Preparation and Characterization of AgNPs In Situ Synthesis on Polyelectrolyte Membrane Coated Sericin/Agar Film for Antimicrobial Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
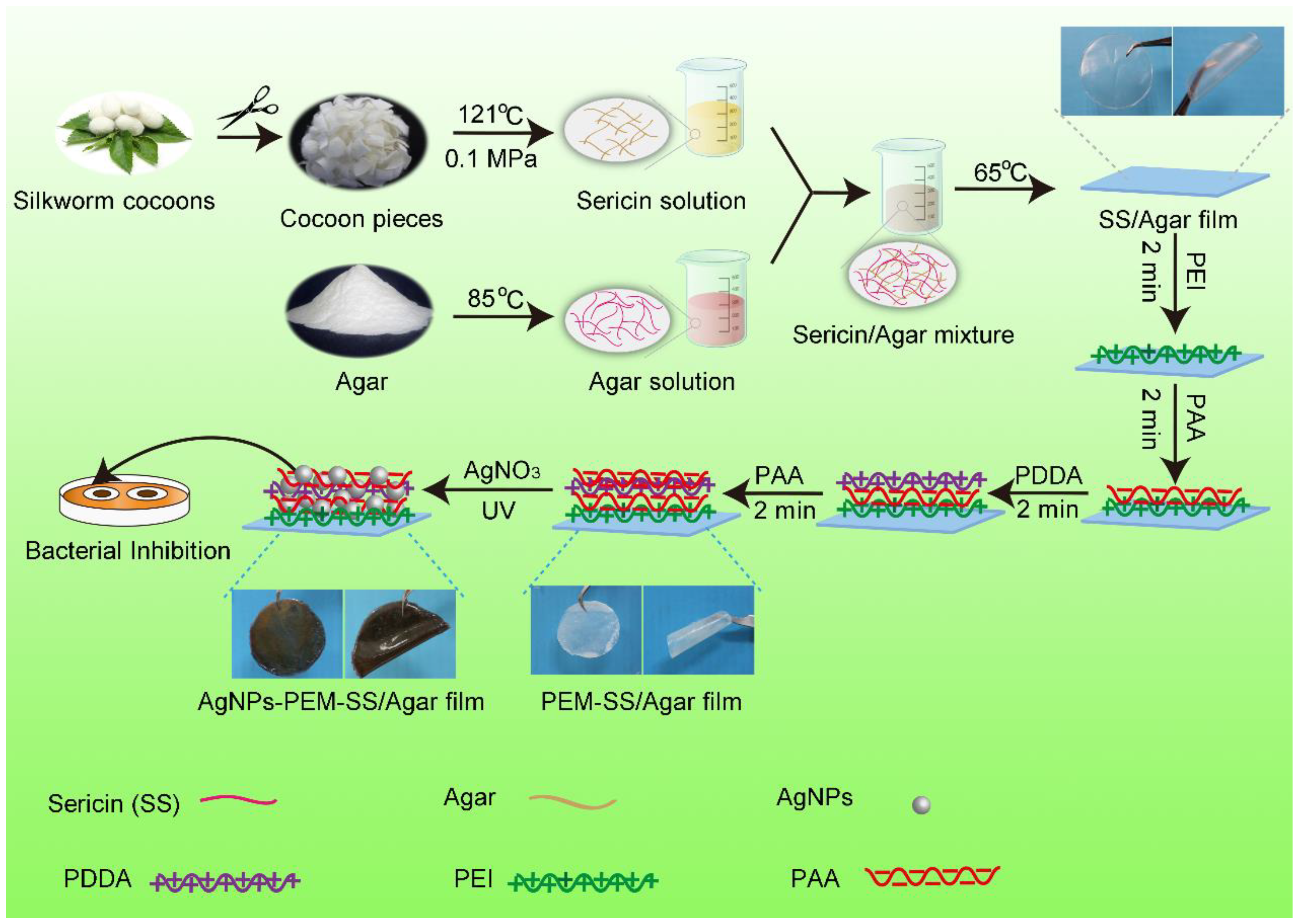
2.2. Fabrication of AgNPs Modified PEM-SS/Agar Film
2.3. Material Characterizations
2.4. XPS Analysis
2.5. Water Contact Angle
2.6. Swelling Ratio
2.7. Mechanical Property
2.8. Inhibition Zone Assay
2.9. Growth Curve Assay
2.10. Antibacterial Stability
2.11. Degradation of AgNPs-PEM-SS/Agar Film
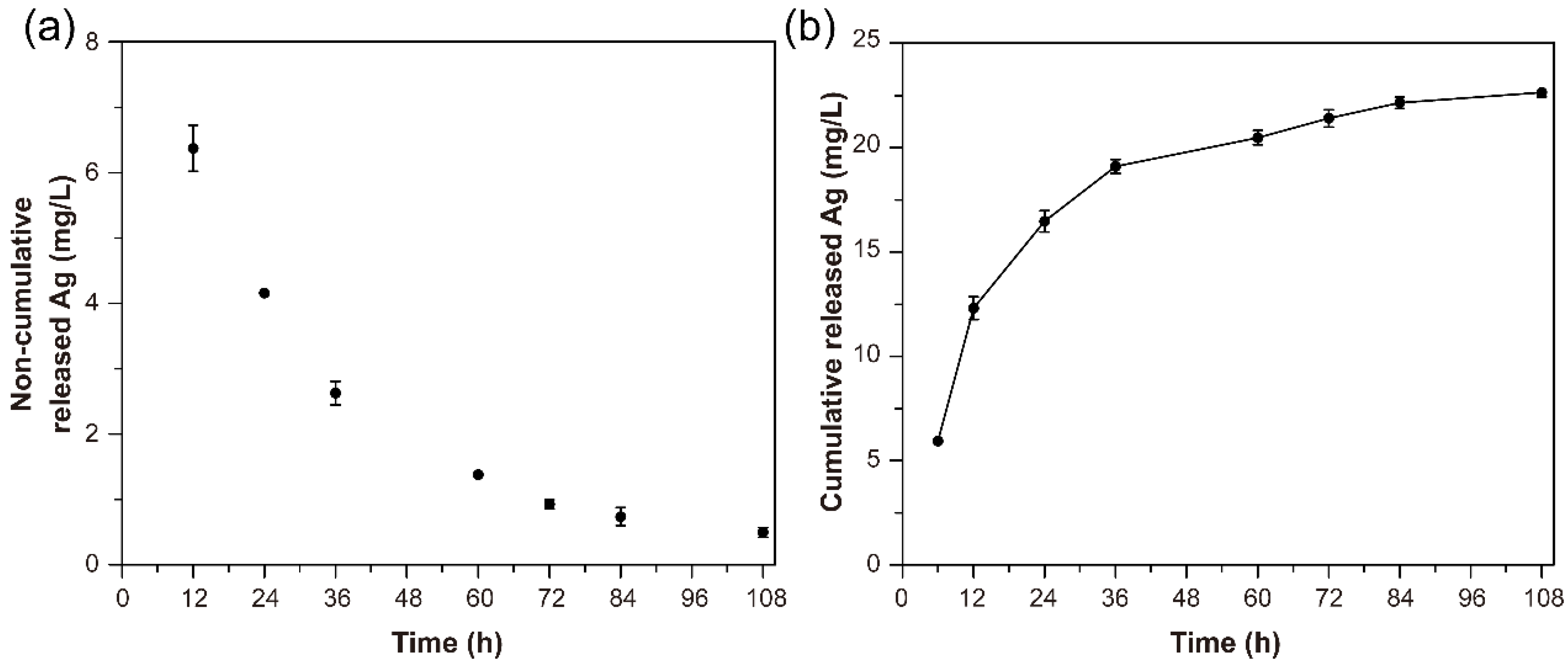
2.12. Release of Silver
2.13. Statistics
3. Results and Discussion
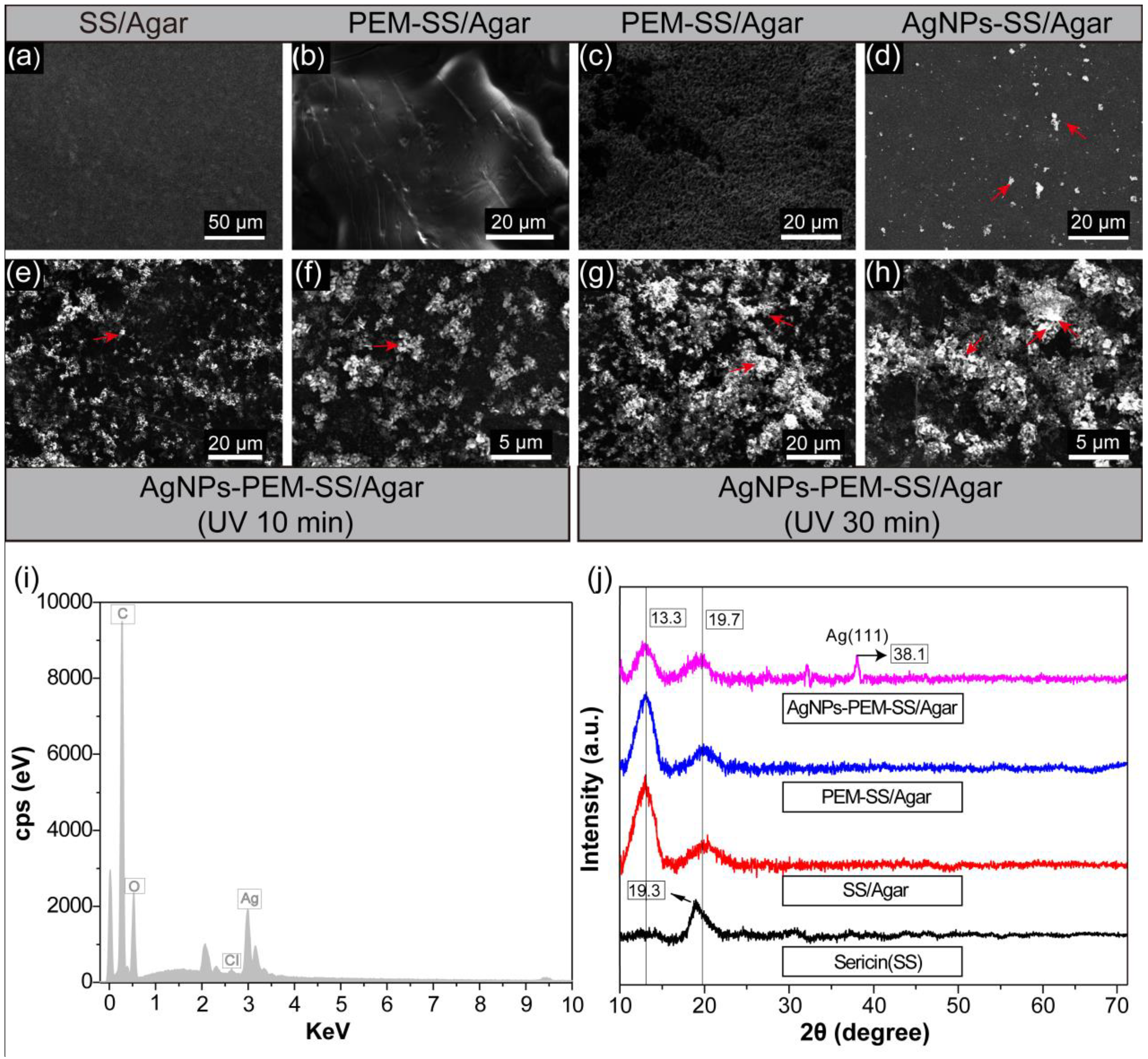
3.1. SEM, EDS and XRD
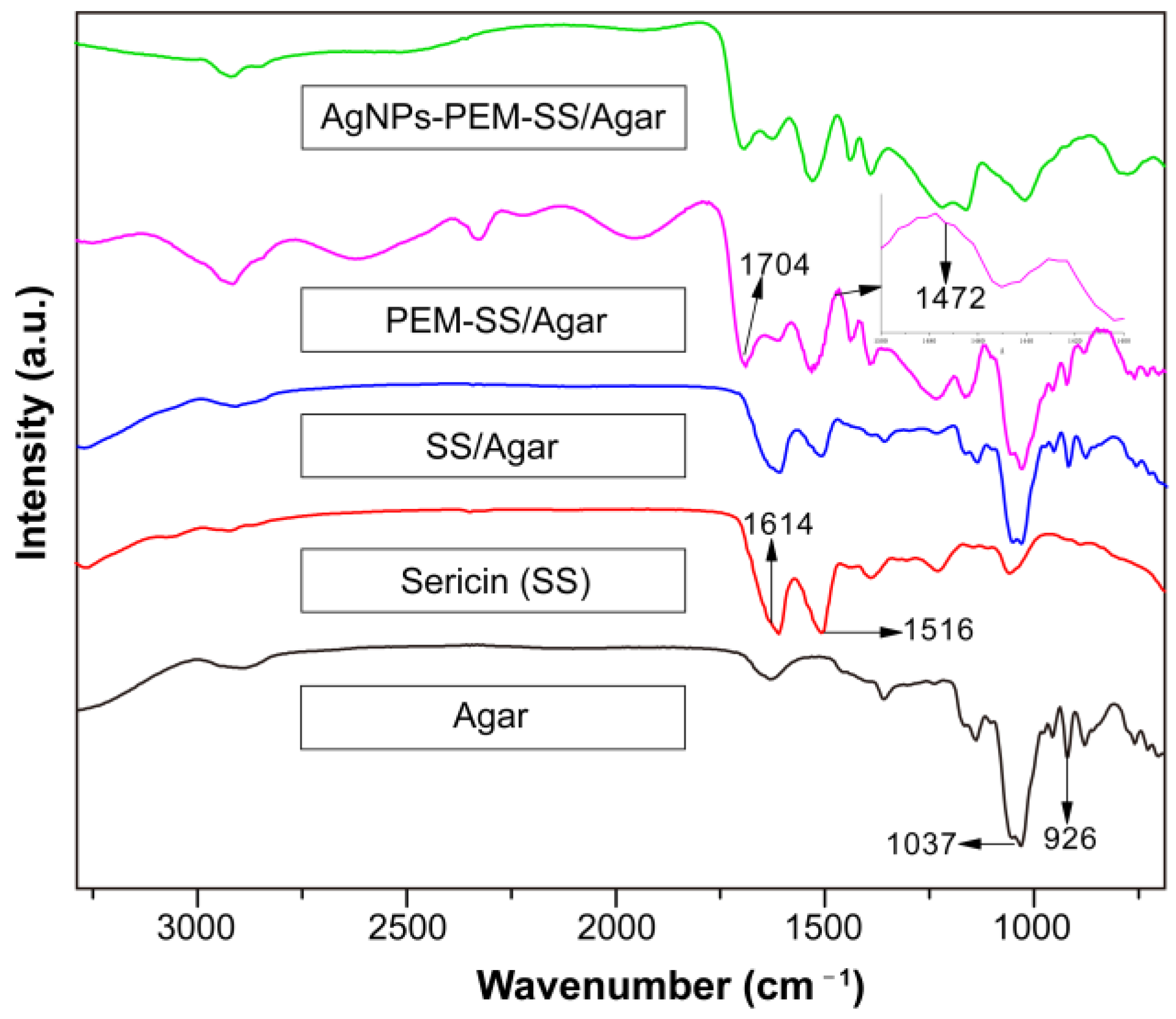
3.2. FT-IR
3.3. XPS
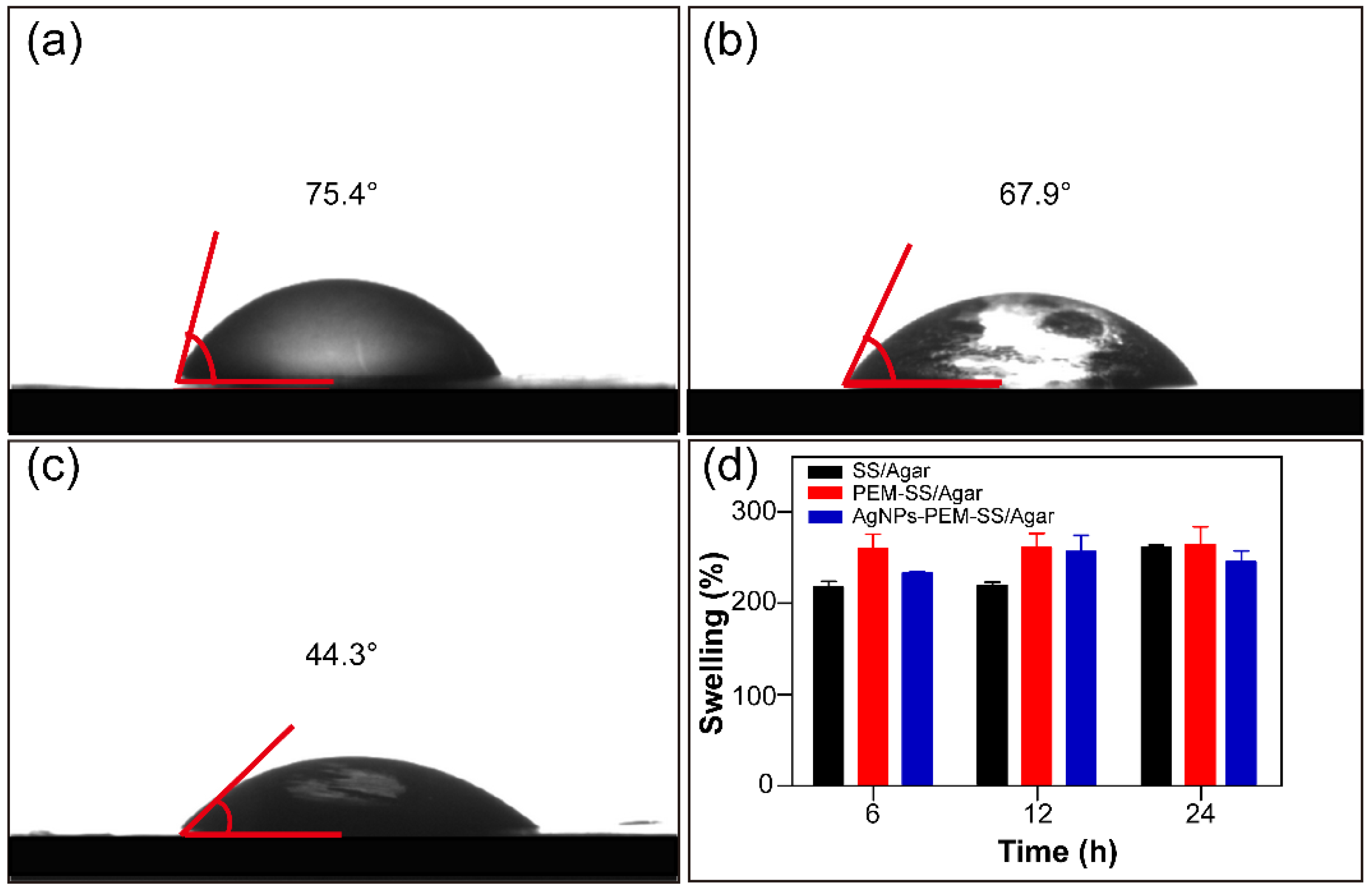
3.4. Wettability and Water Uptake Ability
3.5. Mechanical Property
3.6. Inhibition Zone Assay
3.7. Bacterial Growth Curve
3.8. Antibacterial Stability
3.9. Degradation of AgNPs-PEM-SS/Agar Film
3.10. Release of Silver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Q.; Wu, Z.; Chen, H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology 2007, 18, 285604. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Cai, R.; Wang, Y.; Song, K.; Guo, P.; Zhao, P.; Zuo, H.; He, H. Biosynthesis and characterization of AgNPs–silk/PVA film for potential packaging application. Materials 2017, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Tao, G.; Wang, Y.; Cai, R.; Guo, P.; Chen, L.; Zuo, H.; Zhao, P.; Xia, Q. In situ green synthesis and characterization of sericin-silver nanoparticle composite with effective antibacterial activity and good biocompatibility. Mater. Sci. Eng. C 2017, 80, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Kang, B.; Dai, Y.; Chen, D. Synthesis of antimicrobial silver nanoparticles on silk fibers via γ-radiation. J. Appl. Polym. Sci. 2009, 112, 2511–2515. [Google Scholar] [CrossRef]
- Cintas, P.; Palmisano, G.; Cravotto, G. Power ultrasound in metal-assisted synthesis: From classical barbier-like reactions to click chemistry. Ultrason. Sonochem. 2011, 18, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Tao, M.L.; Shen, W.D.; Zhou, Y.Z.; Ding, Y.; Ma, Y.; Zhou, W.L. Immobilization of l-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 2004, 25, 3751–3759. [Google Scholar] [CrossRef] [PubMed]
- Akturk, O.; Tezcaner, A.; Bilgili, H.; Deveci, M.S.; Gecit, M.R.; Keskin, D. Evaluation of sericin/collagen membranes as prospective wound dressing biomaterial. J. Biosci. Bioeng. 2011, 112, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Gamo, T.; Inokuchi, T.; Laufer, H. Polypeptides of fibroin and sericin secreted from the different sections of the silk gland in bombyx mori. Insect Biochem. 1977, 7, 285–295. [Google Scholar] [CrossRef]
- Kundu, B.; Kundu, S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 2012, 33, 7456–7467. [Google Scholar] [CrossRef] [PubMed]
- Nagura, M.; Ohnishi, R.; Gitoh, Y.; Ohkoshi, Y. Structures and physical properties of cross-linked sericin membranes. J. Insect Biotechnol. Sericol. 2001, 70, 149–153. [Google Scholar]
- Teramoto, H.; Nakajima, K.I.; Takabayashi, C. Chemical modification of silk sericin in lithium chloride/dimethyl sulfoxide solvent with 4-cyanophenyl isocyanate. Biomacromolecules 2004, 5, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, M.L.; Liu, L.; Feng, X. Sericin/poly (vinyl alcohol) blend membranes for pervaporation separation of ethanol/water mixtures. J. Membr. Sci. 2007, 295, 71–79. [Google Scholar] [CrossRef]
- Zhaorigetu, S.; Yanaka, N.; Sasaki, M.; Watanabe, H.; Kato, N. Inhibitory effects of silk protein, sericin on uvb-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J. Photochem. Photobiol. B 2003, 71, 11–17. [Google Scholar] [CrossRef]
- Tamada, Y.; Sano, M.; Niwa, K.; Imai, T.; Yoshino, G. Sulfation of silk sericin and anticoagulant activity of sulfated sericin. J. Biomater. Sci. Polym. Ed. 2004, 15, 971–980. [Google Scholar] [CrossRef]
- Terada, S.; Sasaki, M.; Yanagihara, K.; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng. 2005, 100, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Padamwar, M.N.; Pawar, A.P.; Daithankar, A.V.; Mahadik, K. Silk sericin as a moisturizer: An in vivo study. J. Cosmet. Dermatol. 2005, 4, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Kameda, T.; Tamada, Y. Preparation of gel film from bombyx mori silk sericin and its characterization as a wound dressing. Biosci. Biotechnol. Biochem. 2008, 72, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Priya, A.S.; Kundu, S. Novel silk sericin/gelatin 3-d scaffolds and 2-d films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009, 5, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Tao, G.; He, H.; Guo, P.; Yang, M.; Ding, C.; Zuo, H.; Wang, L.; Zhao, P.; Wang, Y. In situ synthesis of silver nanoparticles on the polyelectrolyte-coated sericin/pva film for enhanced antibacterial application. Materials 2017, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Dey, T.; Naskar, D.; Kundu, S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials 2013, 34, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; He, H.; Xiao, J.; Zhao, P.; Xie, J.; Lu, Z. Controllable in situ synthesis of silver nanoparticles on multilayered film-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 2016, 461, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Stokols, S.; Tuszynski, M.H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 2006, 27, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Tao, G.; He, H.; Song, K.; Zuo, H.; Jiang, W.; Wang, Y. One-step synthesis of silver nanoparticles on polydopamine-coated sericin/polyvinyl alcohol composite films for potential antimicrobial applications. Molecular 2017, 22, 721. [Google Scholar] [CrossRef] [PubMed]
- Abalde-Cela, S.; Ho, S.; Rodríguez-González, B.; Correa-Duarte, M.A.; Álvarez-Puebla, R.A.; Liz-Marzán, L.M.; Kotov, N.A. Loading of exponentially grown lbl films with silver nanoparticles and their application to generalized sers detection. Angew. Chem. Int. Ed. 2009, 48, 5326–5329. [Google Scholar] [CrossRef] [PubMed]
- Dubas, S.T.; Kumlangdudsana, P.; Potiyaraj, P. Layer-by-layer deposition of antimicrobial silver nanoparticles on textile fibers. Colloids Surf. A 2006, 289, 105–109. [Google Scholar] [CrossRef]
- Tao, G.; Wang, Y.; Liu, L.; Chang, H.; Zhao, P.; He, H. Preparation and characterization of silver nanoparticles composited on polyelectrolyte film coated sericin gel for enhanced antibacterial application. Sci. Adv. Mater. 2016, 8, 1547–1552. [Google Scholar] [CrossRef]
- Lai, X.; Gao, G.; Watanabe, J.; Liu, H.; Shen, H. Hydrophilic polyelectrolyte multilayers improve the elisa system: Antibody enrichment and blocking free. Polymers 2017, 9, 51. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, Z.; Xu, S.Y. Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem. 2007, 103, 1255–1262. [Google Scholar] [CrossRef]
- Schillinger, U.; Lücke, F.K. Antibacterial activity of lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901. [Google Scholar] [PubMed]
- Machado, G.; Beppu, M.M.; Feil, A.F.; Figueroa, C.A.; Correia, R.R.B.; Teixeira, S.R. Silver nanoparticles obtained in pah/paa-based multilayers by photochemical reaction. J. Phys. Chem. C 2009, 113, 19005–19010. [Google Scholar] [CrossRef]
- Tao, W.; Li, M.; Xie, R. Preparation and structure of porous silk sericin materials. Macromol. Mater. Eng. 2005, 290, 188–194. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Rhim, J.W. Properties and characterization of agar/cunp bionanocomposite films prepared with different copper salts and reducing agents. Carbohydr. Polym. 2014, 114, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. The influence of lyophilized emugel silica microspheres on the physicomechanical properties, in vitro bioactivity and biodegradation of a novel ciprofloxacin-loaded pcl/paa scaffold. Polymers 2016, 8, 232. [Google Scholar] [CrossRef]
- He, H.; Cai, R.; Wang, Y.; Tao, G.; Guo, P.; Zuo, H.; Chen, L.; Liu, X.; Zhao, P.; Xia, Q. Preparation and characterization of silk sericin/pva blend film with silver nanoparticles for potential antimicrobial application. Int. J. Biol. Macromol. 2017, 104, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zeng, X.; Tan, W.; Luo, J.; Liu, S. Quaternized chitosan/rectorite/agnp nanocomposite catalyst for reduction of 4-nitrophenol. J. Alloys Compd. 2015, 647, 463–470. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, Z.K.; Liu, G.; Shen, B.; Kang, P.D.; Li, J.; Li, Q.; Pei, F.X. High-value utilization of lignin to synthesize ag nanoparticles with detection capacity for hg2+. Acs Appl. Mater. Interfaces 2014, 6, 16147–16155. [Google Scholar]
- Penchev, H.; Paneva, D.; Manolova, N.; Rashkov, I. Electrospun hybrid nanofibers based on chitosan or n-carboxyethylchitosan and silver nanoparticles. Macromol. Biosci. 2009, 9, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jahid, M.A.; Cai, G.; Wu, J.; Wang, X. Surface characterisation of polyelectrolyte/silver nanocomposite films. Polym. Polym. Compos. 2017, 25, 635–642. [Google Scholar]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Shao, X.S.; Zhou, Q.; Li, M.Z.; Zhang, Q.Q. The double effects of silver nanoparticles on the pvdf membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Mecha, C.A.; Pillay, V.L. Development and evaluation of woven fabric microfiltration membranes impregnated with silver nanoparticles for potable water treatment. J. Membr. Sci. 2014, 458, 149–156. [Google Scholar] [CrossRef]
- Jin, Y.; Deng, D.; Cheng, Y.; Kong, L.; Xiao, F. Annealing-free and strongly adhesive silver nanowire networks with long-term reliability by introduction of a nonconductive and biocompatible polymer binder. Nanoscale 2014, 6, 4812–4818. [Google Scholar] [CrossRef] [PubMed]
- Don, T.M.; Chen, C.C.; Lee, C.K.; Cheng, W.Y.; Cheng, L.P. Preparation and antibacterial test of chitosan/paa/pegda bi-layer composite membranes. J. Biomater. Sci. Polym. Ed. 2005, 16, 1503–1519. [Google Scholar] [CrossRef]
- Elashnikov, R.; Lyutakov, O.; Kalachyova, Y.; Solovyev, A.; Svorcik, V. Tunable release of silver nanoparticles from temperature-responsive polymer blends. React. Funct. Polym. 2015, 93, 163–169. [Google Scholar] [CrossRef]
- Elashnikov, R.; Lyutakov, O.; Ulbrich, P.; Svorcik, V. Light-activated polymethylmethacrylate nanofibers with antibacterial activity. Mater. Sci. Eng. C 2016, 64, 229. [Google Scholar] [CrossRef] [PubMed]
- Hadipour-Goudarzi, E.; Montazer, M.; Latifi, M.; Aghaji, A.A. Electrospinning of chitosan/sericin/pva nanofibers incorporated with in situ synthesis of nano silver. Carbohydr. Polym. 2014, 113, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.K.; Singh, R.P.; Reddy, C.R.; Jha, B. Synthesis and characterization of agar-based silver nanoparticles and nanocomposite film with antibacterial applications. Bioresour. Technol. 2012, 107, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Y.; Cai, R.; Tao, G.; Chang, H.; Ding, C.; Zuo, H.; Shen, H.; Zhao, P.; He, H. Preparation and characterization of silk sericin/glycerol films coated with silver nanoparticles for antibacterial application. Sci. Adv. Mater. 2018, 10, 761–768. [Google Scholar] [CrossRef]


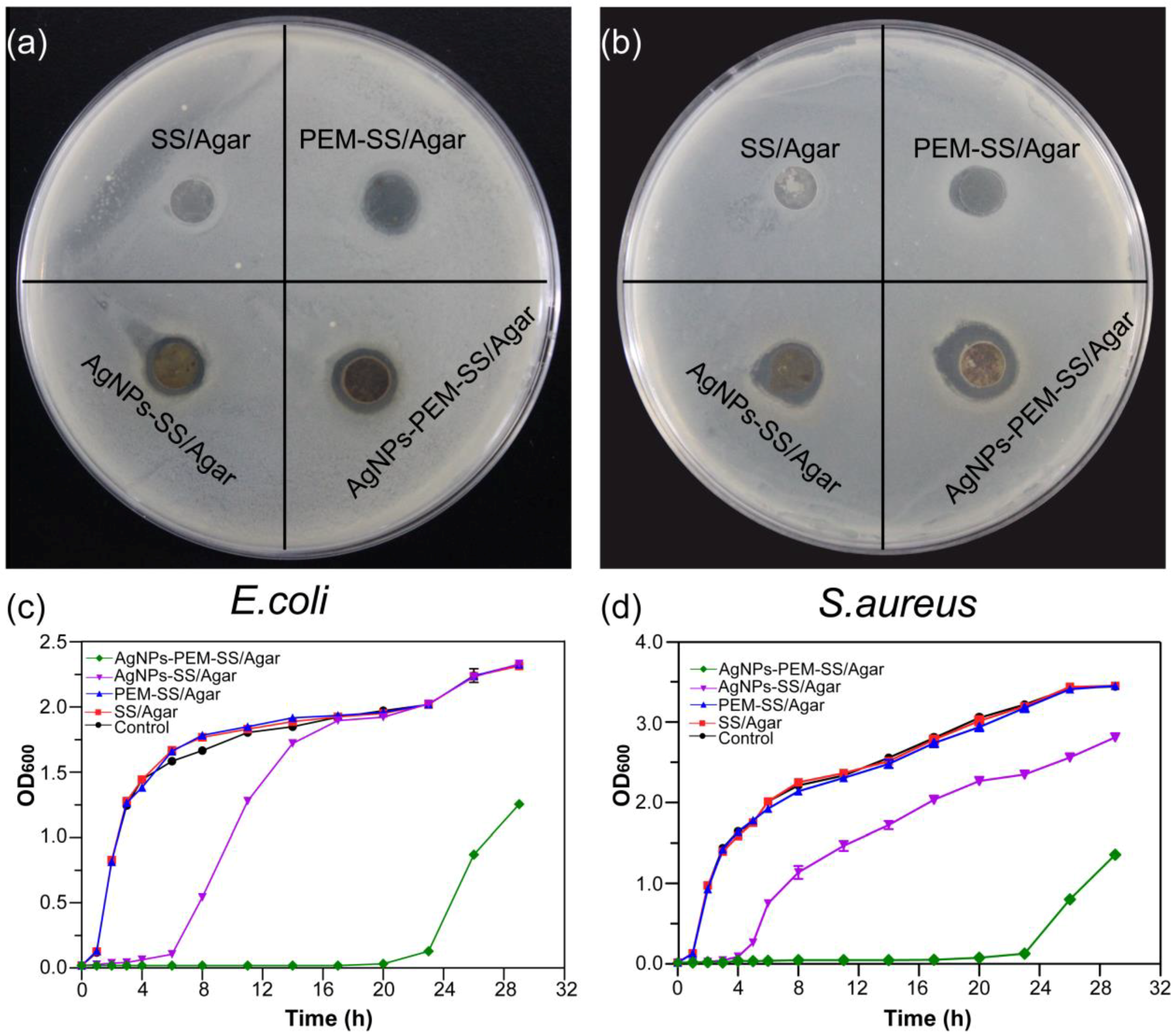


| Bacteria | Control (cm) | PEM-SS/Agar (cm) | AgNPs-SS/Agar (cm) | AgNPs-PEM-SS/Agar (cm) |
|---|---|---|---|---|
| E. coli | 1.10 ± 0.00 | 1.31 ± 0.01 | 1.51 ± 0.03 | 1.68 ± 0.04 |
| S. aureus | 1.10 ± 0.00 | 1.20 ± 0.10 | 1.65 ± 0.05 | 1.98 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Cai, R.; Wang, Y.; Tao, G.; Ai, L.; Wang, P.; Yang, M.; Zuo, H.; Zhao, P.; Shen, H.; et al. Preparation and Characterization of AgNPs In Situ Synthesis on Polyelectrolyte Membrane Coated Sericin/Agar Film for Antimicrobial Applications. Materials 2018, 11, 1205. https://doi.org/10.3390/ma11071205
Liu L, Cai R, Wang Y, Tao G, Ai L, Wang P, Yang M, Zuo H, Zhao P, Shen H, et al. Preparation and Characterization of AgNPs In Situ Synthesis on Polyelectrolyte Membrane Coated Sericin/Agar Film for Antimicrobial Applications. Materials. 2018; 11(7):1205. https://doi.org/10.3390/ma11071205
Chicago/Turabian StyleLiu, Liying, Rui Cai, Yejing Wang, Gang Tao, Lisha Ai, Peng Wang, Meirong Yang, Hua Zuo, Ping Zhao, Hong Shen, and et al. 2018. "Preparation and Characterization of AgNPs In Situ Synthesis on Polyelectrolyte Membrane Coated Sericin/Agar Film for Antimicrobial Applications" Materials 11, no. 7: 1205. https://doi.org/10.3390/ma11071205





