The Corrosion Behavior of AZ91D Magnesium Alloy in Simulated Haze Aqueous Solution
Abstract
:1. Introduction
2. Experimental Methods
2.1. Samples and Solution
2.2. Electrochemical Measurements
2.3. Morphology Observations
3. Results
3.1. The Corrosion Process of AZ91D
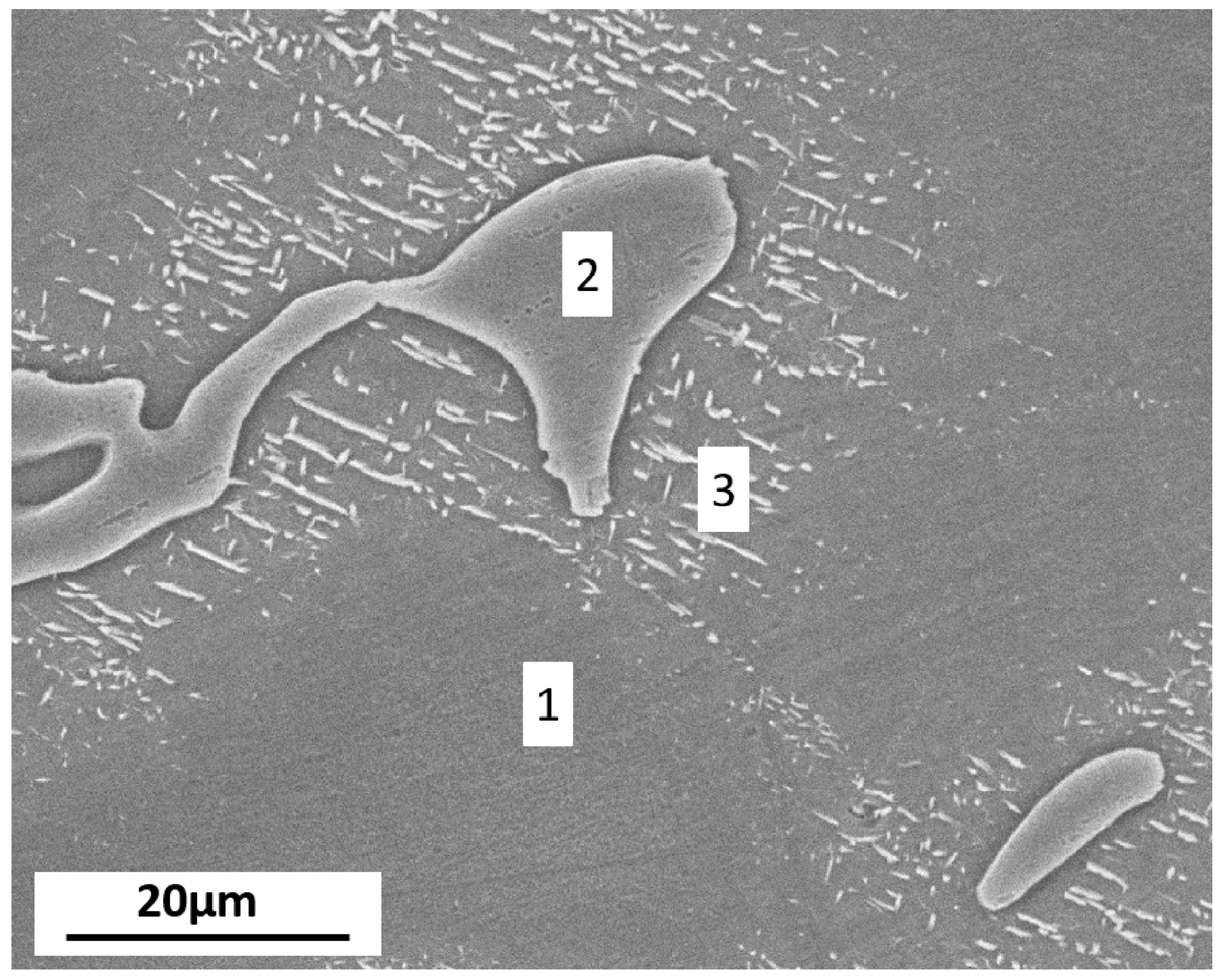
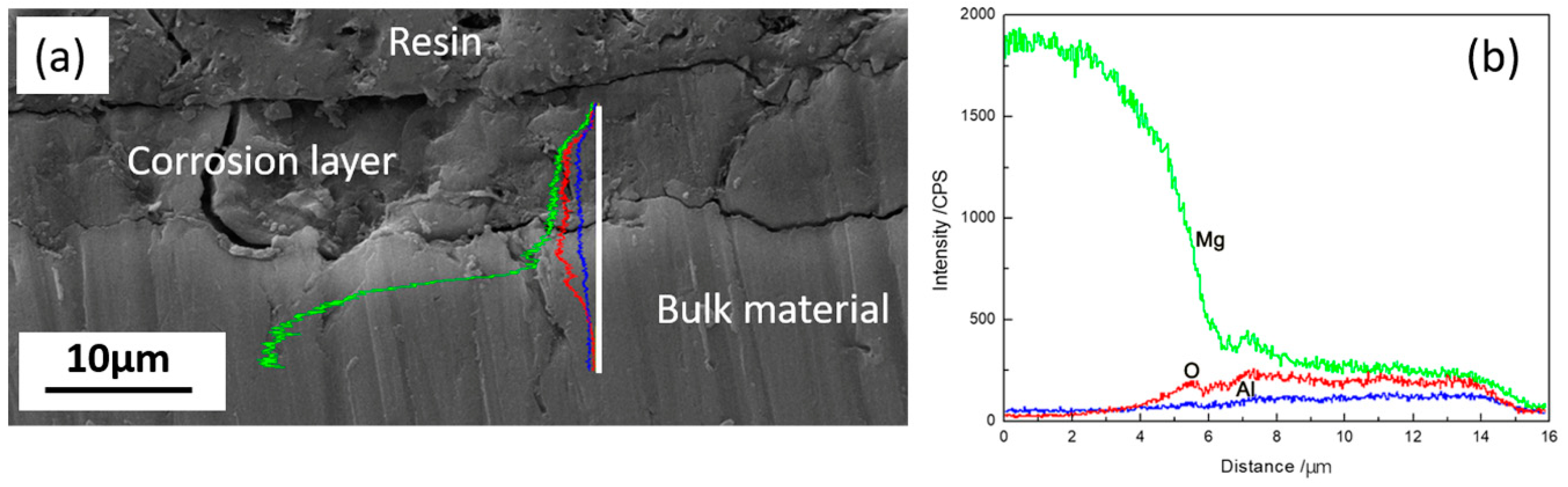
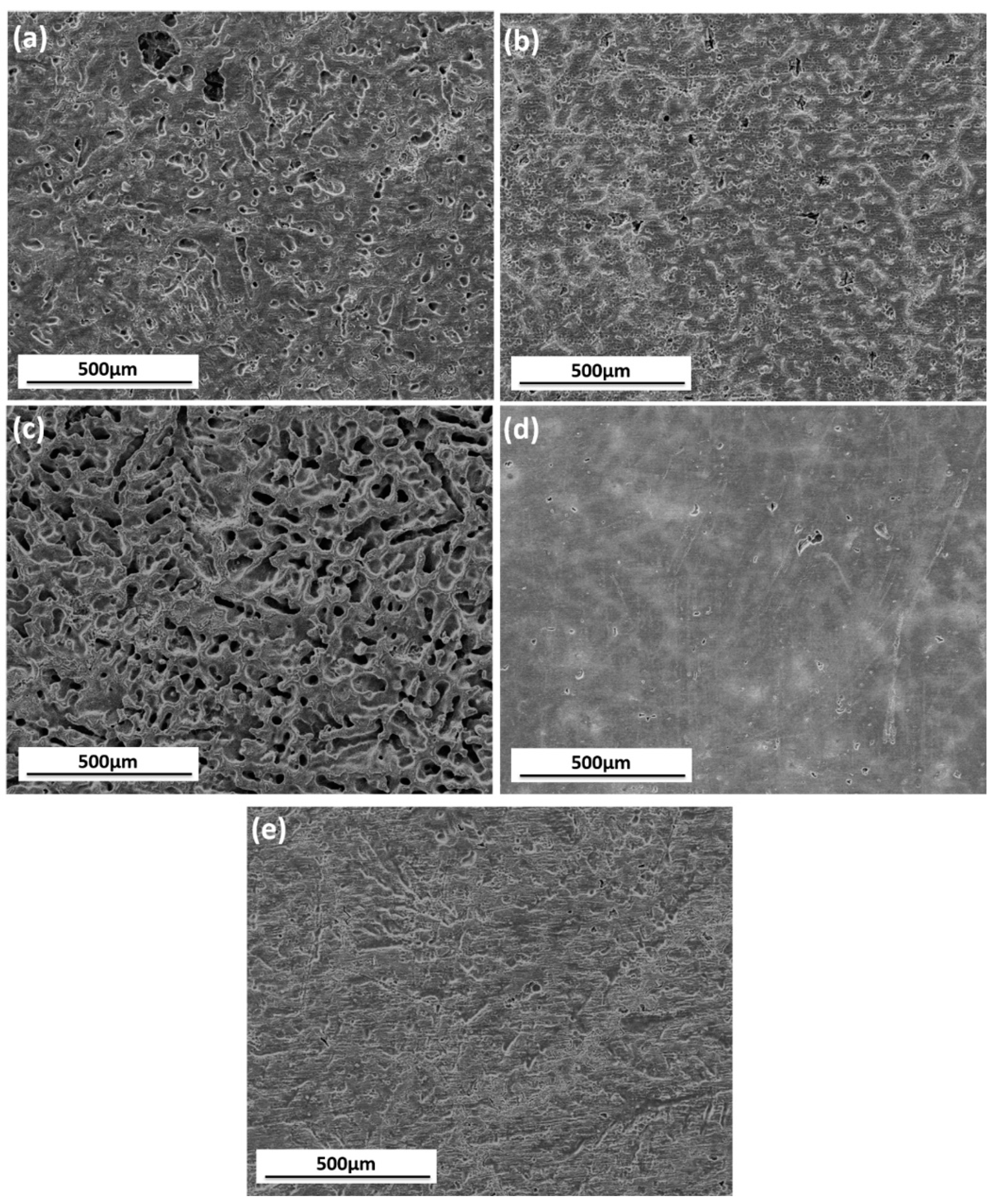
3.1.1. Microstructural Characterization
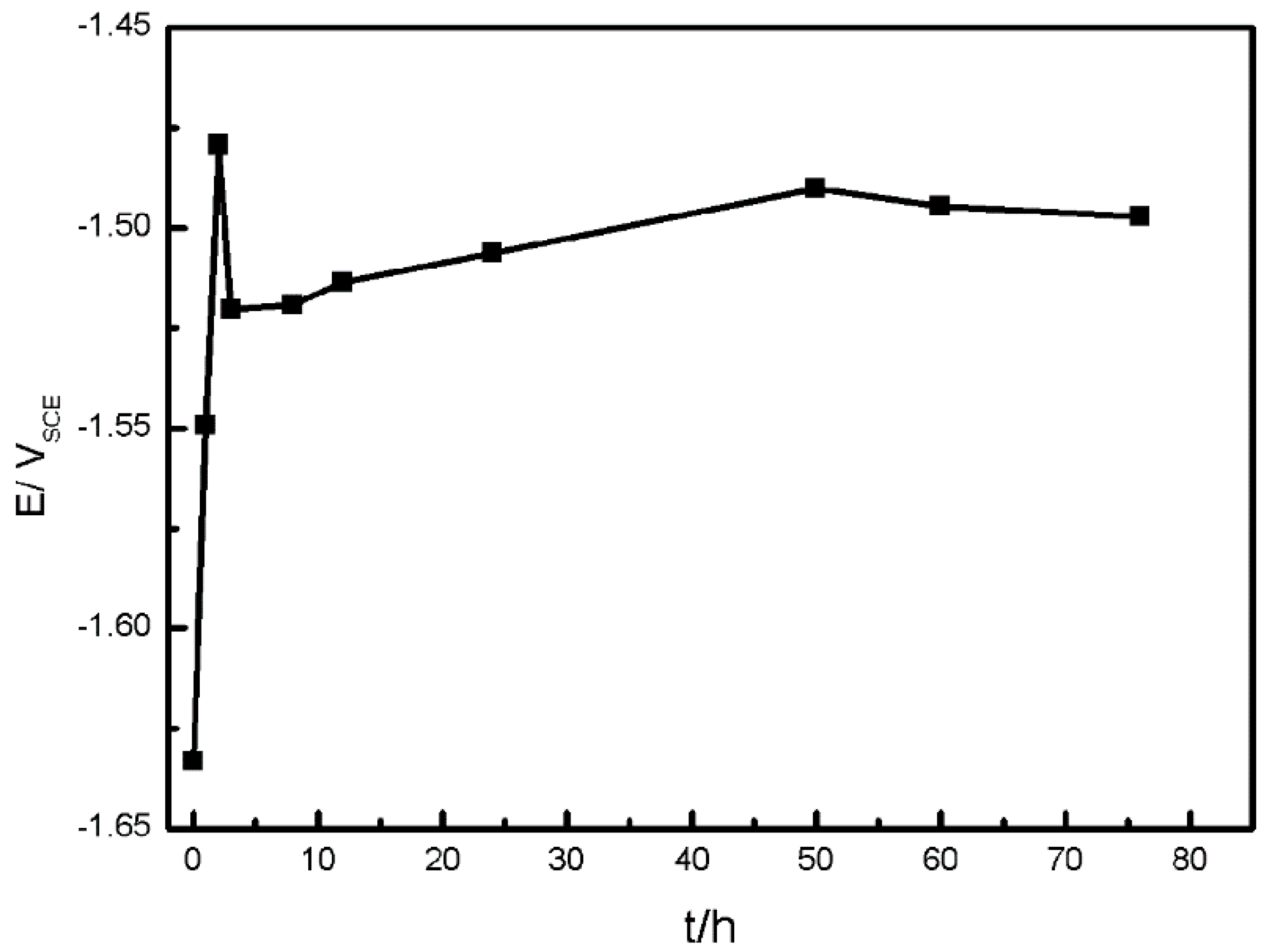
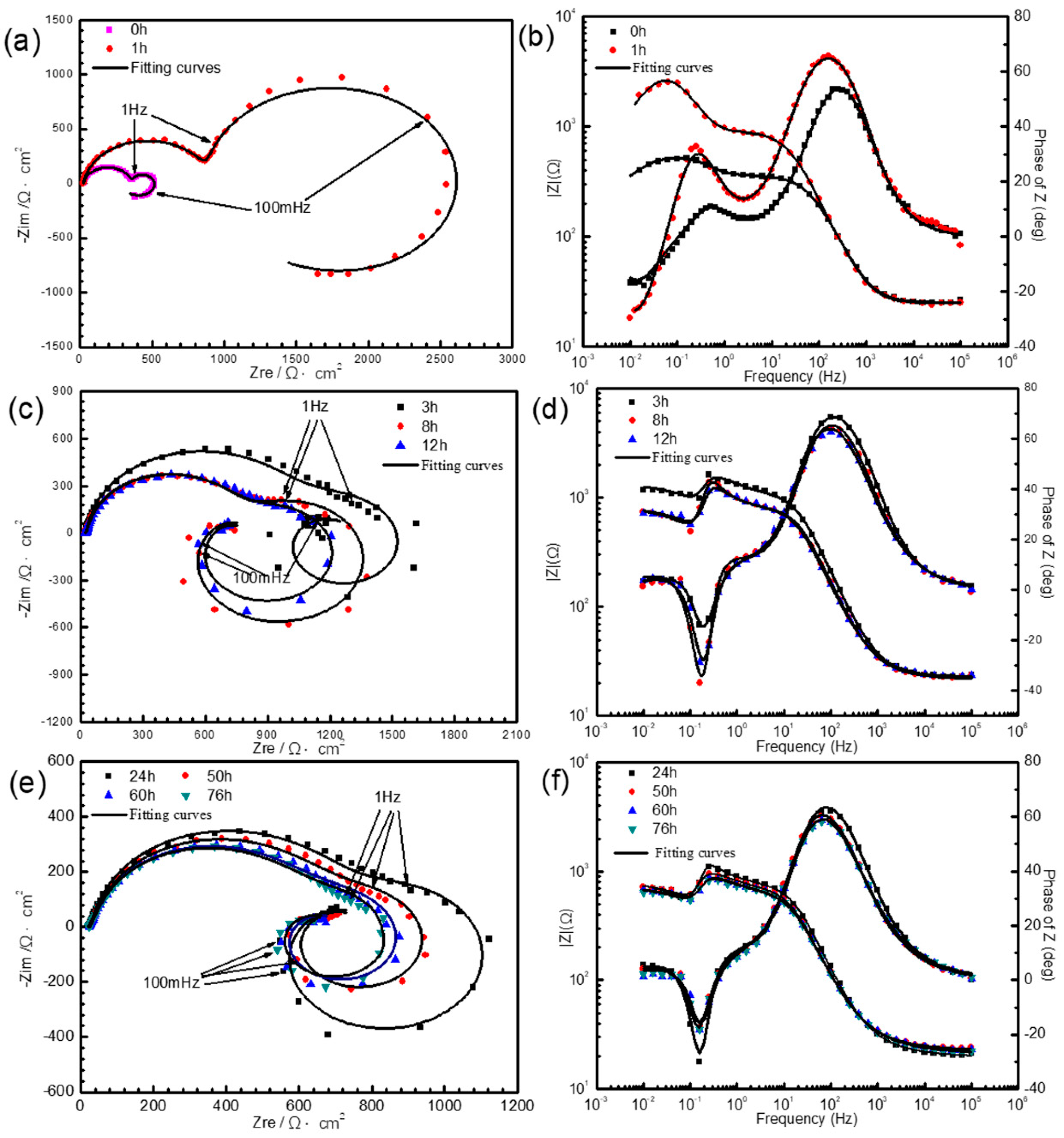
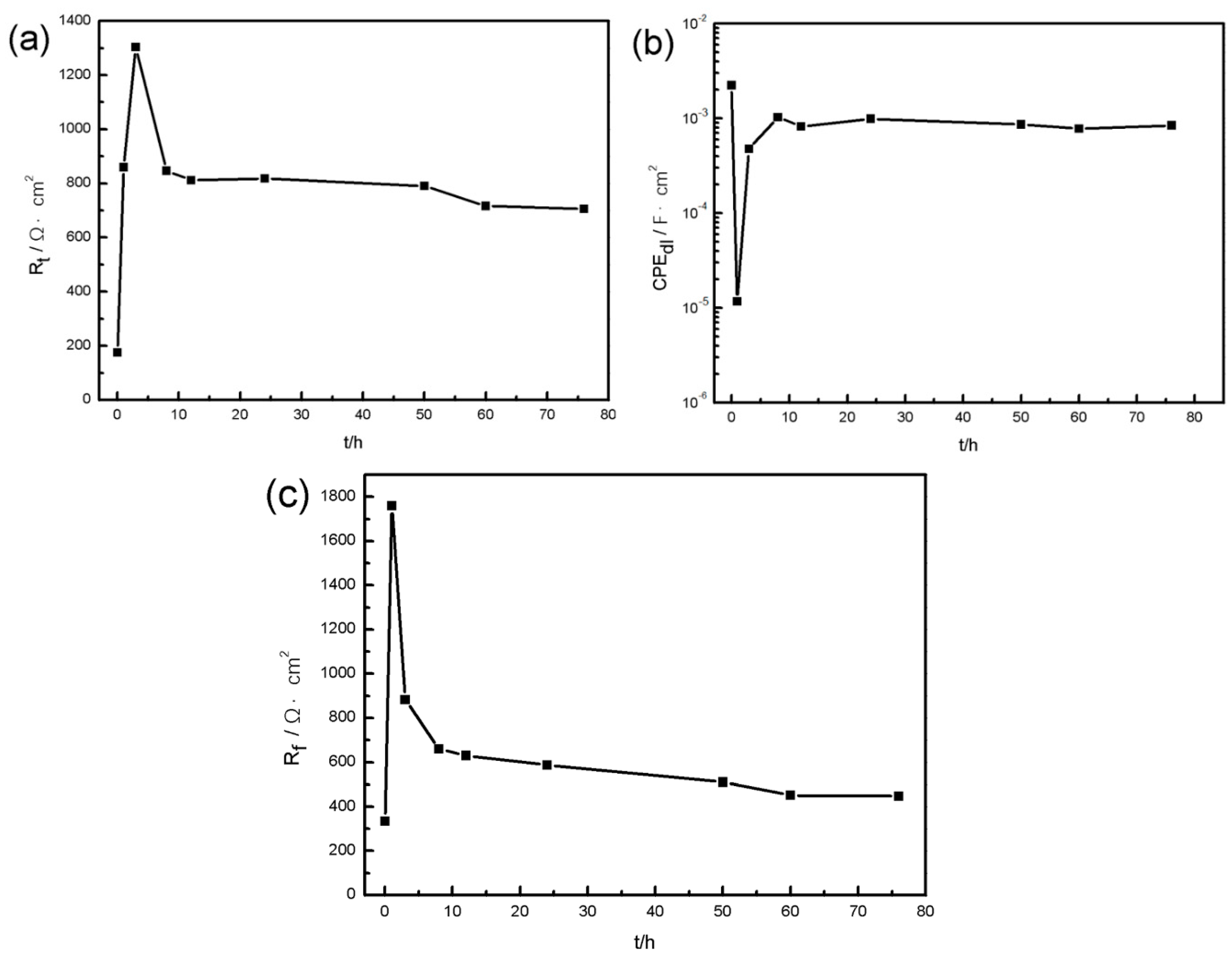
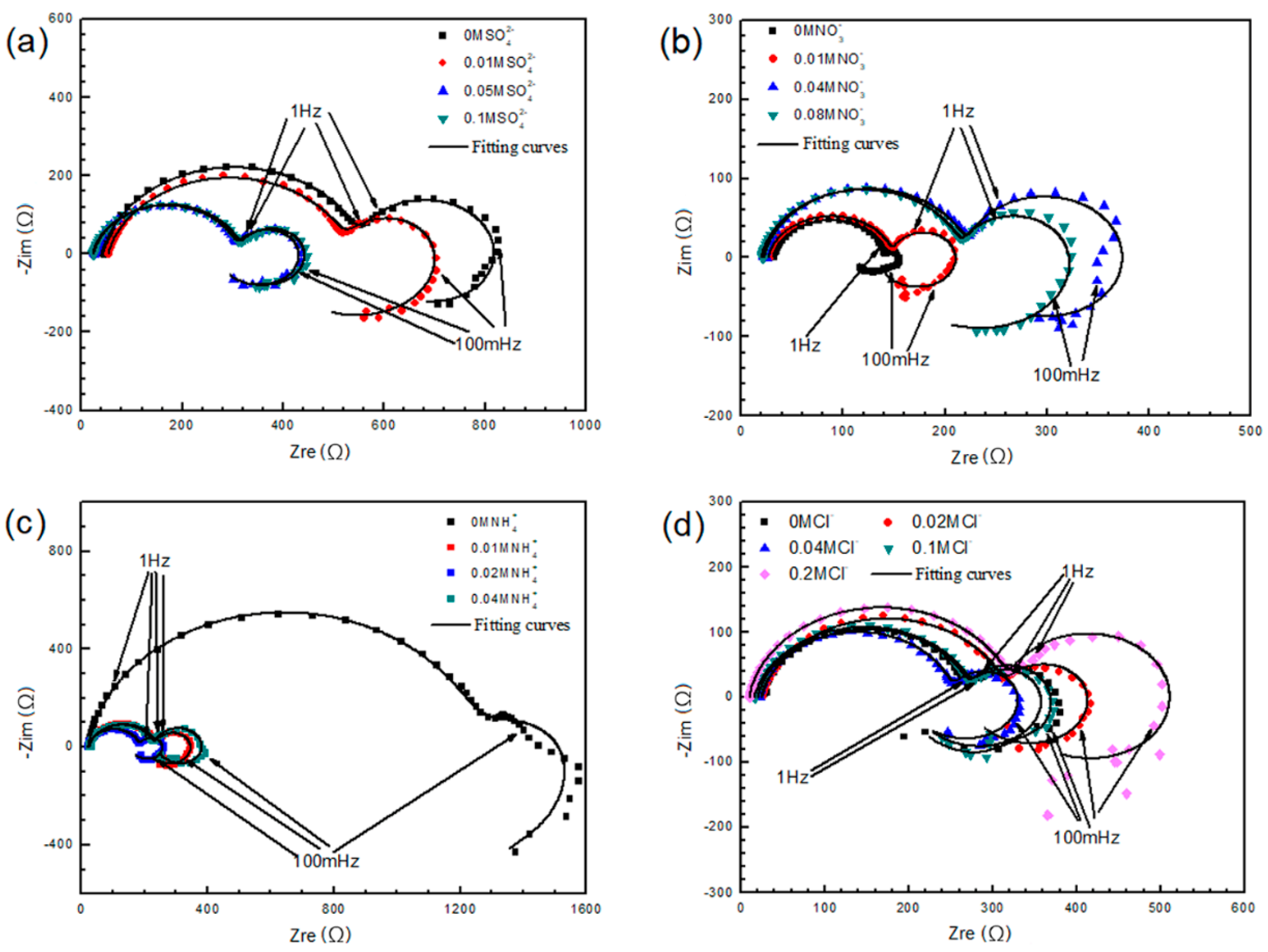
3.1.2. Electrochemical Measurements
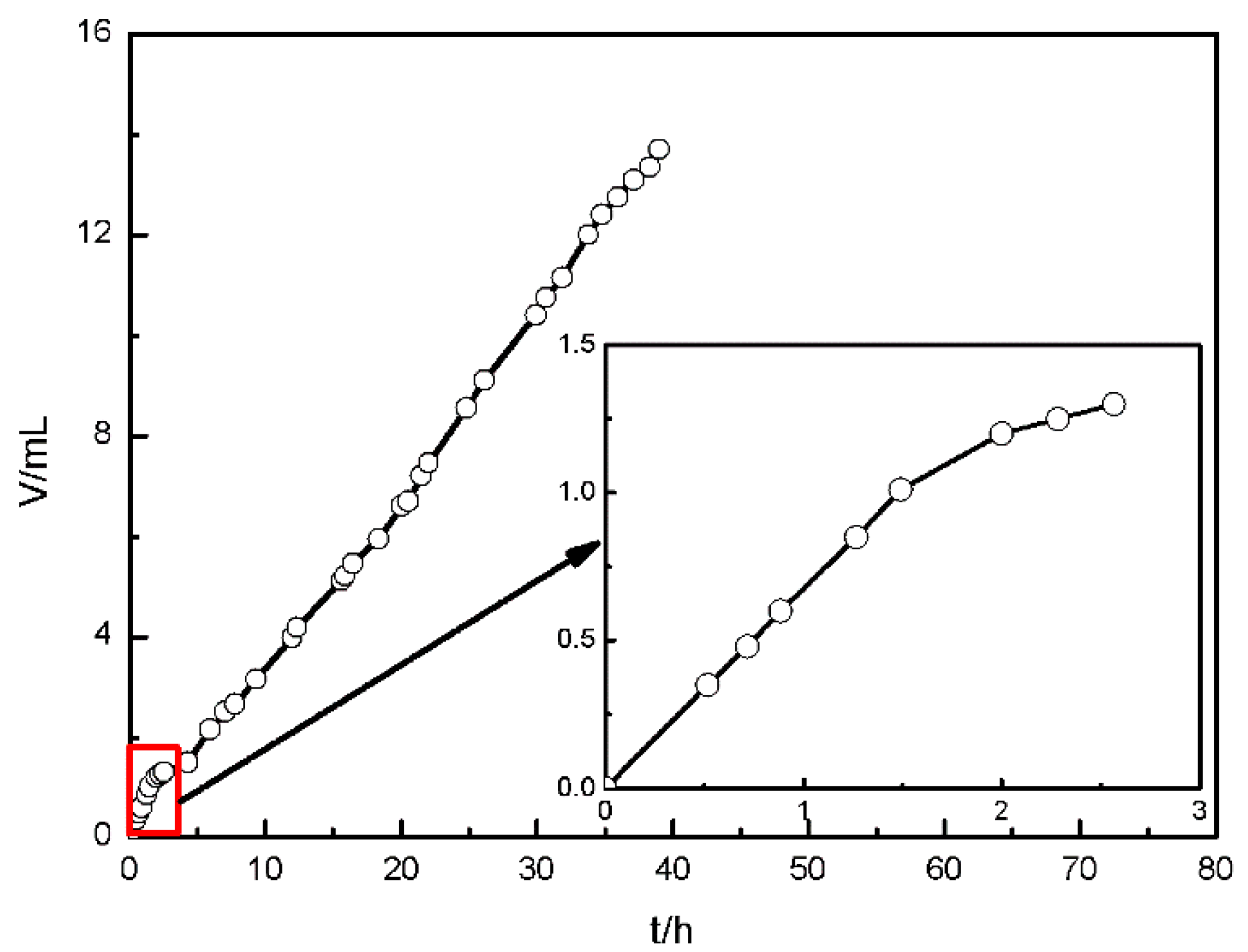
3.1.3. Hydrogen Collection
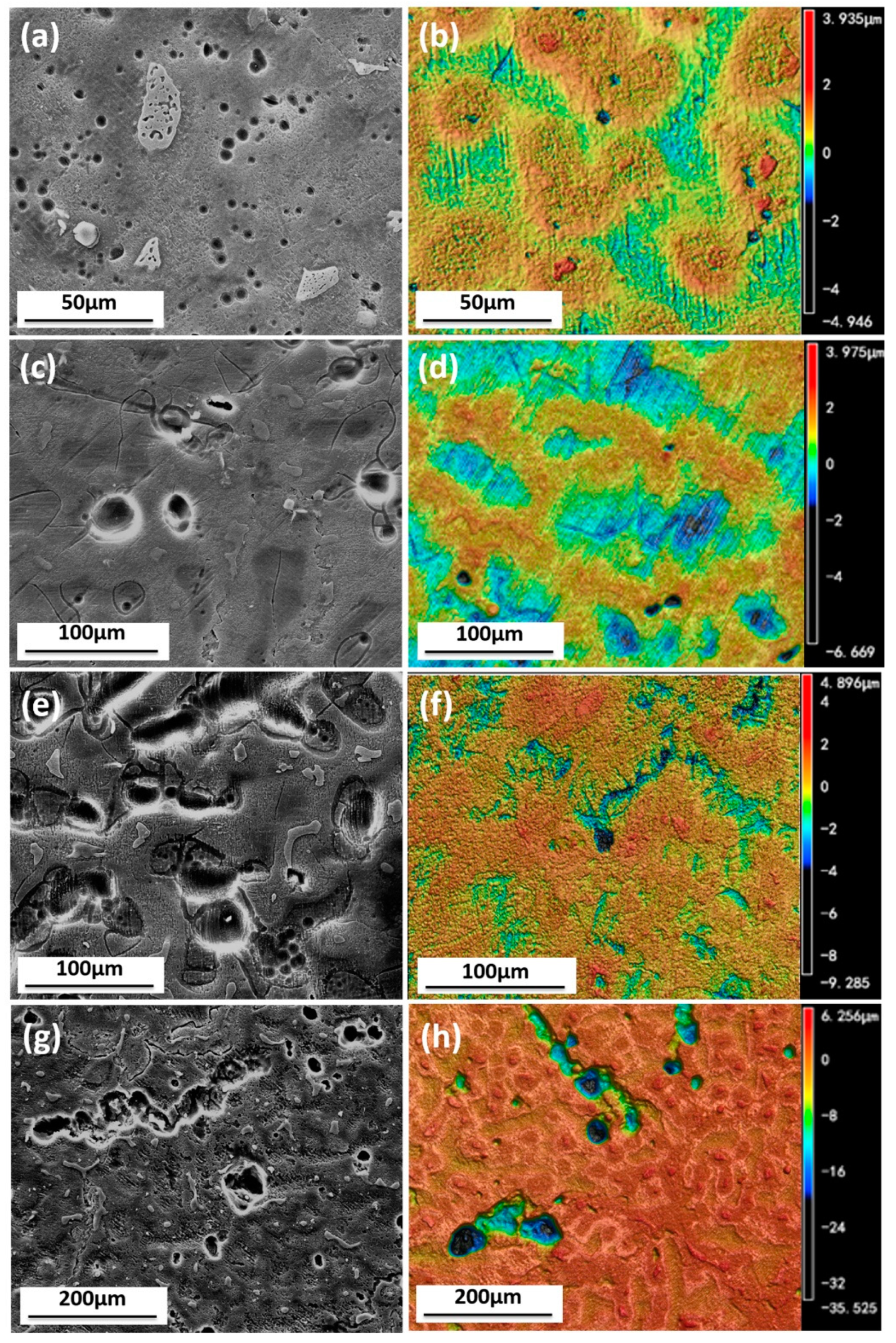
3.1.4. Surface Appearance of AZ91D Immersed for Various Time
3.2. The Influence of Ions
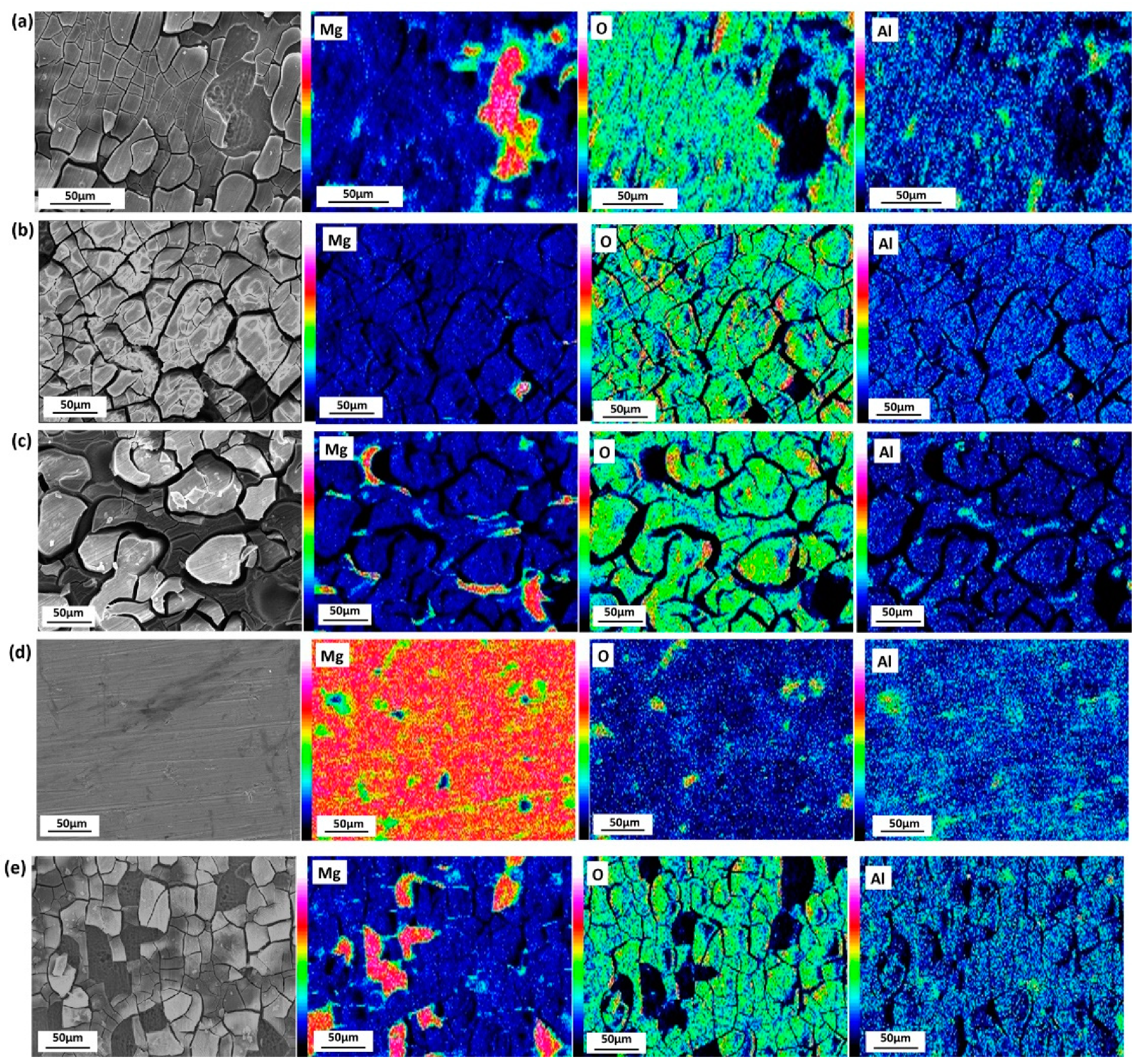
3.2.1. Surface Appearance of AZ91D Immersed in Various Solutions
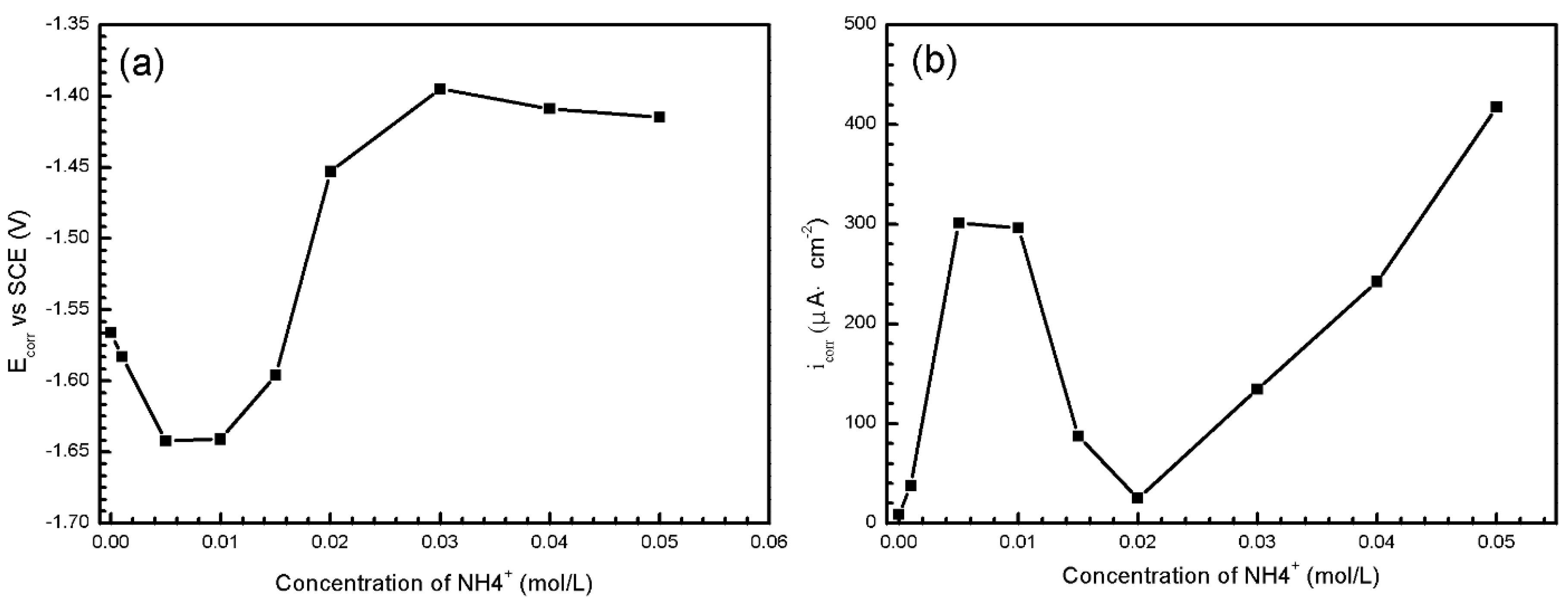
3.2.2. Electrochemical Measurements
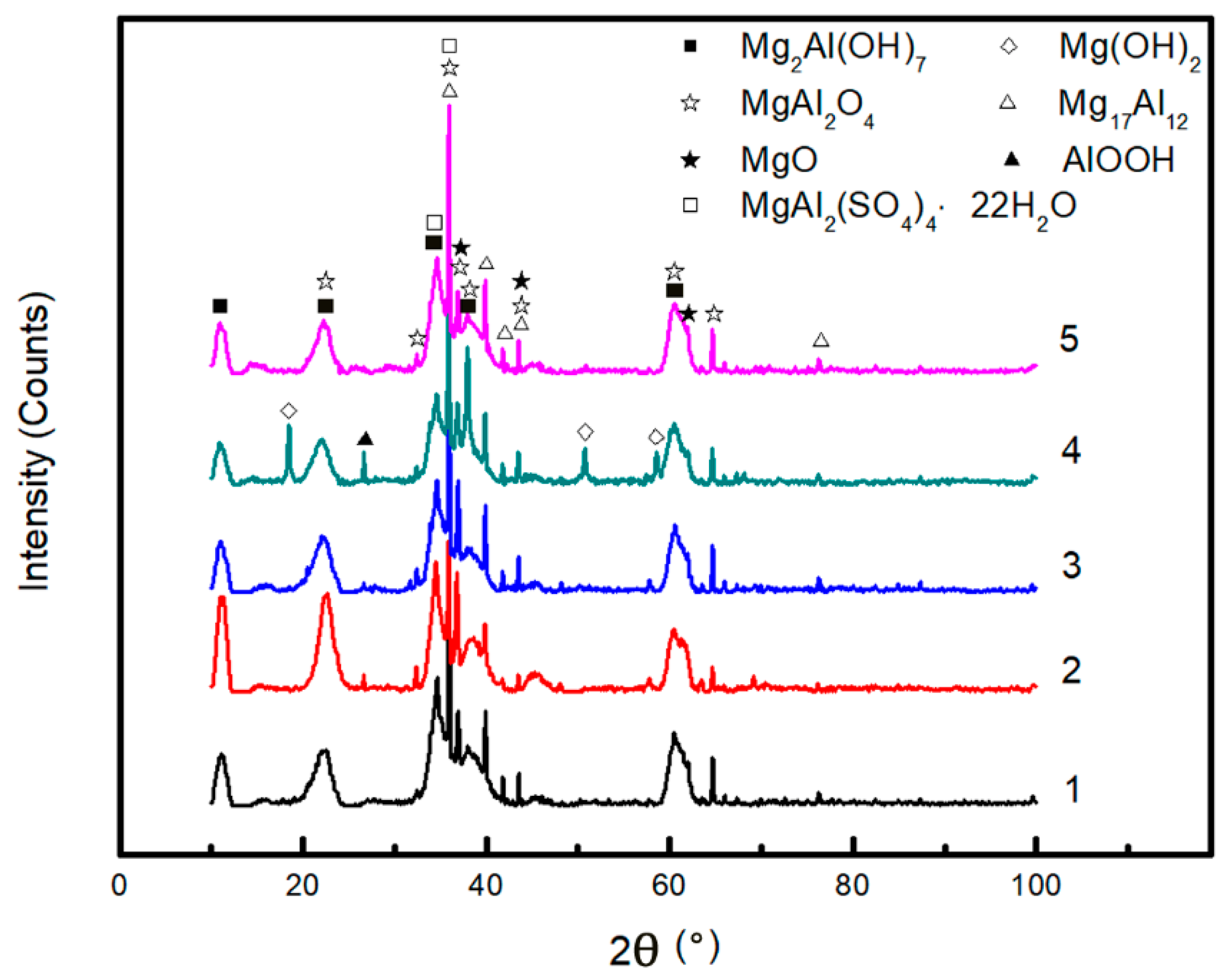
3.2.3. XRD Analysis
4. Discussion
4.1. The Corrosion Behavior of AZ91D Alloy in the Simulated HA Solution
4.2. The Corrosion Mechanism of AZ91D in the Simulated HA Solution
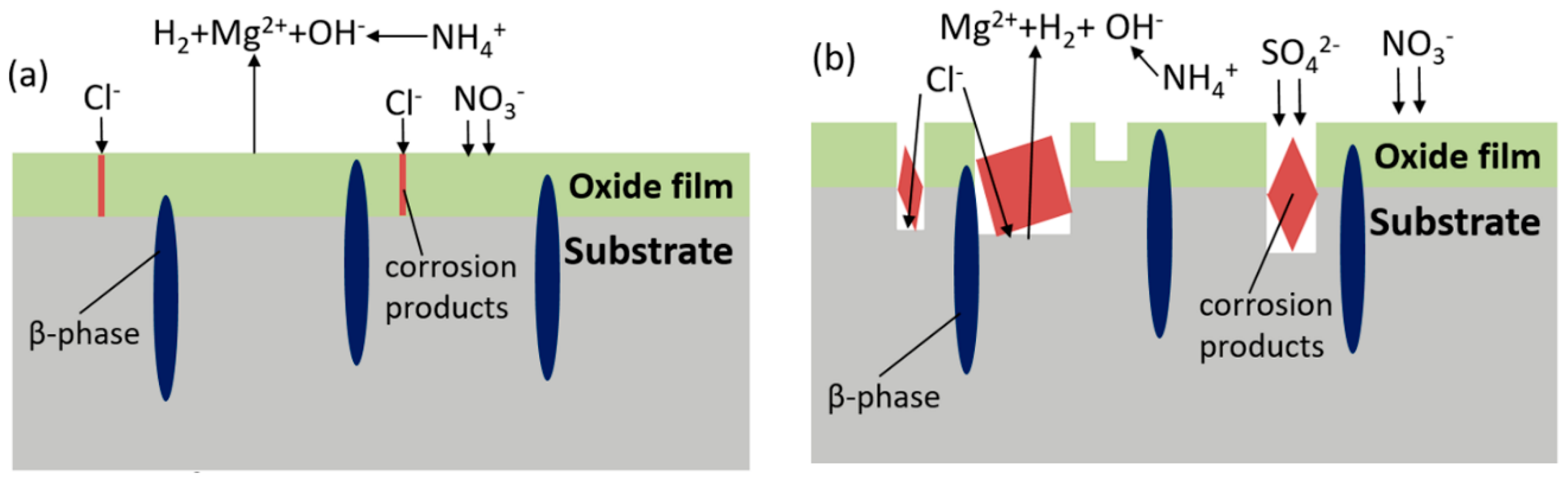
- First stage (shown in Figure 16a). According to Song [17], the anodic reaction becomes the following equations:The overall reaction:The cathodic reaction:Mg(OH)2 is supposed to be formed on the surface, but the process is thwarted by :Therefore, the product film is formed once the sample is exposed to the solution, and the main components refer to MgO instead of Mg(OH)2. The existence of helps to form a passive film on the surface, so the corrosion rate is soon limited. Cl− brings about the localized corrosion, therefore tiny corrosion pits appear where the sites are active.
- Second stage (shown in Figure 16b). As the corrosion develops, the resistant β-Mg17Al12 dissolves, thus aluminum concentration elevates and the precipitation of MgAl2(SO4)4·22H2O is formed according to Reaction (2), resulting in a higher corrosion rate. Corrosion continues to develop in pits, where bare α-matrix is firstly to be corroded and is consumed up by OH−, then β-phase reacts with OH−, generating Mg2Al(OH)7 and AlOOH. Cracks generally appear on the coverage and film drops out, even in a severe process, Mg particle surrounded by completely corroded material falls into solution, resulting in bare Mg exposed to the solution. Meanwhile, and Cl− keep attacking to form deeper pits.
5. Conclusions
- , , , Cl− are the four main water-soluble ions in simulated HA solution. and Cl− are aggressive towards AZ91D, causing and aggravating pitting corrosion. The absorption of prevents samples, especially α-matrix from severe corrosion by generating passive film on the surface. The combination of and OH− blocks the formation of Mg(OH)2, therefore corrosion process accelerates drastically.
- The corrosion attack of AZ91D immersed in simulated HA solution mainly takes place in α-phase matrix. Pitting corrosion is the main damage taking place on the surface. In addition, shallow pits resulting from Cl− are superimposed to form deep pits due to , and Mg particle undermining might take place. The main corrosion products are MgO, MgAl2O4 and MgAl2(SO4)4·22H2O.
- With the development of the corrosion, α-matrix and β-Mg17Al12 are dissolved, and localized corrosion aggravates, so the corrosion rate rises and finally stabilizes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turan, M.E.; Sun, Y.; Akgul, Y. Mechanical, tribological and corrosion properties of fullerene reinforced magnesium matrix composites fabricated by semi powder metallurgy. J. Alloys Compd. 2018, 740, 1149–1158. [Google Scholar] [CrossRef]
- Heakal, F.E.T.; Fekry, A.M.; Fatayerji, M.Z. Influence of halides on the dissolution and passivation behavior of AZ91D magnesium alloy in aqueous solutions. Electrochim. Acta 2009, 54, 1545–1557. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Blawert, C.; Tang, S.W. Influence of surface pre-treatment on the deposition and corrosion properties of hydrophobic coatings on a magnesium alloy. Corros. Sci. 2016, 112, 483–494. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Xiao, G.Y.; Lu, Y.P. Phosphate chemical conversion coatings on metallic substrates for biomedical application: A review. Mater. Sci. Eng. C 2015, 47, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Yerokhin, A.; Matthews, A. Deposition and evaluation of duplex hydroxyapatite and plasma electrolytic oxidation coatings on magnesium. Surf. Coat. Technol. 2015, 269, 170–182. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Cerium-based sealing of PEO coated AM50 magnesium alloy. Surf. Coat. Technol. 2015, 269, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Amaravathy, P.; Sowndarya, S.; Sathyanarayanan, S.; Rajendran, N. Novel sol gel coating of Nb2O5 on magnesium alloy for biomedical applications. Surf. Coat. Technol. 2014, 244, 131–141. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Baiocco, G.; Rubino, G.; Tagliaferri, V.; Ucciardello, N. Al2O3 coatings on magnesium alloy deposited by the Fluidized Bed (FB) technique. Materials 2018, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Han, G.M.; Huang, W.G. T6 treatment and its effects on corrosion properties of an Mg–4Sn–4Zn–2Al Alloy. Materials 2018, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Merino, M.C.; Coy, A.E. Influence of microstructure and composition on the corrosion behavior of Mg/Al alloys in chloride media. Electrochim. Acta 2008, 53, 7890–7902. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, F.L.; Zhao, M.C. Rare earth element yttrium modified Mg-Al-Zn alloy: microstructure, degradation properties and hardness. Materials 2017, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Anawati, A.; Asoh, H.; Ono, S. Effects of alloying element Ca on the corrosion behavior and bioactivity of anodic films formed on AM60 Mg alloys. Materials 2017, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, Q.; Chen, F.N.; Dai, Y.; Luo, F.; Li, L.Q. Study of the corrosion inhibition effect of sodium silicate on AZ91D magnesium alloy. Corros. Sci. 2011, 53, 1401–1407. [Google Scholar] [CrossRef]
- Liao, J.S.; Hotta, M. Corrosion products of field-exposed Mg-Al series magnesium alloys. Corros. Sci. 2016, 112, 276–288. [Google Scholar] [CrossRef]
- Hara, N.; Kobayashi, Y.; Kagaya, D.; Akao, N. Formation and breakdown of surface films on magnesium and its alloys in aqueous solutions. Corros. Sci. 2007, 49, 166–175. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Santamaria, M.; Quarto, F.D.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Ghali, E.; Dietzel, W.; Kainer, K.U. General and localized corrosion of magnesium alloys: A critical review. J. Mater. Eng. Perform. 2004, 13, 7–23. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Chang, Q.; Yang, F.M.; Li, X.H. Characteristics of mass and chemical species size distributions of particulate matter during haze pollution in the winter in Beijing. Acta Sci. Circumst. 2015, 35, 363–370. [Google Scholar] [CrossRef]
- Fekry, A.M.; Fatayerji, M.Z. Electrochemical corrosion behavior of AZ91D alloy in ethylene glycol. Electrochim. Acta 2009, 54, 6522–6528. [Google Scholar] [CrossRef]
- Retter, U.; Widmann, A.; Siegler, K.; Kahlert, H. On the impedance of potassium nickel(II) hexacyanoferrate(II) composite electrodes—the generalization of the Randles model referring to inhomogeneous electrode materials. J. Electroanal. Chem. 2003, 546, 87–96. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion of Magnesium Alloys; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Liu, Z.Y.; Li, X.G.; Du, C.W. Effect of inclusions on initiation of stress corrosion cracks in X70 pipeline steel in an acidic soil environment. Corros. Sci. 2009, 51, 895–900. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.Z.; Du, C.W. Effect of hydrogen-induced plasticity on the stress corrosion cracking of X70 pipeline steel in simulated soil environments. Mater. Sci. Eng. A 2016, 658, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.C.; Liu, Z.Y.; Du, C.W.; Li, X.G.; Cui, Z.Y. Comparative study of the SCC behavior of E690 steel and simulated HAZ microstructures in a SO2-polluted marine atmosphere. Mater. Sci. Eng. A 2016, 650, 93–101. [Google Scholar] [CrossRef]
- Shi, Z.M.; Jia, J.X.; Atrens, A. Galvanostatic anodic polarization curves and galvanic corrosion of high purity Mg in 3.5% NaCl saturated with Mg(OH)2. Corros. Sci. 2012, 60, 296–308. [Google Scholar] [CrossRef]
- Shi, Z.M.; Jia, J.X.; Atrens, A. Galvanostatic anodic polarisation and galvanic corrosion of AZ31B in 0.01 M Na2SO4 saturated with Mg(OH)2. Adv. Eng. Mater. 2012, 14, 324–334. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.Q.; Han, E.H. AC impedance spectroscopy study of the corrosion behavior of an AZ91 magnesium alloy in 0.1 M sodium sulfate solution. Electrochim. Acta 2007, 52, 3299–3309. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; John, D.; Wu, X.; Nairn, J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros. Sci. 1997, 39, 1981–2004. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate electrochemical measurement of magnesium corrosion rates; a combined impedance, mass-loss and hydrogen collection atudy. Electrochim. Acta 2014, 121, 394–460. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion of Magnesium Alloys; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- El-Naggar, M.M. Effects of Cl−, and anions on the anodic behavior of carbon steel in deaerated 0.50 M NaHCO3 solutions. Appl. Surf. Sci. 2006, 252, 6179–6194. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion Prevention of Magnesium Alloys; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Shi, Z.M.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Z.J.; Wang, J.J. Slow positron beam study of corrosion behavior of AM60B magnesium alloy in NaCl solution. Corros. Sci. 2016, 106, 271–280. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Chen, R.S.; Han, E.H. Corrosion characterization of Mg–8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]



| Al | Zn | Mn | Si | Ni | Fe | Cu | Mg |
|---|---|---|---|---|---|---|---|
| 8.56 | 0.54 | 0.22 | 0.054 | <0.005 | <0.005 | <0.005 | Bal. |
| Region | Mg | Al | Zn |
|---|---|---|---|
| 1 | 97.72 | 1.56 | – |
| 2 | 70.21 | 27.25 | 1.86 |
| Concentration (mol/L) | ||||||
|---|---|---|---|---|---|---|
| Ecorr (V vs. SCE) | icorr (μA/cm2) | rc (mm/a) | Ecorr (V vs. SCE) | icorr (μA/cm2) | rc (mm/a) | |
| 0 | −1.566 | 8.68 | 0.1983 | −1.665 | 246.7 | 5.637 |
| 0.001 | −1.583 | 37.63 | 0.8598 | −1.670 | 224.0 | 5.118 |
| 0.005 | −1.642 | 301.2 | 6.882 | −1.670 | 265.2 | 6.060 |
| 0.01 | −1.641 | 296.2 | 6.768 | −1.653 | 172.7 | 3.946 |
| 0.015 | −1.596 | 87.52 | 2.000 | −1.645 | 203.0 | 4.639 |
| 0.02 | −1.453 | 25.07 | 0.5729 | −1.648 | 239.7 | 5.477 |
| 0.03 | −1.395 | 134.5 | 3.073 | −1.657 | 268.5 | 6.135 |
| 0.04 | −1.409 | 242.3 | 5.537 | −1.652 | 244.1 | 5.578 |
| 0.05 | −1.415 | 417.6 | 9.542 | −1.648 | 280.2 | 6.403 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Liu, Z.; Hu, P.; Shao, J.; Li, X.; Du, C.; Jiang, B. The Corrosion Behavior of AZ91D Magnesium Alloy in Simulated Haze Aqueous Solution. Materials 2018, 11, 970. https://doi.org/10.3390/ma11060970
Cui L, Liu Z, Hu P, Shao J, Li X, Du C, Jiang B. The Corrosion Behavior of AZ91D Magnesium Alloy in Simulated Haze Aqueous Solution. Materials. 2018; 11(6):970. https://doi.org/10.3390/ma11060970
Chicago/Turabian StyleCui, Liying, Zhiyong Liu, Peng Hu, Jiamin Shao, Xiaogang Li, Cuiwei Du, and Bin Jiang. 2018. "The Corrosion Behavior of AZ91D Magnesium Alloy in Simulated Haze Aqueous Solution" Materials 11, no. 6: 970. https://doi.org/10.3390/ma11060970






