Microstructure and Mechanical Properties of Si3N4-Fe3Si Composites Prepared by Gas-Pressure Sintering
Abstract
:1. Introduction
2. Materials and Methods
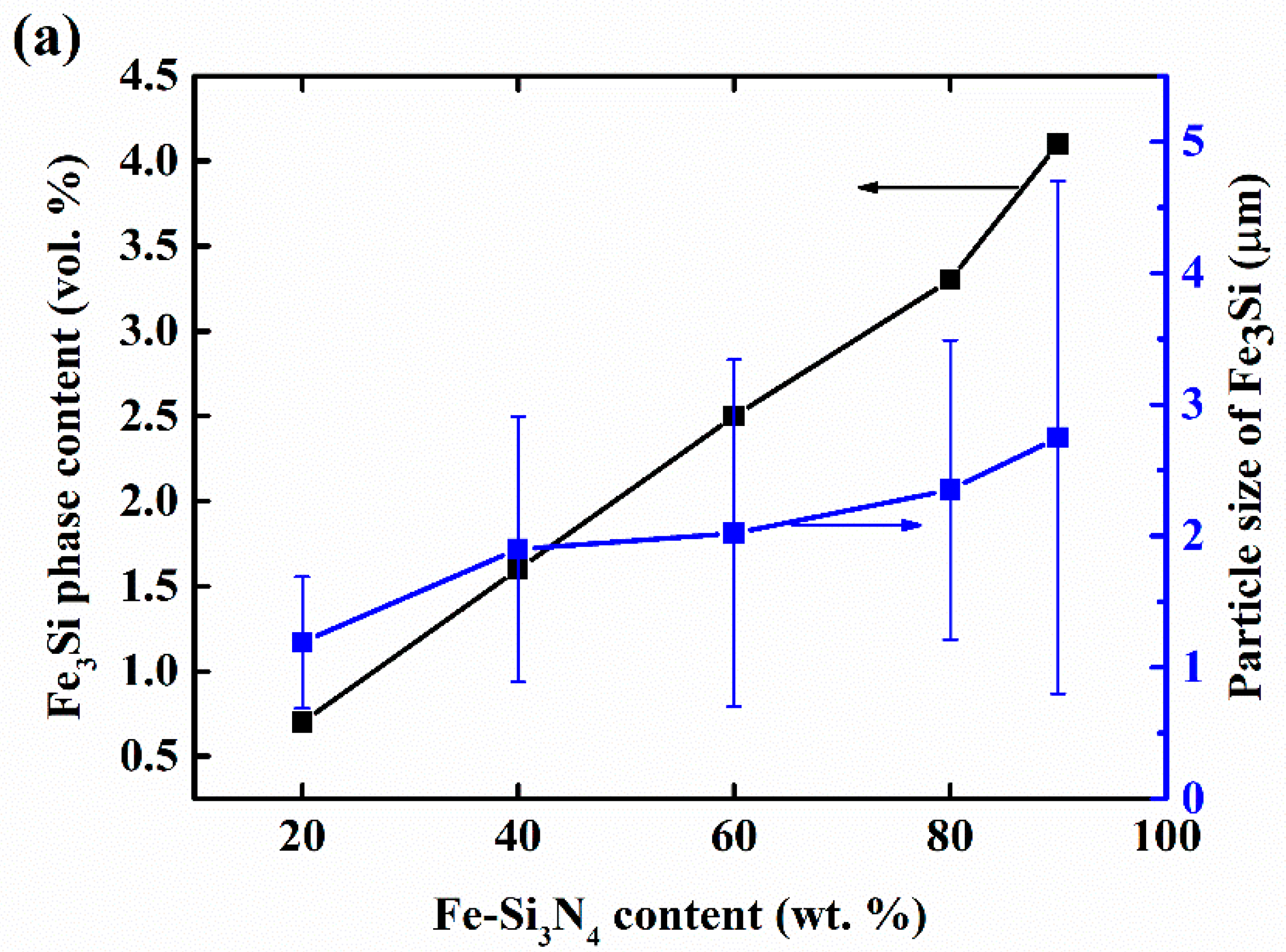
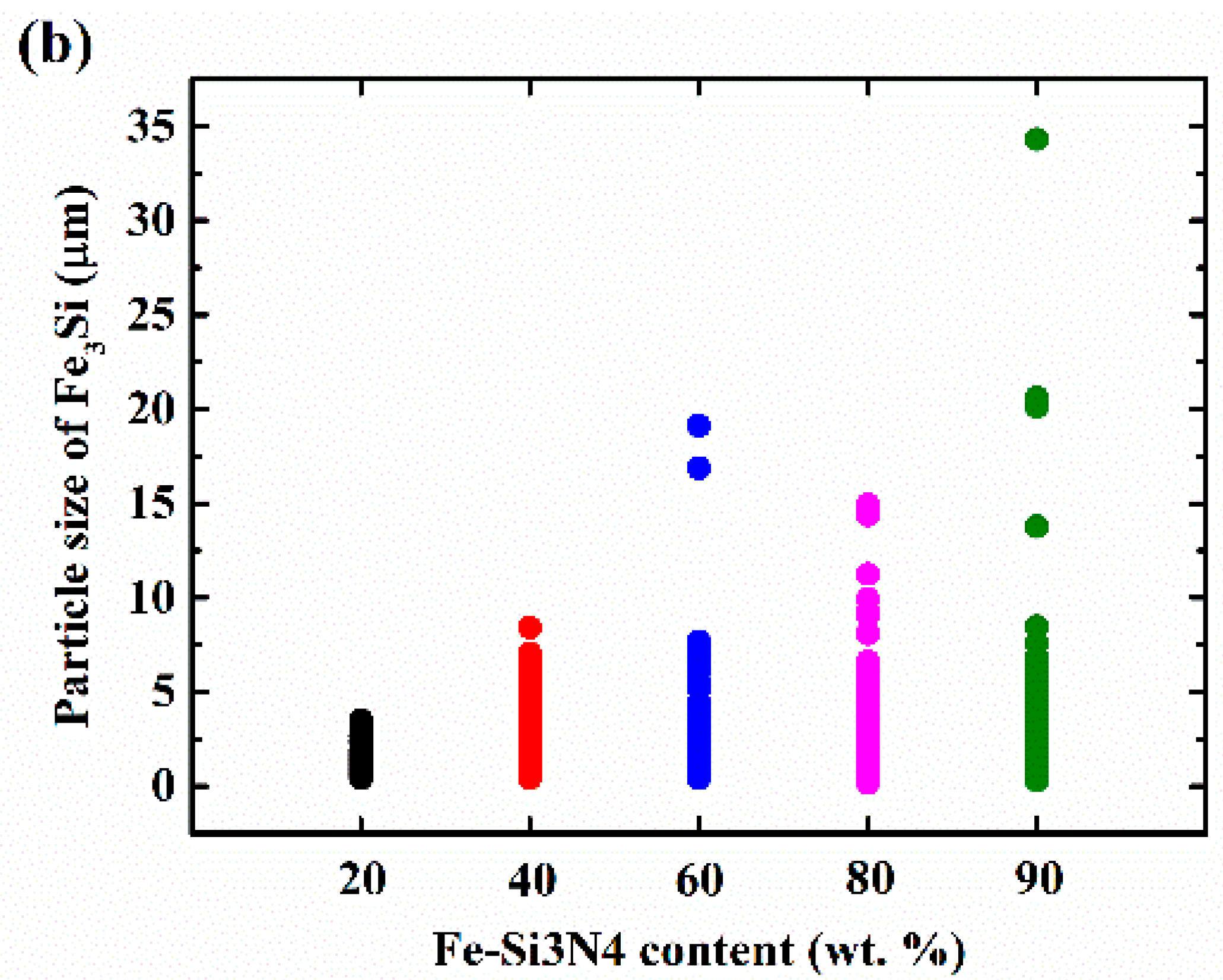
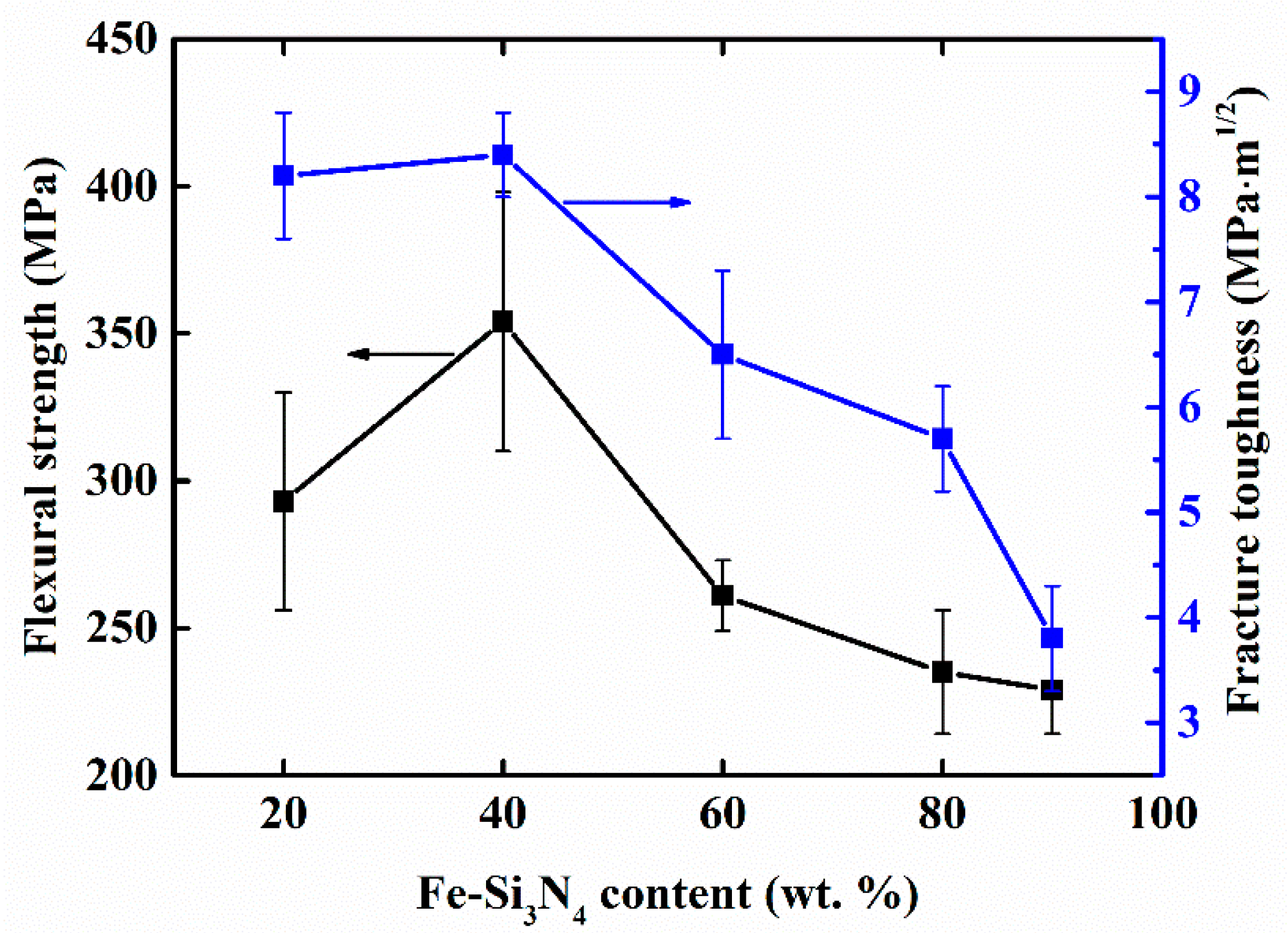
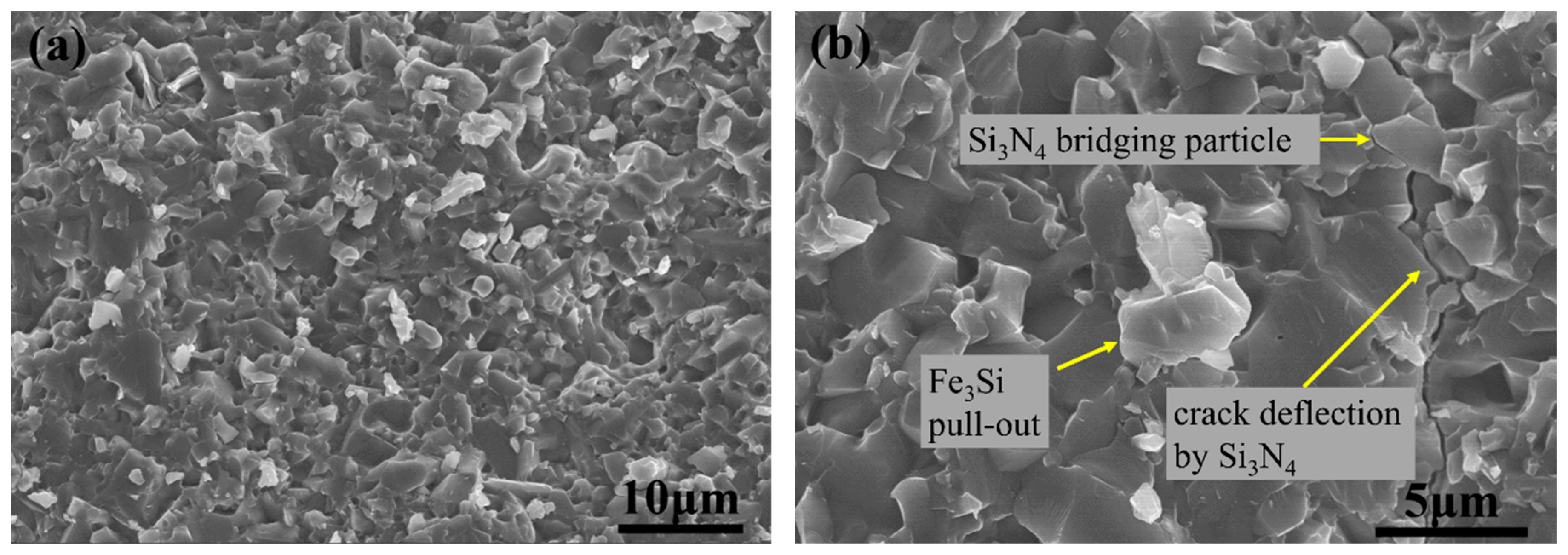
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Riley, F.L. Silicon nitride and related materials. J. Am. Ceram. Soc. 2000, 83, 245–265. [Google Scholar] [CrossRef]
- Klemm, H. Silicon Nitride for High-Temperature Applications. J. Am. Ceram. Soc. 2010, 93, 1501–1522. [Google Scholar] [CrossRef]
- Shen, Z.; Zhao, Z.; Peng, H.; Nygren, M. Formation of tough interlocking microstructures in silicon nitride ceramics by dynamic ripening. Nature 2002, 417, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Petzow, G.; Herrmann, M. Silicon nitride ceramics. In High Performance Non-Oxide Ceramics II; Springer: Berlin/Heidelberg, Germany, 2002; pp. 47–167. [Google Scholar]
- Kometani, K.; Iizuka, K.; Kaga, T. Behavior of Ferro-Si3N4 in Taphole Mud of Blast Furnace. Refractories 1998, 50, 326–330. [Google Scholar]
- Ziatdinov, M.K.; Shatokhin, I. Using ferrosilicon nitride of nitro-fesil grade in gate and spout components. Refract. Ind. Ceram. 2008, 49, 383–387. [Google Scholar] [CrossRef]
- Mitomo, M. Effect of Fe and Al additions on nitridation of silicon. J. Mater. Sci. 1977, 12, 273–276. [Google Scholar] [CrossRef]
- Vlasova, M.; Lavrenko, V.; Dyubova, L.Y.; Gonzalez-Rodriguez, J.; Kakasey, M. Nitriding of ferrosilicon powders. J. Mater. Synth. Process. 2001, 9, 111–117. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Guan, J.; Zhang, L. Effect of dilution and additive on direct nitridation of ferrosilicon. J. Eur. Ceram. Soc. 2014, 34, 1115–1122. [Google Scholar] [CrossRef]
- Chukhlomina, L. Fundamental aspects of silicon nitride synthesis by combustion of an iron-silicon alloy in nitrogen. Russ. J. Appl. Chem. 2007, 80, 1793–1797. [Google Scholar] [CrossRef]
- Chukhlomina, L.; Maksimov, Y.M.; Kitler, V.; Vitushkina, O. Mechanism and features of nitriding of ferrosilicon in the combustion regime. Combust. Explos. Shock Waves 2006, 42, 309–316. [Google Scholar] [CrossRef]
- Chukhlomina, L.; Maksimov, Y.M. Combustion of the Fe-Si alloy in nitrogen gas. Int. J. Self-Propagating High-Temp. Synth. 2007, 16, 18–22. [Google Scholar] [CrossRef]
- Chukhlomina, L. Chemically and thermally conjugate synthesis of silicon nitride based compositions using ferrosilicon. Glass Ceram. 2009, 66, 288–292. [Google Scholar] [CrossRef]
- Pavarajarn, V.; Kimura, S. Catalytic effects of metals on direct nitridation of silicon. J. Am. Ceram. Soc. 2001, 84, 1669–1674. [Google Scholar] [CrossRef]
- Lin, S.S. Comparative studies of metal additives on the nitridation of silicon. J. Am. Ceram. Soc. 1977, 60, 78–81. [Google Scholar] [CrossRef]
- Chen, J.H.; Wen, S.; Liu, X.G. Formation mechanism of FexSi particles in ferro-silicon nitride prepared via flashing combustion. J. Univ. Sci. Technol. Beijing 2009, 31, 597–601. [Google Scholar]
- Chukhlomina, L.; Maksimov, Y.M.; Vitushkina, O.; Golobokov, N.; Vereshchagin, V.I. Phase composition and morphology of products of combustion of ferrosilicon in nitrogen. Glass Ceram. 2007, 64, 63–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, D.Y.; Wen, H.J. Sintering process of special ceramics Fe-Si3N4 bonded SiC. J. Iron Steel Res. 2002, 14, 25–28. [Google Scholar]
- Li, Y.; Zhu, X.Y.; Zhai, Y.W.; Wang, J.P.; Xue, W.D.; Chen, J.H.; Sun, J.L. Study on High Performance Fe3Si-Si3N4-SiC Composite Preparation and its Application in Blast Furnace. Adv. Mater. Res. 2011, 194–196, 1547–1553. [Google Scholar] [CrossRef]
- Vitushkina, O.; Chukhlomina, L.; Vereshchagin, V.I. Preparation of Si3N4–ZrO2 ceramic composites by self-propagating high-temperature synthesis. Refract. Ind. Ceram. 2012, 52, 402–404. [Google Scholar] [CrossRef]
- Daniel, B.; Murthy, V.; Murty, G. Metal-ceramic composites via in-situ methods. J. Mater. Process. Technol. 1997, 68, 132–155. [Google Scholar] [CrossRef]
- Burkes, D.E.; Gottoli, G.; Moore, J.J.; Yi, H.C. Combustion synthesis and mechanical properties of dense NiTi-TiC intermetallic-ceramic composites. Metall. Mater. Trans. A 2006, 37, 235–242. [Google Scholar] [CrossRef]
- Schneibel, J.; Carmichael, C.; Specht, E.; Subramanian, R. Liquid-phase sintered iron aluminide-ceramic composites. Intermetallics 1997, 5, 61–67. [Google Scholar] [CrossRef]
- Klassen, T.; Günther, R.; Dickau, B.; Gärtner, F.; Bartels, A.; Bormann, R.; Mecking, H. Processing and properties of intermetallic/ceramic composites with interpenetrating microstructure. J. Am. Ceram. Soc. 1998, 81, 2504–2506. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Marino, F.; Zhang, B.R.; Gialanella, S. Ni3Al intermetallic compound as second phase in Al2O3 ceramic composites. Mater. Sci. Eng. 1997, 239–240, 665–671. [Google Scholar] [CrossRef]
- Chen, W.-W.; Cheng, Y.-B.; Tuan, W.-H. Preparation of sialon–transition metal silicide composites. J. Eur. Ceram. Soc. 2006, 26, 193–199. [Google Scholar] [CrossRef]
- Gong, H.; Yin, Y.; Wang, X.; Liu, Y. Fabrication and microstructure of in situ toughened Al2O3/Fe3Al. Mater. Res. Bull. 2004, 39, 513–521. [Google Scholar] [CrossRef]
- Wang, L.; Qi, Q.; Wu, H.; Zhang, H.; Yin, J.; Yang, Y.; Liu, X.; Huang, Z. Stress distribution around Fe5Si3 and its effect on interface status and mechanical properties of Si3N4 ceramics. J. Am. Ceram. Soc. 2018, 101, 856–864. [Google Scholar] [CrossRef]
- Koch, C.C.; Liu, C.T.; Stoloff, N.S.; Wanner, A. High-Temperature Ordered Intermetallic Alloys; Materials Research Society: Warrendale, PA, USA, 1989. [Google Scholar]
- Miglio, L.; D’Heurle, F. Silicides: Fundamentals and Applications. Mater. Trans. 1923, 43, 1023–1029. [Google Scholar]
- Becerro, A.I.; Escudero, A. Phase Transitions in Lu-Doped Y2Si2O7 at High Temperatures. Chem. Mater. 2005, 17, 112–117. [Google Scholar] [CrossRef]
- Bouarroudj, A.; Goursat, P.; Besson, J.L. Oxidation resistance and creep behaviour of a silicon nitride ceramic densified with Y2O3. J. Mater. Sci. 1985, 20, 1150–1159. [Google Scholar] [CrossRef]
- Cinibulk, M.K.; Thomas, G.; Johnson, S.M. Strength and Creep Behavior of Rare-Earth Disilicate–Silicon Nitride Ceramics. J. Am. Ceram. Soc. 1992, 75, 2050–2055. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. (Eds.) Wettability at High Temperatures; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Narciso-Romero, F.J.; Rodriguez-Reinoso, F. Synthesis of SiC from rice husks catalysed by iron, cobalt or nickel. J. Mater. Sci. 1996, 31, 779–784. [Google Scholar] [CrossRef]
- Shimoo, T.; Okamura, K.; Yamasaki, T. Reaction between Si3N4 and Fe-Ni alloy. J. Mater. Sci. 1999, 34, 5525–5532. [Google Scholar] [CrossRef]
- Novgorodova, M.I.; Yusupov, R.G.; Dmitrieva, M.T.; Tsepin, A.I.; Sivtsov, A.V.; Gorshkov, A.I.; Korovushkin, V.V.; Yakubovskaya, N.Y. First occurrence of suessite on the Earth. Int. Geol. Rev. 1984, 26, 98–101. [Google Scholar] [CrossRef]
- Dokken, R.N. A resistance-measurement study of ordering in iron-silicon alloys FeSi-and Fe3Si-type superstructures. Trans. AIME 1965, 233, 1187. [Google Scholar]
- Ziegler, G.; Heinrich, J.; Wötting, G. Relationships between processing, microstructure and properties of dense and reaction-bonded silicon nitride. J. Mater. Sci. 1987, 22, 3041–3086. [Google Scholar] [CrossRef]
- Becher, P.F.; Painter, G.S.; Sun, E.Y.; Hsueh, C.H.; Lance, M.J. The importance of amorphous intergranular films in self-reinforced Si3N4 ceramics. Acta. Mater. 2000, 48, 4493–4499. [Google Scholar] [CrossRef]
- Neshpor, V.S.; Reznichenko, M. Investigating the thermal expansion of some silicides. Refractories 1963, 4, 145–148. [Google Scholar] [CrossRef]
- Mario, C.; Xiang, C.; Narciso, J.; Gupta, N. Reactive melt infiltration as synthesis route for enhanced SiC/CoSi2 composite materials for advanced armor systems. Ceram. Int. 2018, 44, 13182–13190. [Google Scholar]
- Arpon, R.; Molina, J.M.; Saravanan, R.A.; García-Cordovilla, C.; Louis, E.; Narciso, J. Thermal expansion behaviour of aluminium/SiC composites with bimodal particle distributions. Acta Mater. 2003, 51, 3145–3156. [Google Scholar] [CrossRef]
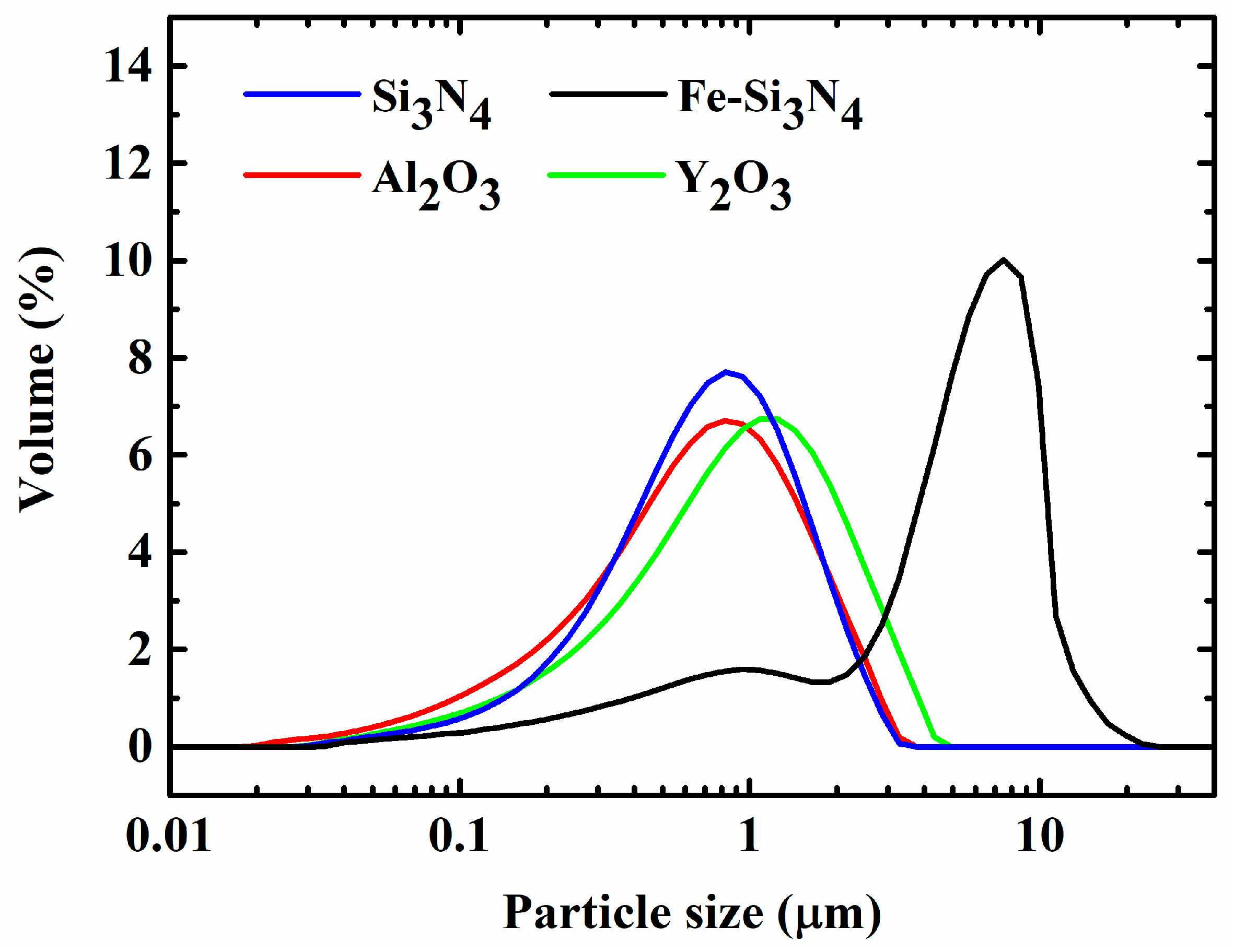
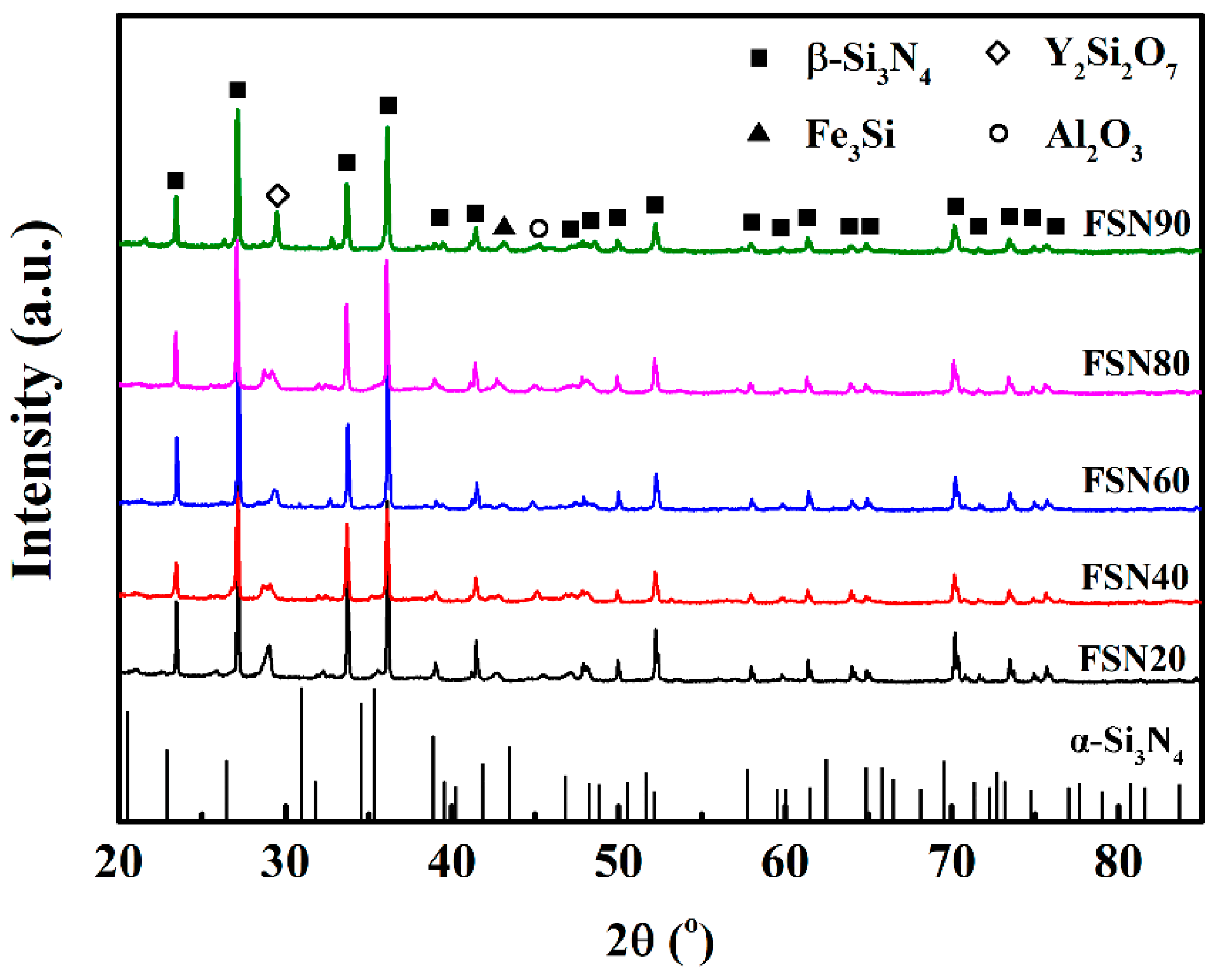
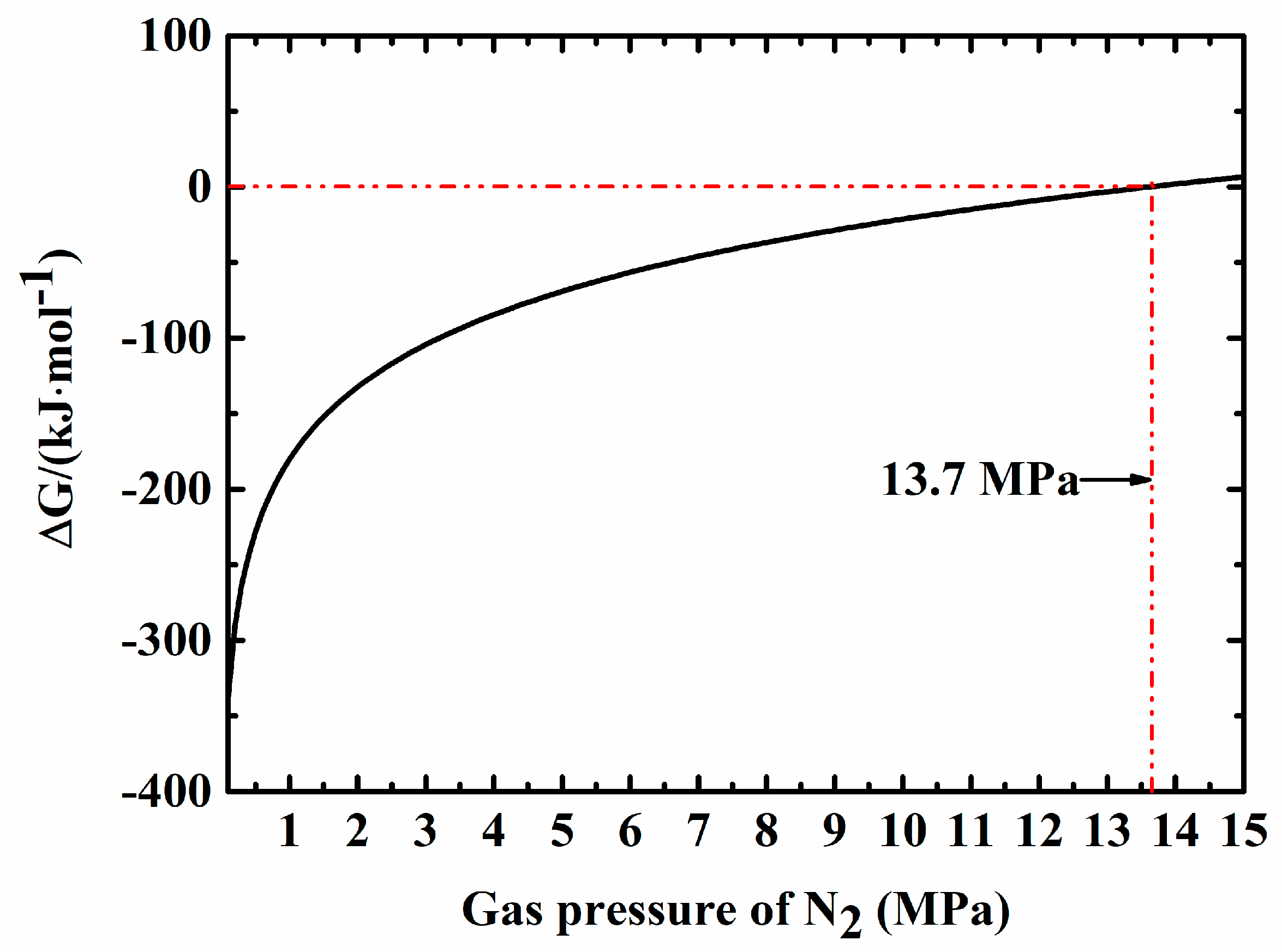
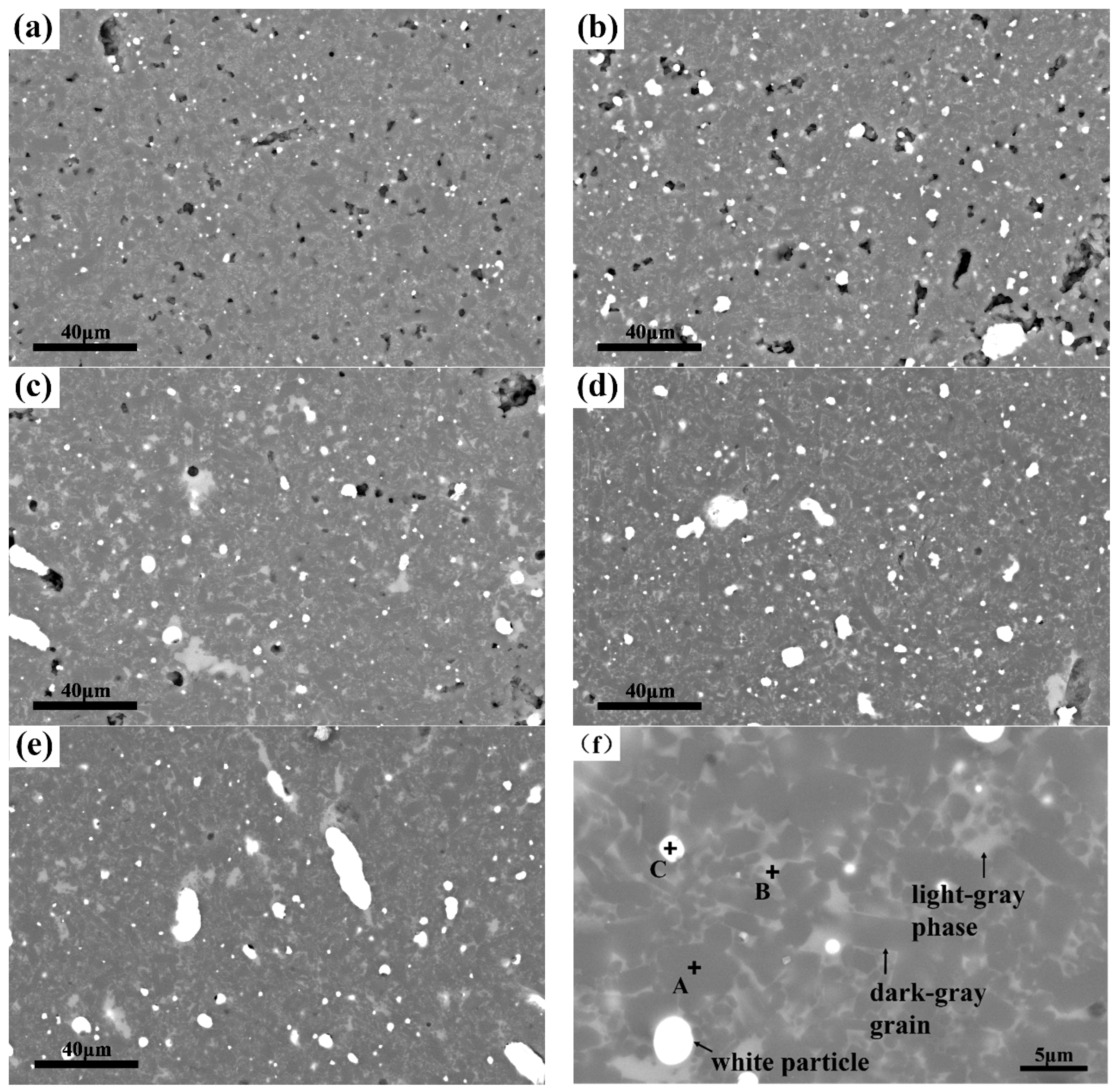

| Samples | Fe-Si3N4 | Si3N4 | Y2O3 | Al2O3 | Fe3Si in Starting Materials |
|---|---|---|---|---|---|
| (wt. %) | (wt. %) | (wt. %) | (wt. %) | (vol. %) | |
| FSN20 | 20 | 70 | 5 | 5 | 1.9 |
| FSN40 | 40 | 50 | 5 | 5 | 3.9 |
| FSN60 | 60 | 30 | 5 | 5 | 5.9 |
| FSN80 | 80 | 10 | 5 | 5 | 8.1 |
| FSN90 | 90 | 0 | 5 | 5 | 9.2 |
| Samples | Density (g/cm3) | Open Porosity (%) | Vickers Hardness (GPa) | Flexural Strength (MPa) | Fracture Toughness (MPa·m1/2) | |
|---|---|---|---|---|---|---|
| SENB | Indentation | |||||
| FSN-20 | 3.20 | 0.67 | 11.21 | 293 ± 37 | 8.2 ± 0.6 | 9.6 ± 0.9 |
| FSN-40 | 3.28 | 0.46 | 9.91 | 354 ± 44 | 8.4 ± 0.4 | 10.1 ± 0.8 |
| FSN-60 | 3.28 | 0.65 | 9.43 | 261 ± 12 | 6.5 ± 0.8 | 8.1 ± 0.8 |
| FSN-80 | 3.29 | 2.06 | 9.19 | 235 ± 21 | 5.7 ± 0.5 | 7.0 ± 0.5 |
| FSN-90 | 3.37 | 1.17 | 8.79 | 229 ± 15 | 3.8 ± 0.5 | 4.6 ± 0.7 |
| Element | Spot A | Spot B | Spot C | |
|---|---|---|---|---|
| Atom ratio (%) | Si | 55.4 | 22.5 | 23.0 |
| N | 44.6 | 18.0 | 0 | |
| Y | - | 8.1 | 0 | |
| Al | - | 11.0 | 0 | |
| O | - | 38.9 | 0 | |
| Fe | - | 1.5 | 77.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, J.; Cheng, L.; Li, M. Microstructure and Mechanical Properties of Si3N4-Fe3Si Composites Prepared by Gas-Pressure Sintering. Materials 2018, 11, 1206. https://doi.org/10.3390/ma11071206
Guan J, Cheng L, Li M. Microstructure and Mechanical Properties of Si3N4-Fe3Si Composites Prepared by Gas-Pressure Sintering. Materials. 2018; 11(7):1206. https://doi.org/10.3390/ma11071206
Chicago/Turabian StyleGuan, Jiasuo, Laifei Cheng, and Mingxing Li. 2018. "Microstructure and Mechanical Properties of Si3N4-Fe3Si Composites Prepared by Gas-Pressure Sintering" Materials 11, no. 7: 1206. https://doi.org/10.3390/ma11071206




