An overview. J. Power Sources 2014, 246, 167–177. [Google Scholar] [CrossRef]
- Haftbaradaran, H.; Xiao, X.; Verbrugge, M.W.; Gao, H. Method to deduce the critical size for interfacial delamination of patterned electrode structures and application to lithiation of thin-film silicon islands. J. Power Sources 2012, 206, 357–366. [Google Scholar] [CrossRef]
- Richards, V. Silicon nanowires: Swallowed whole. Nat. Nanotechnol. 2017. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Wang, C.; Cui, Y. The effect of metallic coatings and crystallinity on the volume expansion of silicon during electrochemical lithiation/delithiation. Nano Energy 2012, 1, 401–410. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Wang, D. Si/TiSi2 heteronanostructures as high-capacity anode material for Li ion batteries. Nano Lett. 2010, 10, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, S.; Qiu, X.; Zhu, W.; Chen, L. Preparation and characterization of silicon-based three-dimensional cellular anode for lithium ion battery. Electrochem. Commun. 2007, 9, 930–934. [Google Scholar] [CrossRef]
- Grupp, R.; Nöthe, M.; Kieback, B.; Banhart, J. Cooperative material transport during the early stage of sintering. Nat. Commun. 2011, 2, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Lee, H.-W.; Ryu, I.; Nix, W.D.; Gao, H.; Cui, Y. Kinetics and fracture resistance of lithiated silicon nanostructure pairs controlled by their mechanical interaction. Nat. Commun. 2015, 6, 7533. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, J.; Liu, H.; Xie, Y.; Tang, W.; Fu, T.; Tang, Y.; Lu, L.; Ding, X.; Tang, X. Three-Dimensional Copper Foil-Powder Sintering Current Collector for a Silicon-Based Anode Lithium-Ion Battery. Materials 2018, 11, 1338. https://doi.org/10.3390/ma11081338
Long J, Liu H, Xie Y, Tang W, Fu T, Tang Y, Lu L, Ding X, Tang X. Three-Dimensional Copper Foil-Powder Sintering Current Collector for a Silicon-Based Anode Lithium-Ion Battery. Materials. 2018; 11(8):1338. https://doi.org/10.3390/ma11081338
Chicago/Turabian StyleLong, Jin, Huilong Liu, Yingxi Xie, Weijin Tang, Ting Fu, Yong Tang, Longsheng Lu, Xinrui Ding, and Xingxian Tang. 2018. "Three-Dimensional Copper Foil-Powder Sintering Current Collector for a Silicon-Based Anode Lithium-Ion Battery" Materials 11, no. 8: 1338. https://doi.org/10.3390/ma11081338




