Application of Ti6Al7Nb Alloy for the Manufacture of Biomechanical Functional Structures (BFS) for Custom-Made Bone Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Manufacturing
2.2. Evaluation of Mechanical Properties
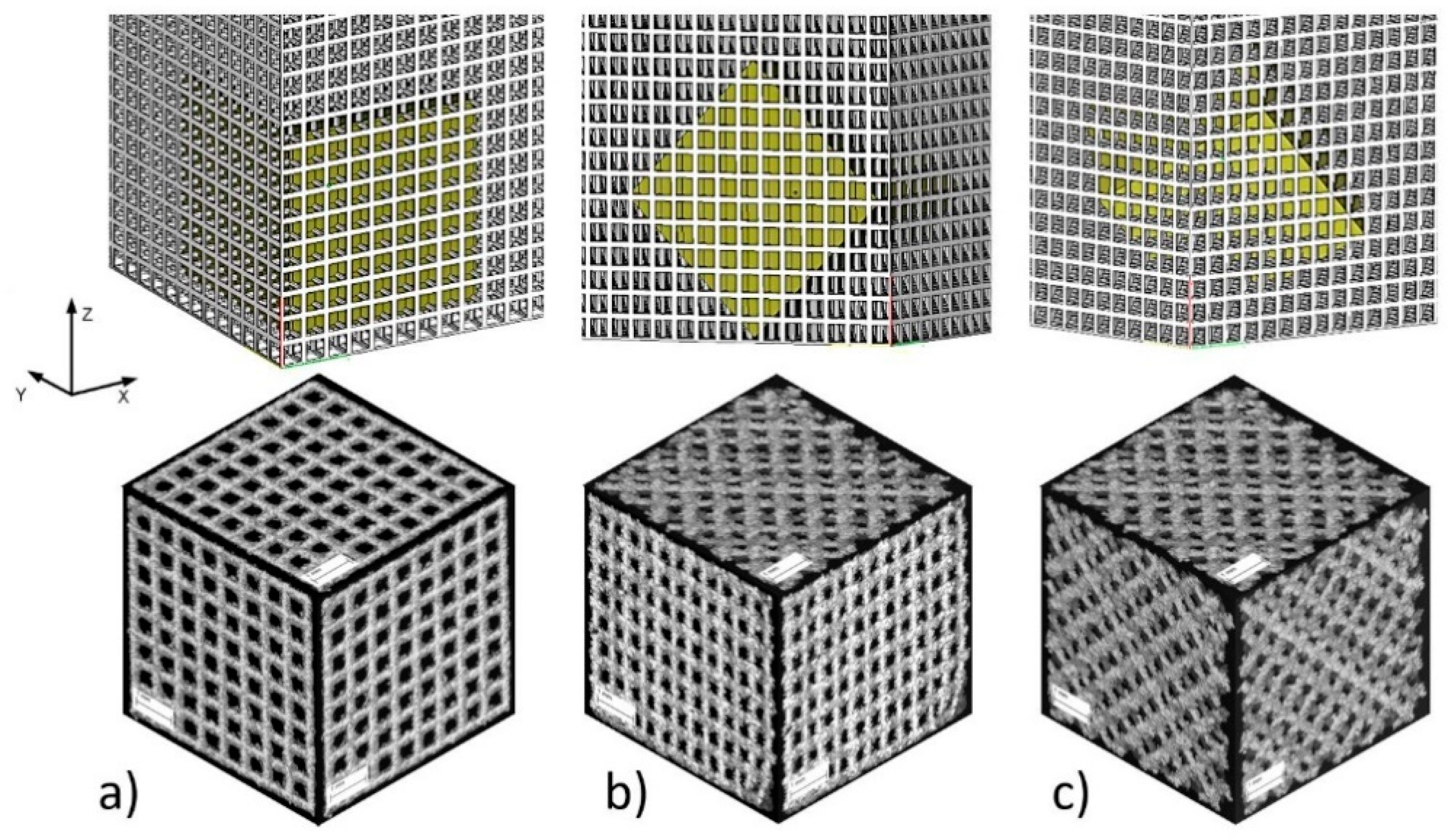
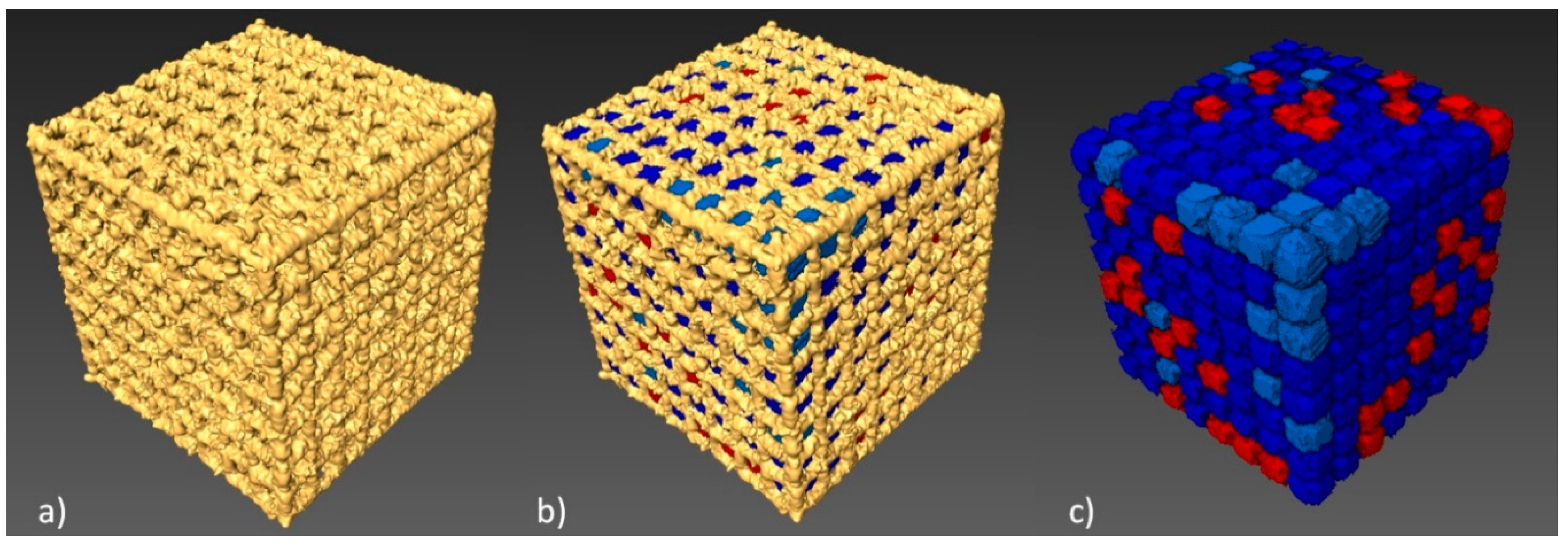
2.3. Geometric Analysis Using CT
2.4. Biological Evaluation
2.4.1. In Vitro Cytotoxicity of BFS Structures against Osteoblast Cell Line
2.4.2. Osteoblast’s Growth and Multiplication on Implants
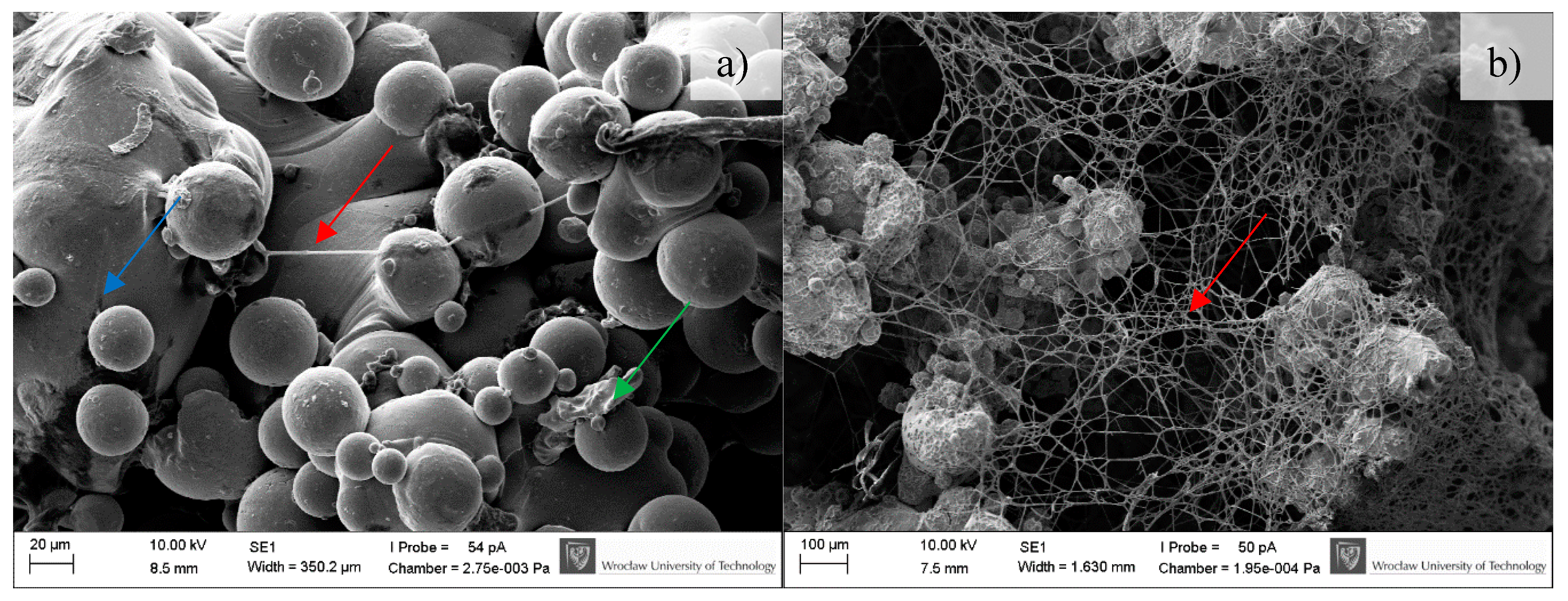
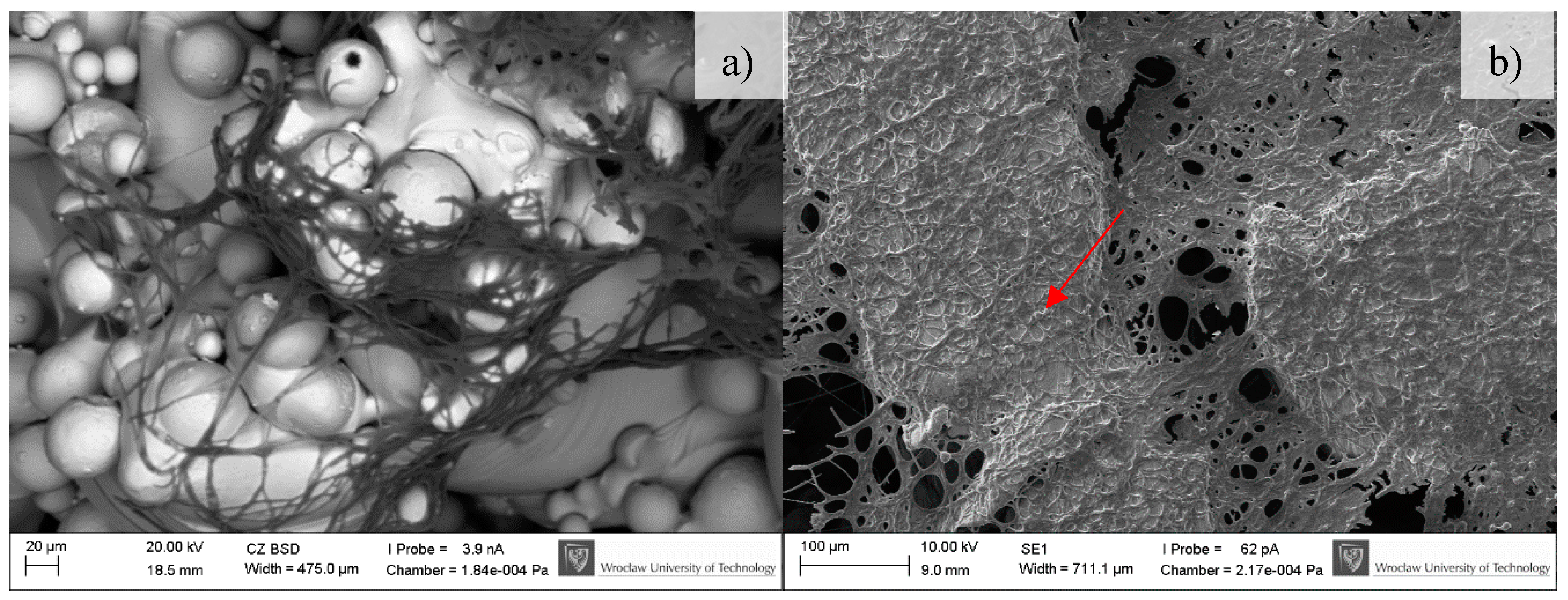
2.4.3. Scanning Electron Microscopy
2.5. Ability of S. epidermidis to form Biofilm
2.6. Statistical Analysis
3. Results
3.1. Mechanical Properties of BFS
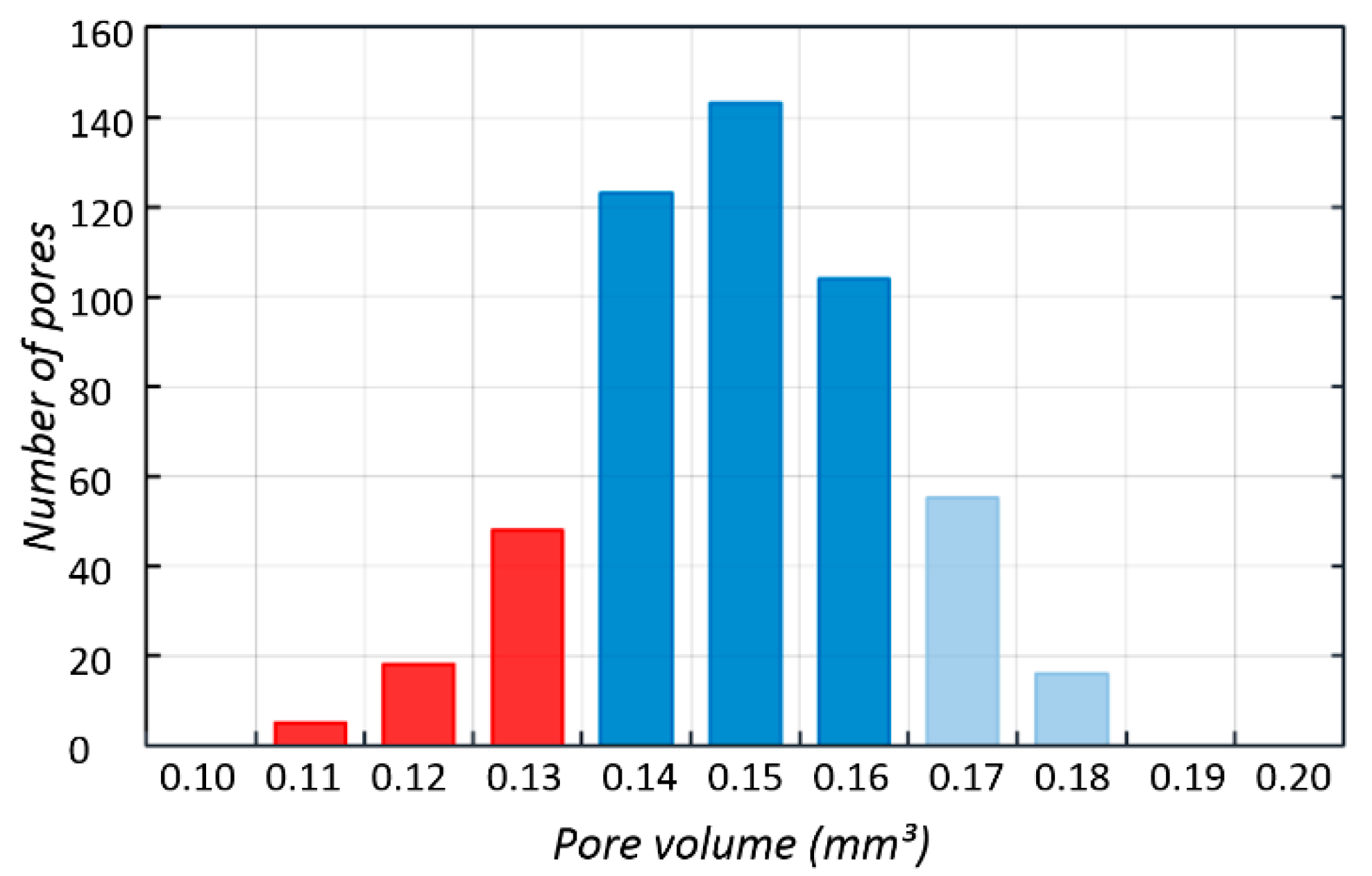
3.2. CT Reconstruction
3.3. In-Vitro Cell Response
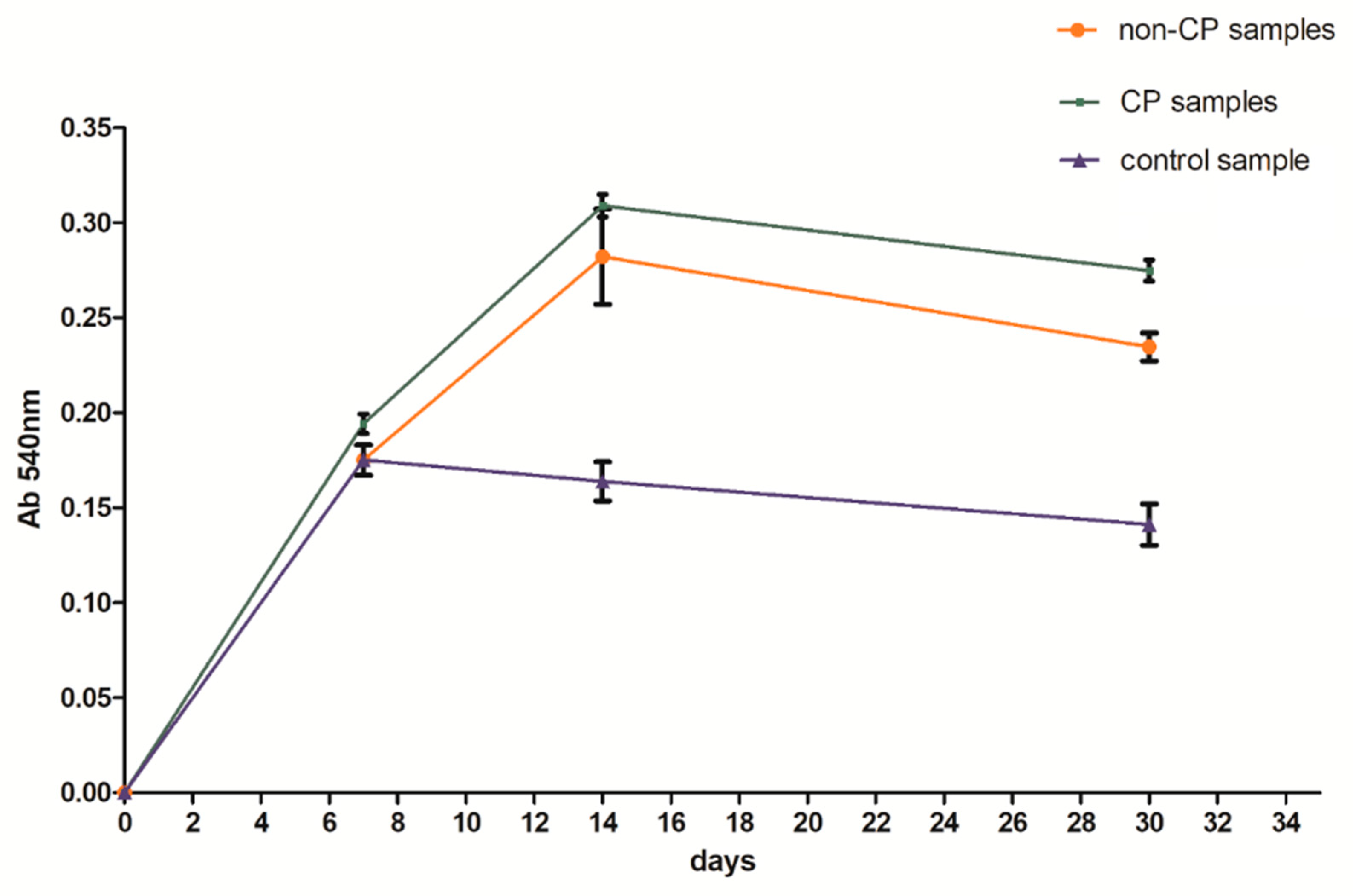
3.4. Microbiological Tests
3.5. Example of Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ryan, G.; Pandit, A.; Apatsidis, D.B. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, T.; Gandhinathan, R.; Ramu, M.; Ananthasubramanian, M.; Banu Pradheepa, K. Design, analysis and fabrication of polyamide/hydroxyapatite porous structured scaffold using selective laser sintering method for bio-medical applications. J. Mech. Sci. Technol. 2016, 30, 5305–5312. [Google Scholar] [CrossRef]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.A.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. A 2014, 102, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Domingos, M.A.N.; Kleina, T.J.; Maldaa, J.; Bartoloc, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef] [Green Version]
- Stuyts, B.; Peersman, G.; Thienpont, E.; Van den Eeden, E.; Van der Bracht, H. Custom-made lateral femoral hemiarthroplasty for traumatic bone loss: A case report. Knee 2015, 22, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Fratzl, P.; Dunlop, J.W. Geometry as a factor for tissue growth: Towards shape optimization of tissue engineering scaffolds. Adv. Healthc. Mater. 2013, 2, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.; van Dongen, J.T.; Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragdon, C.R.; Jasty, M.; Greene, M.; Rubash, H.E.; Harris, W.H. Biologic fixation of total hip implants. Insights gained from a series of canine studies. J. Bone Jt. Surg. 2004, 86, 105–117. [Google Scholar] [CrossRef]
- Tuchinskiy, L.; Loutfy, R. Titanium foams for medical applications. In Medical Device Materials, Proceedings of the Materials &Processes for Medical Devices Conferences, Anaheim, CA, USA, 8–10 September 2003; ASM International: Almere, The Netherlands, 2003; pp. 377–381. [Google Scholar]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopaedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Manjubala, I.; Woesz, A.; Pilz, C.; Rumpler, M.; Fratzl-Zelman, N.; Roschger, P.; Stampfl, J.; Fratzl, P. Biomimetic mineral-organic composite scaffolds with controlled internal architecture. J. Mater. Sci. Mater. Med. 2005, 16, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnke, P.H.; Douglas, T.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S.T.; Springer, I.N.; Wiltfang, J.; Sivananthan, S. Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng. Part C Methods 2009, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Harrysson, O.L.A.; Cansizoglu, O.; Marcellin-Little, D.J.; Cormier, D.R.; West, H.A. Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Mater. Sci. Eng. C 2008, 28, 366–373. [Google Scholar] [CrossRef]
- Hollander, D.A.; von Walter, M.; Wirtz, T.; Sellei, R.; Schmidt-Rohlfing, B.; Paar, O.; Erlia, H. Structural, mechanical and in vitro characterization of individually structured Ti-6Al-4V produced by direct laser forming. Biomaterials 2006, 27, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Heredia, M.A.; Goyenvalle, E.; Aguado, E.; Leroux, C.; Dorget, M.; Layrolle, P. Bone Growth in Porous Titanium Implants made by Rapid Prototyping. Key Eng. Mater. 2006, 309–311, 1099–1104. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.; Toksvig-Larsen, S.; Aspenberg, P. Ingrowth of bone into pores in titanium chambers implanted in rabbits: Effect of pore cross-sectional shape in the presence of dynamic shear. J. Biomed. Mater. Res. 1993, 27, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Van Cleynenbreugel, T.; Van Oosterwyck, H.; Vander Sloten, J.; Schrooten, J. Trabecular bone scaffolding using a biomimetic approach. J. Mater. Sci. Mater. Med. 2002, 13, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Chlebus, E.; Kuźnicka, B.; Kurzynowski, T.; Dybała, B. Microstructure and mechanical behaviour of Ti-6Al-7Nb Alloy produced by selective laser melting. Mater. Charact. 2011, 62, 5488–5495. [Google Scholar] [CrossRef]
- Leordean, D.; Marcu, T.; Radu, S.A.; Berce, P. Porous metal structures from Ti alloys produced by SLM technology. Acad. J. Manuf. Eng. 2011, 9, 10–15. [Google Scholar] [CrossRef]
- Pawlak, A.; Szymczyk, P.; Ziółkowski, G.; Dybała, B.; Chlebus, E. Fabrication of microscaffolds from Ti-6Al-7Nb alloy by SLM. Rapid Prototyp. J. 2015, 21, 393–401. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Kerckhofs, G.; Van Bael, S.; Pyka, G.; Schrooten, J.; Moesen, M.; Loeck, D.; Wevers, M. Non-destructive characterization of the influence of surface modification on the morphology and mechanical behavior of rapid prototyped Ti6Al4V bone tissue engineering scaffolds. In Proceedings of the European Conference for Non-Destructive Testing, Moscow, Russia, 7–11 June 2010; pp. 1–9. [Google Scholar]
- Sitting, C.; Textor, M.; Spencer, N.D.; Wieland, M.; Vallotton, P.H. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J. Mater. Sci. Mater. Med. 1999, 10, 35–46. [Google Scholar] [CrossRef]
- Szymczyk, P.; Junka, A.; Ziółkowski, G.; Bartoszewicz, M.; Smutnicka, D.; Chlebus, E. The ability of S.aureus to form biofilm on the Ti-6Al-7Nb bone scaffolds produced by Selective Laser Melting and subjected to the different types of surface modifications. Acta Bioeng. Biomech. 2013, 15, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.F.; Szymczyk, P.; Secewicz, A.; Pawlak, A.; Smutnicka, D.; Ziółkowski, G.; Bartoszewicz, M.; Chlebus, E. The chemical digestion of Ti6Al7Nb scaffolds produced by Selective Laser Melting reduces significantly ability of Pseudomonas aeruginosa to form biofilm. Acta Bioeng. Biomech. 2016, 18, 1115–1120. [Google Scholar] [CrossRef]
- Miao, G.; Xiang, L. Development of porous Ti6Al4V/chitosan sponge composite scaffold for orthopedic applications. Mater. Sci. Eng. C 2016, 58, 1177–1181. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wirtz, T.; LaMarca, F.; Hollister, S.J. Structural and mechanical evaluation of topology optimized titanium interbody fusion cage fabricated by selective laser melting process. J. Biomed. Mater. Res. 2007, 83A, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowski, G.; Szymczyk, P.; Dybała, B.; Chlebus, E.; Pawlak, A. Geometric Characteristics of Scaffolds Made by Additive Manufacturing. Powder Metall. Met. Ceram. 2015, 54, 12–16. [Google Scholar] [CrossRef]
- Liua, X.; Chub, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Ellingsen, J.E. Pre-treatment of titanium implants with fluoride improves their retention in bone. J. Mater. Sci. Mater. Med. 1995, 6, 749–753. [Google Scholar] [CrossRef]
- Rumiński, S.; Noga, M.; Ostrowska, B.; Pawlak, A.; Dybała, B.; Dąbrowski, B.; Święszkowski, W.; Lewandowska-Szumieł, M. Osteogenic-like behaviour of adipose derived stem cells in selected scaffolds obtained by 3D-printing. Eur. Cells Mater. 2013, 26, 62. [Google Scholar]
- Rotaru, H.; Armencea, G.; Spîrchez, D.; Berce, C.; Marcu, T.; Leordean, D.; Seong-Gon, K.; Sang-Woon, L.; Dinu, C.; Băciu, G.; et al. In vivo behavior of surface modified Ti alloys used in selective laser melting for custom-made implants. A preliminary study. Rom. J. Morphol. Embryol. 2013, 54, 791–795. [Google Scholar] [PubMed]
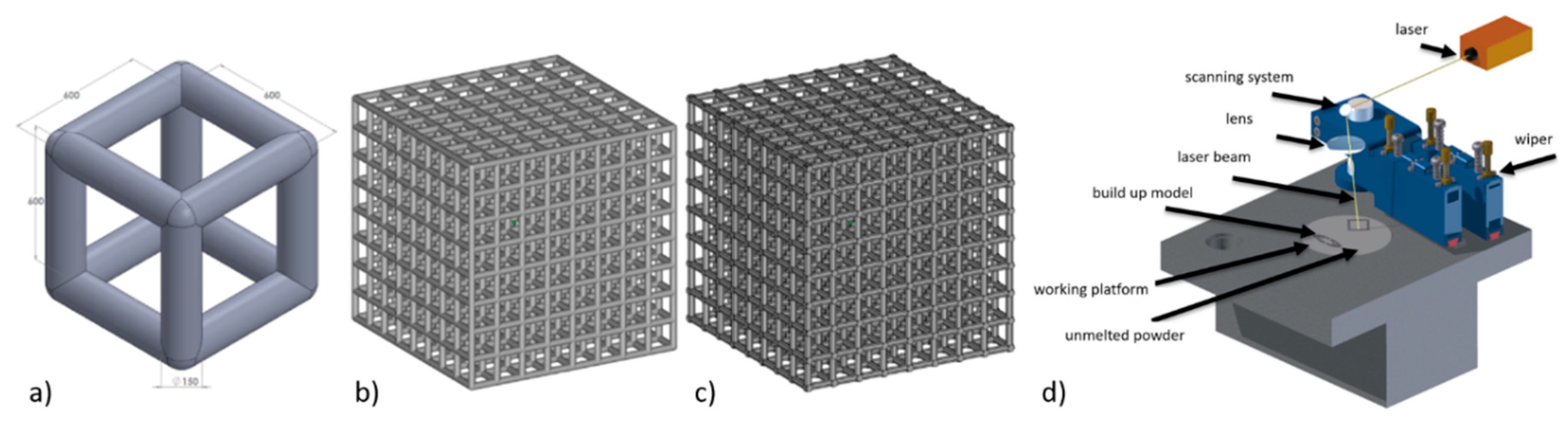







| Process Parameter | Laser Power | Spot Size | Scan Velocity | Layer Thickness | Overlap | Scanning Strategy |
|---|---|---|---|---|---|---|
| unit | W | µm | mm/s | µm | % | - |
| value | 25 | 100 | 200 | 50 | 30 | XY |
| Force Direction | Parameters | BFS Types | ||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | B | C | ||
| 1 | σ [MPa] | 85 ± 31 | 50 ± 6 | 100 ± 3 | 32 ± 5 | 29 ± 5 |
| E [MPa] | 2430 ± 727 | 1752 ± 671 | 2860 ± 174 | 855 ± 130 | 817 ± 108 | |
| 2 | σ [MPa] | 158 ± 14 | 51 ± 6 | 107 ± 4 | 29 ± 2 | 29 ± 9 |
| E [MPa] | 743 ± 270 | 2139 ± 173 | 3009 ± 202 | 817 ± 195 | 648 ± 78 | |
| 3 | σ [MPa] | 82 ± 30 | 28 ± 7 | 108 ± 5 | 81 ± 2 | 28 ± 8 |
| E [MPa] | 2193 ± 867 | 485 ± 112 | 3303 ± 145 | 2356 ± 129 | 790 ± 102 | |
| BFS Type A3 | Strut Diameter µm | Total Porosity P % | Volume of Single Pore mm3 | Total Surface mm2 |
|---|---|---|---|---|
| CAD model | 150 | 85% | 0.202 | 431.34 |
| CT data | 225 | 56% | 0.150 | 849.49 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczyk, P.; Ziółkowski, G.; Junka, A.; Chlebus, E. Application of Ti6Al7Nb Alloy for the Manufacture of Biomechanical Functional Structures (BFS) for Custom-Made Bone Implants. Materials 2018, 11, 971. https://doi.org/10.3390/ma11060971
Szymczyk P, Ziółkowski G, Junka A, Chlebus E. Application of Ti6Al7Nb Alloy for the Manufacture of Biomechanical Functional Structures (BFS) for Custom-Made Bone Implants. Materials. 2018; 11(6):971. https://doi.org/10.3390/ma11060971
Chicago/Turabian StyleSzymczyk, Patrycja, Grzegorz Ziółkowski, Adam Junka, and Edward Chlebus. 2018. "Application of Ti6Al7Nb Alloy for the Manufacture of Biomechanical Functional Structures (BFS) for Custom-Made Bone Implants" Materials 11, no. 6: 971. https://doi.org/10.3390/ma11060971





