Damping Analysis of Some Inorganic Particles on Poly(butyl-methacrylate)
Abstract
:1. Introduction
2. Materials and Methods
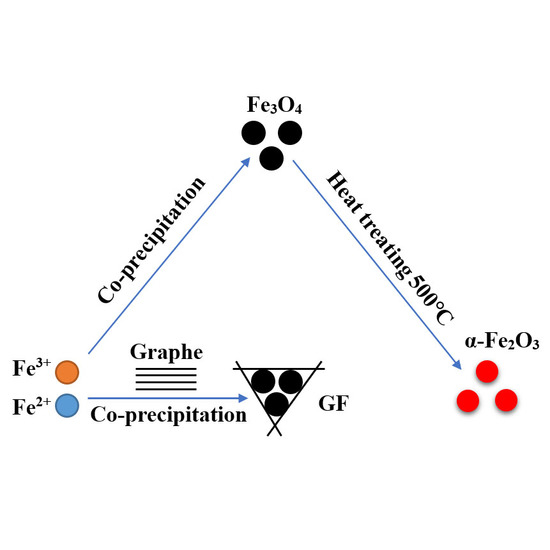
2.1. Synthesis of Graphene/Fe3O4 Hybrid Particles
2.2. Preparation of Samples of Poly(butyl-methacrylate)-Based Hybrid Material
2.3. Characterization
3. Results and Discussion
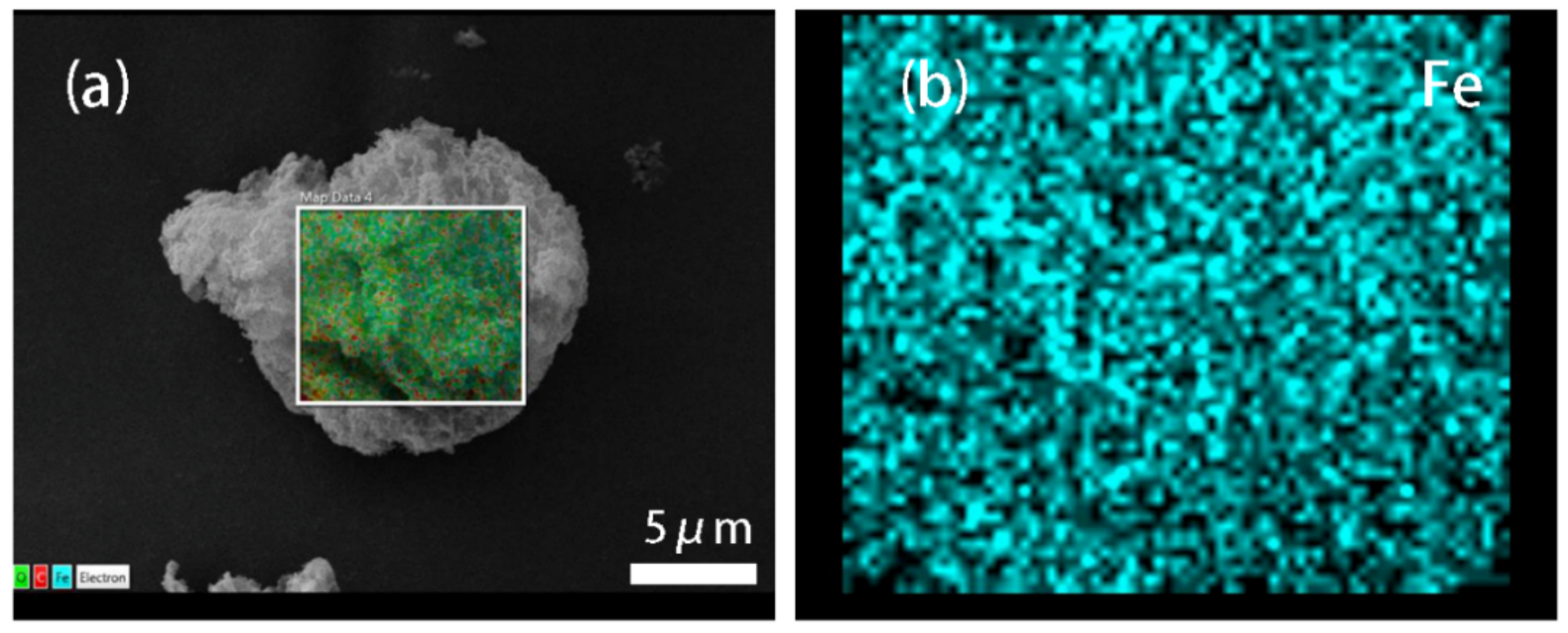
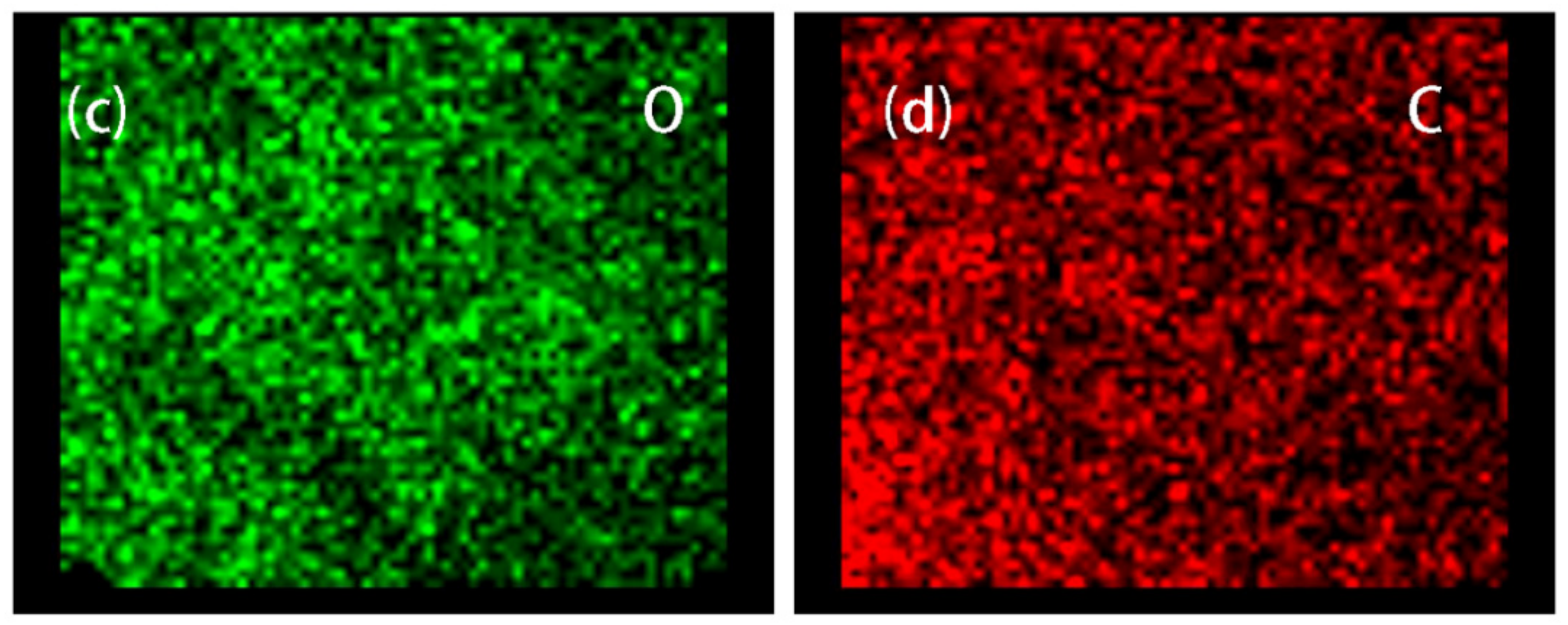
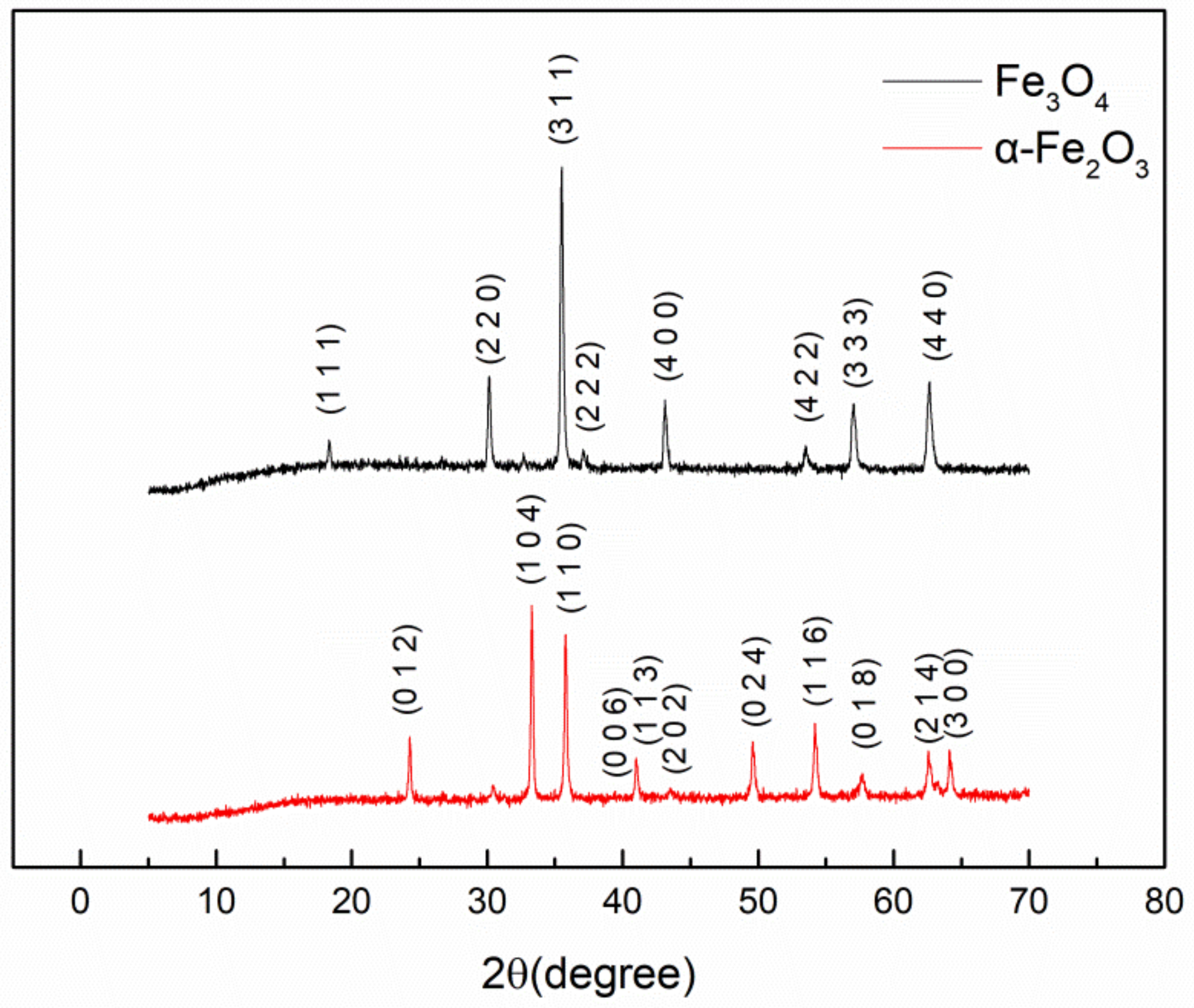
3.1. Morphologies and EDS of Graphene/Fe3O4 Hybrid Particles
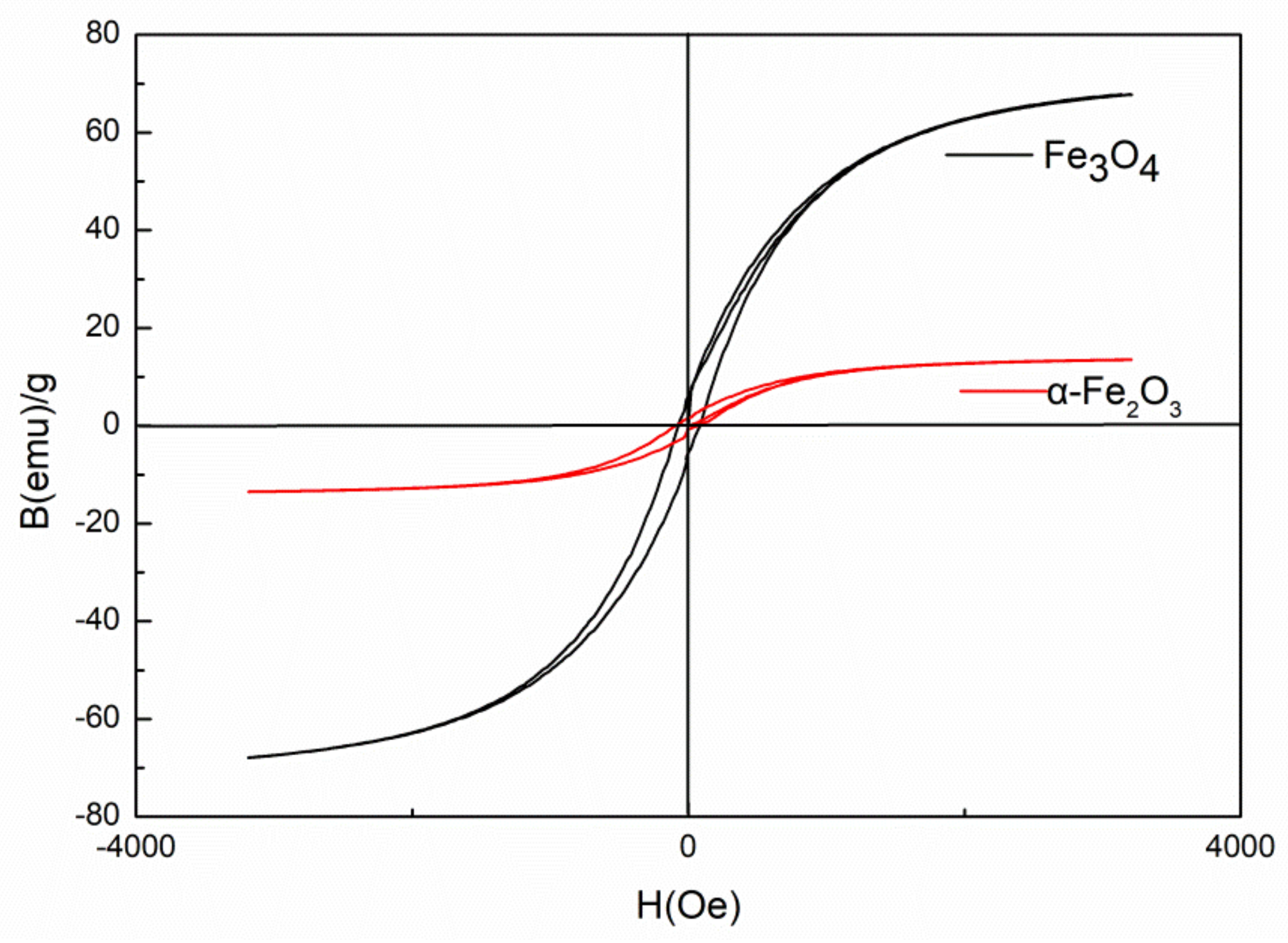
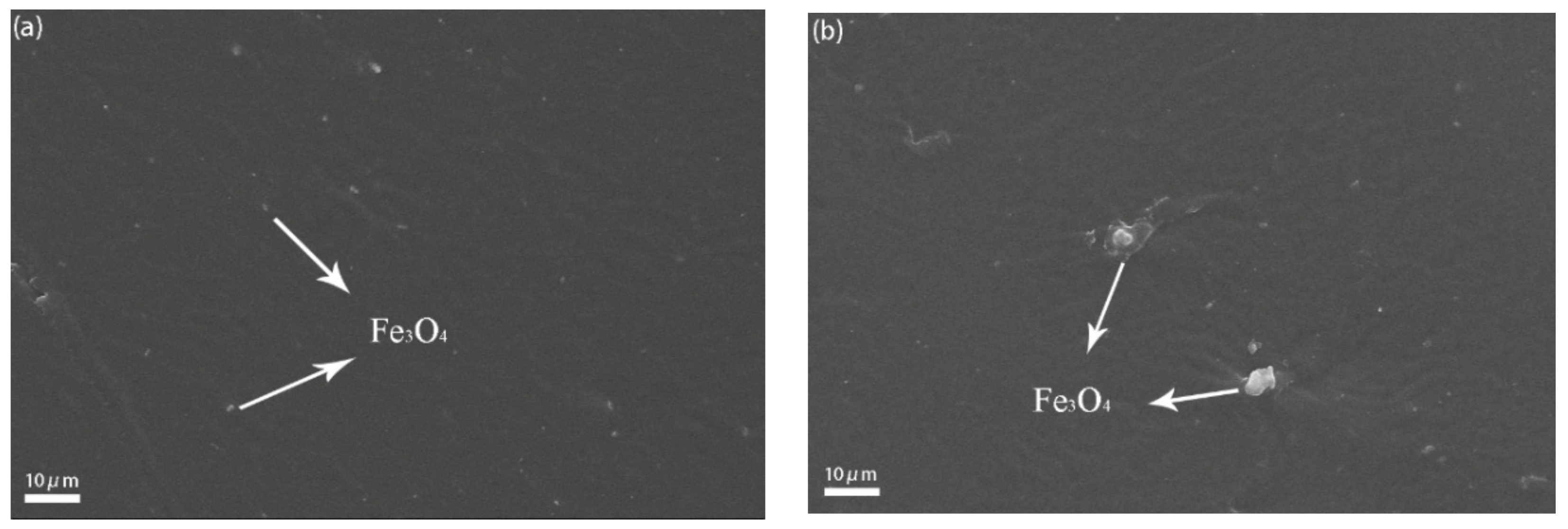
3.2. Demagnetization of Fe3O4
3.3. Morphology
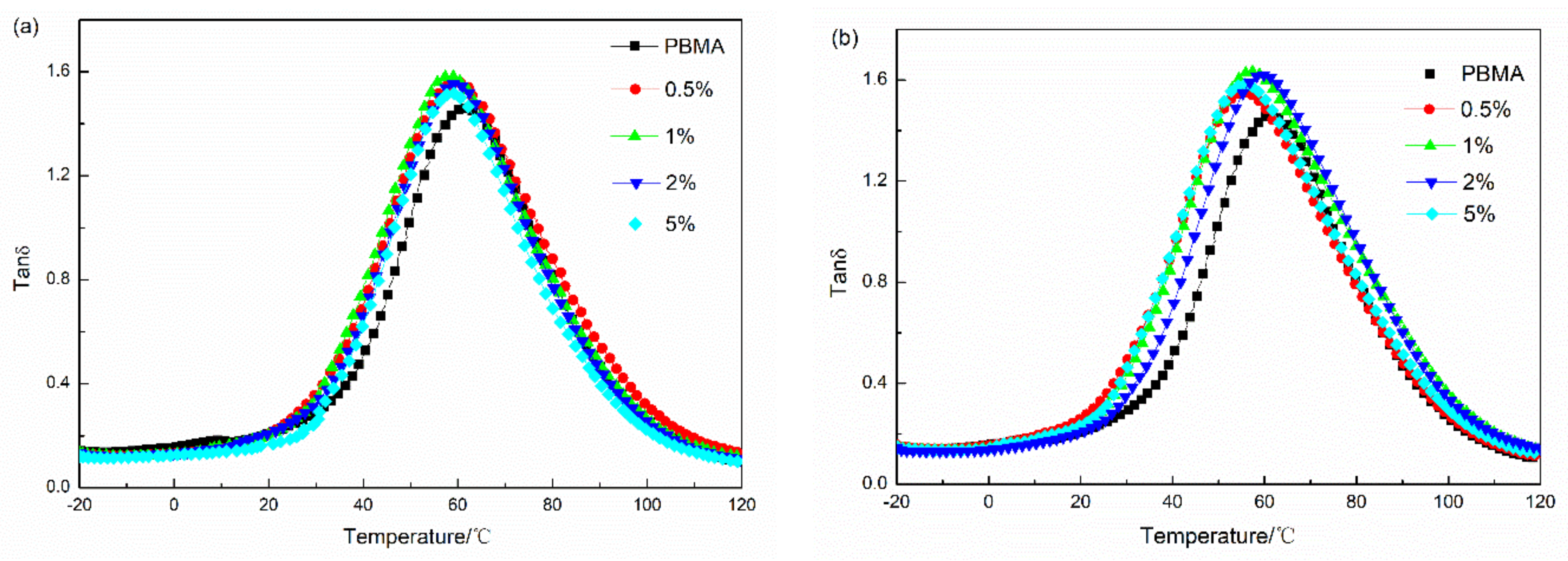
3.4. Damping Property of PBMA/Fe3O4 and PBMA/α-Fe2O3
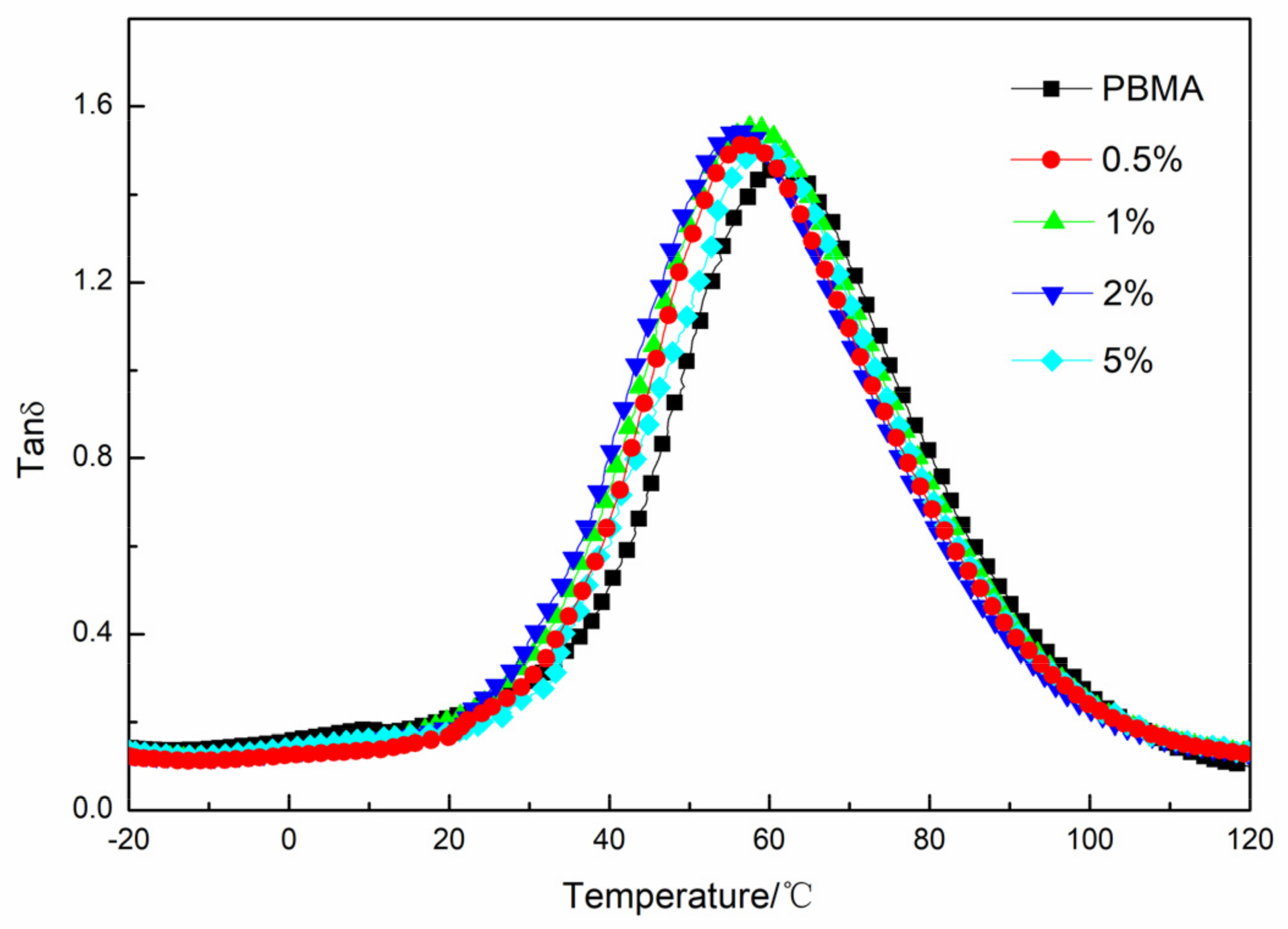
3.5. Damping Property of PBMA/GF
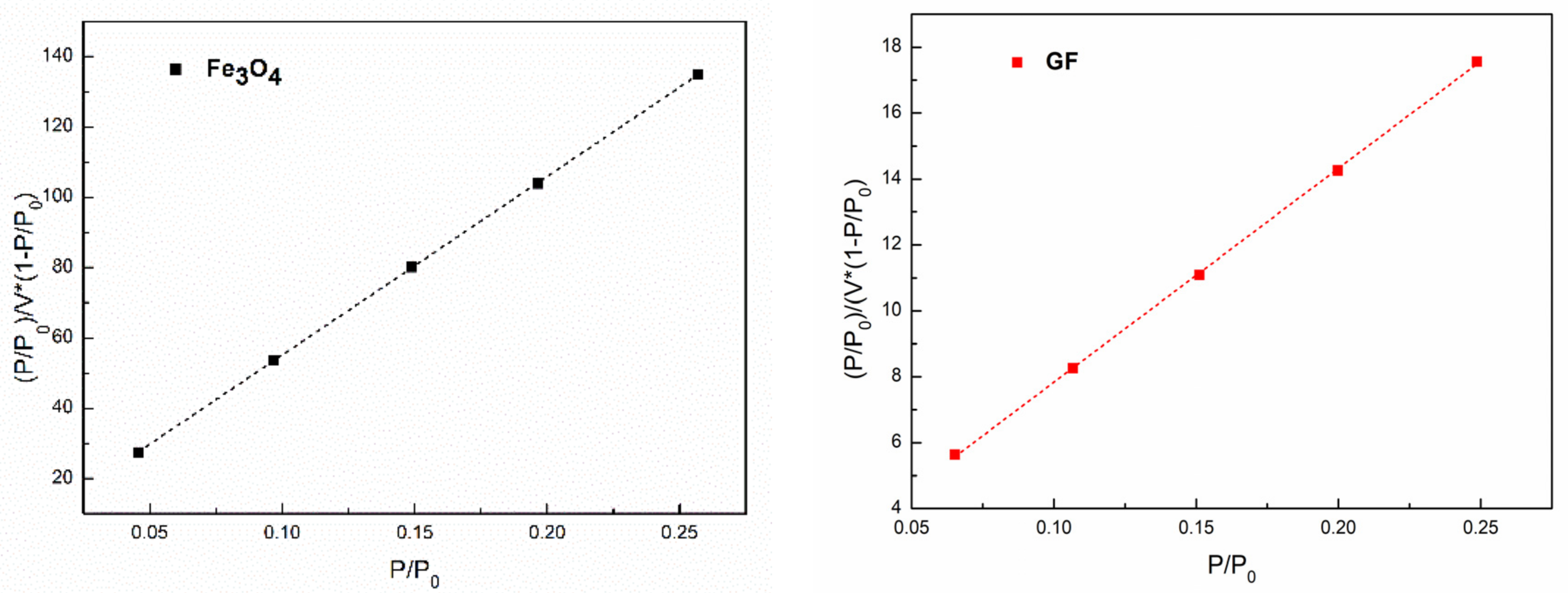
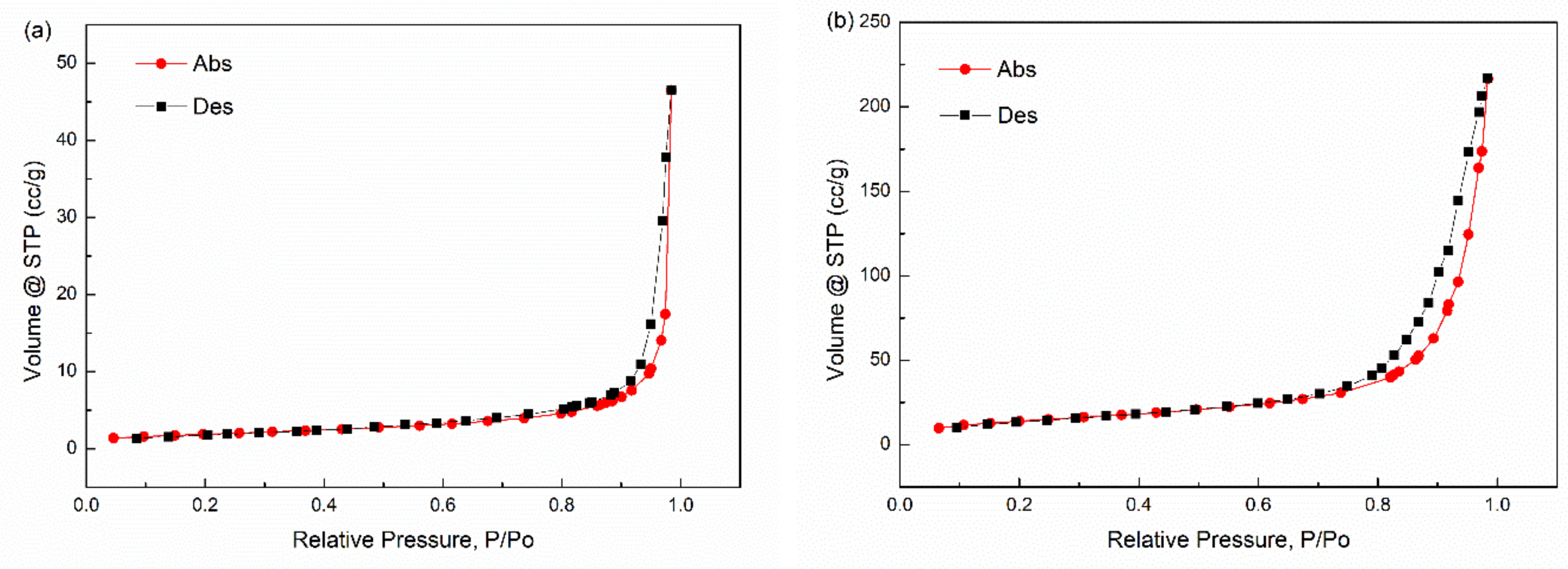
3.6. Surface Properties of Inorganic Particles
- Vm → single layer adsorption volume
- V → all adsorption volume
- P → partial pressure of adsorption thing
- P0 → saturated vapor pressure of adsorption thing
- C → BET constant
- A → Avogodro constant (/mol)
- → the sectional area of an adsorption thing (for the adsorption of , = .
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahn, S.K.; Jeon, E.; Park, J.; Kim, H.; Kho, H. Investigation of damping in the polymer concrete sleeper for use in reduction of rolling noise from railway. J. Acoust. Soc. Am. 2014, 136, 2209–2210. [Google Scholar] [CrossRef]
- Kishi, H.; Kuwata, M.; Matsuda, S.; Asami, T.; Murakami, A. Damping properties of thermoplasticelastomer interleaved carbon fiber-reinforced epoxy composites. Compos. Sci. Technol. 2003, 2003, 2517–2523. [Google Scholar]
- Qin, C.L.; Cai, W.M.; Cai, J.; Tang, D.Y.; Zhang, J.S.; Qin, M. Damping properties and morphology of polyurethane/vinyl ester resin interpenetrating polymer network. Mater. Chem. Phys. 2004, 85, 402–409. [Google Scholar] [CrossRef]
- Nashif, A.D.; Jones, D.I.G.; Henderson, J.P. Vibration Damping. J. Vib. Acous. 1987, 109, 240–253. [Google Scholar] [CrossRef]
- Ratna, D.; Manoj, N.R.; Chandrasekhar, L.; Chakraborty, B.C. Novel epoxy compositions for vibration damping applications. Polym. Adv. Technol. 2004, 15, 583–586. [Google Scholar] [CrossRef]
- Zhang, F.S.; He, G.S.; Xu, K.M.; Wu, H.; Guo, S.Y.; Zhang, C.L. Damping Mechanism and Different Modes of Molecular Motion through the Glass Transition of Chlorinated Butyl Rubber and Petroleum Resin Blends. J. Appl. Polym. Sci. 2014, 131, 378–387. [Google Scholar] [CrossRef]
- Ward, I.M.; Sweeney, J. An Introducion to the Mechanical Properties of Solid Polymers. Choice 2004, 12, 694. [Google Scholar]
- Li, Z.; Lu, X.; Tao, G.; Guo, J.; Jiang, H. Damping elastomer with broad temperature range based on irregular networks formed by end-linking of hydroxyl-terminated poly(dimethylsiloxane). Polym. Eng. Sci. 2016, 56, 97–102. [Google Scholar] [CrossRef]
- Yin, X.; Liu, C.; Lin, Y.; Guan, A.; Wu, G. Influence of hydrogen bonding interaction on the damping properties of poly(N-butyl methacrylate)/small molecule hybrids. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Su, C.; Zong, D.; Xu, L.; Zhang, C. Dynamic mechanical properties of semi-interpenetrating polymer network-based on nitrile rubber and poly(methyl methacrylate-co-butyl acrylate). J. Appl. Polym. Sci. 2014, 131, 742–751. [Google Scholar] [CrossRef]
- Borah, J.S.; Chaki, T.K. Dynamic mechanical, thermal, physico-mechanical and morphological properties of LLDPE/EMA blends. J. Polym. Res. 2011, 18, 569–578. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.R.; Yu, H. Why tandof poly (butyl acrylate) and poly (ethyl acrylate) with little double bonds are becoming asymmetric? Polymer 2014, 55, 4720–4727. [Google Scholar] [CrossRef]
- Li, S.; Zeng, W. Effect of crosslinker, buffer, and blending on damping properties of poly(styrene-acrylonitrile)/poly(ethyl acrylate-N-butyl acrylate) latex interpenetrating polymer networks. J. Appl. Polym. Sci. 2002, 84, 2347–2351. [Google Scholar] [CrossRef]
- Li, Y.; Oono, Y.; Nakayama, K.; Shimizu, H.; Inoue, T. Dual lamellar crystal structure in poly(vinylidene fluoride)/acrylic rubber blends and its biaxial orientation behavior. Polymer 2006, 47, 3946–3953. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Chen, Q.; Feng, C.; Meng, L. Crystallization Kinetics of Functionalized Fe3O4/Ethylene-vinyl Acetate Copolymer Nanocomposites Adhesives. J. Macromol. Sci. Part B 2016, 55, 55–72. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Lai, Z.; Yang, D. Effect of some inorganic particles on the softening dispersion of the dynamics of butyl rubber. Polym. Bull. 2016, 74, 1–13. [Google Scholar] [CrossRef]
- Huang, Z.C.; Liu, W.Q.; Yue, J.J.; Zhou, Q.; Zhang, W.; Lu, Y.; Sui, Y.; Zhai, Y.; Chen, Q.; Dong, S. Enhancing the spin-orbit coupling in Fe3O4 epitaxial thin films by interface engineering. ACS Appl. Mater. Interfaces 2016, 8, 27354–27359. [Google Scholar] [CrossRef] [PubMed]
- He, X.R.; Lu, X.B.; Chen, Q.; Zhang, R. Adhesive and viscoelastic performance of surface functionalized nano-Fe3O4 induced orientated ethylene vinyl-acetate composite hot melt adhesives. J. Appl. Polym. Sci. 2016, 43931, 1–10. [Google Scholar]
- Zhang, Z.; He, X.R.; Zhang, J.J.; Lu, X.B.; Yang, C.H.; Liu, T.; Wang, X.; Zhang, R. Influence of graphene/ferriferrous oxide hybrid particles on properties of nitrile rubber. RSC Adv. 2016, 6, 91798–91805. [Google Scholar] [CrossRef]
- Terrier, E.; Liu, Y.; Pichon, B.P.; Bégin-Colin, S.; Halté, V. Ultrafast demagnetization in Fe3O4 and γ-Fe2O3 nanoparticles: The role of enhanced antiferromagnetic exchange interaction. J. Phys. D Appl. Phys. 2016, 49, 505001. [Google Scholar] [CrossRef]
- Can, M.M.; Coşkun, M.; Fırat, T. A comparative study of nanosized iron oxide particles; magnetite (Fe3O4), maghemite (γ-Fe2O3) and hematite (α-Fe2O3), using ferromagnetic resonance. J. Alloys Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Song, Y.; Zhang, X.; Ma, Y.; Liang, J.; Chen, Y. Room-Temperature Ferromagnetism of Graphene. Nano Lett. 2009, 9, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.A.; Ghorbani, A.; Ghasemi, I. Mech. Mechanical, Thermal and Dynamic Mechanical Properties of PP/GF/xGnP Nanocomposites. Compos. Mater. 2017, 53, 131–138. [Google Scholar] [CrossRef]
- Kaneko, H.; Inoue, K.; Tominaga, Y.; Asai, S.; Sumita, M. Damping performance of polymer blend/organic filler hybrid materials with selective compatibility. Mater. Lett. 2002, 52, 96–99. [Google Scholar] [CrossRef]
- Rahman, N.A.; Hassan, A.; Yahya, R.; Lafiaaraga, R.A.; Hornsby, P.R. Micro-structural, thermal, and mechanical properties of injection-molded glass fiber/nanoclay/polypropylene composites. J. Reinf. Plast. Compos. 2012, 31, 269–281. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Huang, G. Dynamics of Poly (butyl acrylate) and Poly (ethyl acrylate) with internal double bonds. J. Polym. Res. 2014, 21, 1–11. [Google Scholar] [CrossRef]
- Cao, F.H.; Wang, J.C. Preparation and characterization of hyperbranched polymer modified montmorillonite/chlorinated butyl rubber damping composites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Jiang, P.; Yang, C.; He, X.; Rodrigues, A.M.; Zhang, R. Viscoelastic changes in chlorinated butyl rubber modified with graphene oxide. Iran. Polym. J. 2017, 26, 861–870. [Google Scholar] [CrossRef]


| Material | Weight (g) |
|---|---|
| PBMA | 19.9/19.8/19.6/19.0 |
| Fe3O4 | 0.1/0.2/0.4/1.0 |
| α-Fe2O3 | 0.1/0.2/0.4/1.0 |
| GF | 0.1/0.2/0.4/1.0 |
| Sample | d (0.5) |
|---|---|
| Fe3O4 | 2.202 μm |
| α-Fe2O3 | 2.110 μm |
| Sample Code | Tan δ Max | Temperature Range of Tan δ > 0.3 | TA (Tan δ > 1.0) | |||
|---|---|---|---|---|---|---|
| PBMA/Fe3O4 | Value | T/°C | T1/°C | T2/°C | ΔT/°C | |
| 0% | 1.46 | 61.19 | 30.80 | 98.00 | 67.20 | 7.39 |
| 0.5% | 1.56 | 59.66 | 26.87 | 100.83 | 73.96 | 10.59 |
| 1% | 1.58 | 57.72 | 28.30 | 97.97 | 69.67 | 10.58 |
| 2% | 1.55 | 59.06 | 28.43 | 97.02 | 68.59 | 9.21 |
| 5% | 1.52 | 58.63 | 30.84 | 94.86 | 64.02 | 8.08 |
| PBMA/α-Fe2O3 | ||||||
| 0% | 1.46 | 61.19 | 30.80 | 98.00 | 67.20 | 7.39 |
| 0.5% | 1.55 | 55.3 | 22.79 | 98.34 | 75.55 | 10.59 |
| 1% | 1.63 | 56.81 | 25.19 | 103.00 | 77.81 | 13.43 |
| 2% | 1.62 | 59.05 | 27.83 | 102.51 | 74.68 | 12.71 |
| 5% | 1.58 | 55.58 | 25.16 | 99.80 | 74.64 | 11.74 |
| Sample Code | Tanδ Max | Temperature Range of Tanδ > 0.3 | TA (Tanδ > 1.0) | |||
|---|---|---|---|---|---|---|
| PBMA/GF | Value | T/°C | T1/°C | T2/°C | ΔT/°C | |
| 0.5% | 1.52 | 56.88 | 30.13 | 95.68 | 65.55 | 8.11 |
| 1% | 1.55 | 57.63 | 27.95 | 96.36 | 68.41 | 9.78 |
| 2% | 1.54 | 55.95 | 27.08 | 94.68 | 67.60 | 9.27 |
| 5% | 1.50 | 58.72 | 33.74 | 96.12 | 62.38 | 7.80 |
| Samples | SBET |
|---|---|
| Fe3O4 | 6.808 |
| GF | 52.565 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Yang, C.; Hu, J.; He, X.; Zhang, R. Damping Analysis of Some Inorganic Particles on Poly(butyl-methacrylate). Materials 2018, 11, 992. https://doi.org/10.3390/ma11060992
Zhou S, Yang C, Hu J, He X, Zhang R. Damping Analysis of Some Inorganic Particles on Poly(butyl-methacrylate). Materials. 2018; 11(6):992. https://doi.org/10.3390/ma11060992
Chicago/Turabian StyleZhou, Saisai, Chunhua Yang, Jia Hu, Xianru He, and Rui Zhang. 2018. "Damping Analysis of Some Inorganic Particles on Poly(butyl-methacrylate)" Materials 11, no. 6: 992. https://doi.org/10.3390/ma11060992