Ion Doping Effects on the Lattice Distortion and Interlayer Mismatch of Aurivillius-Type Bismuth Titanate Compounds
Abstract
:1. Introduction
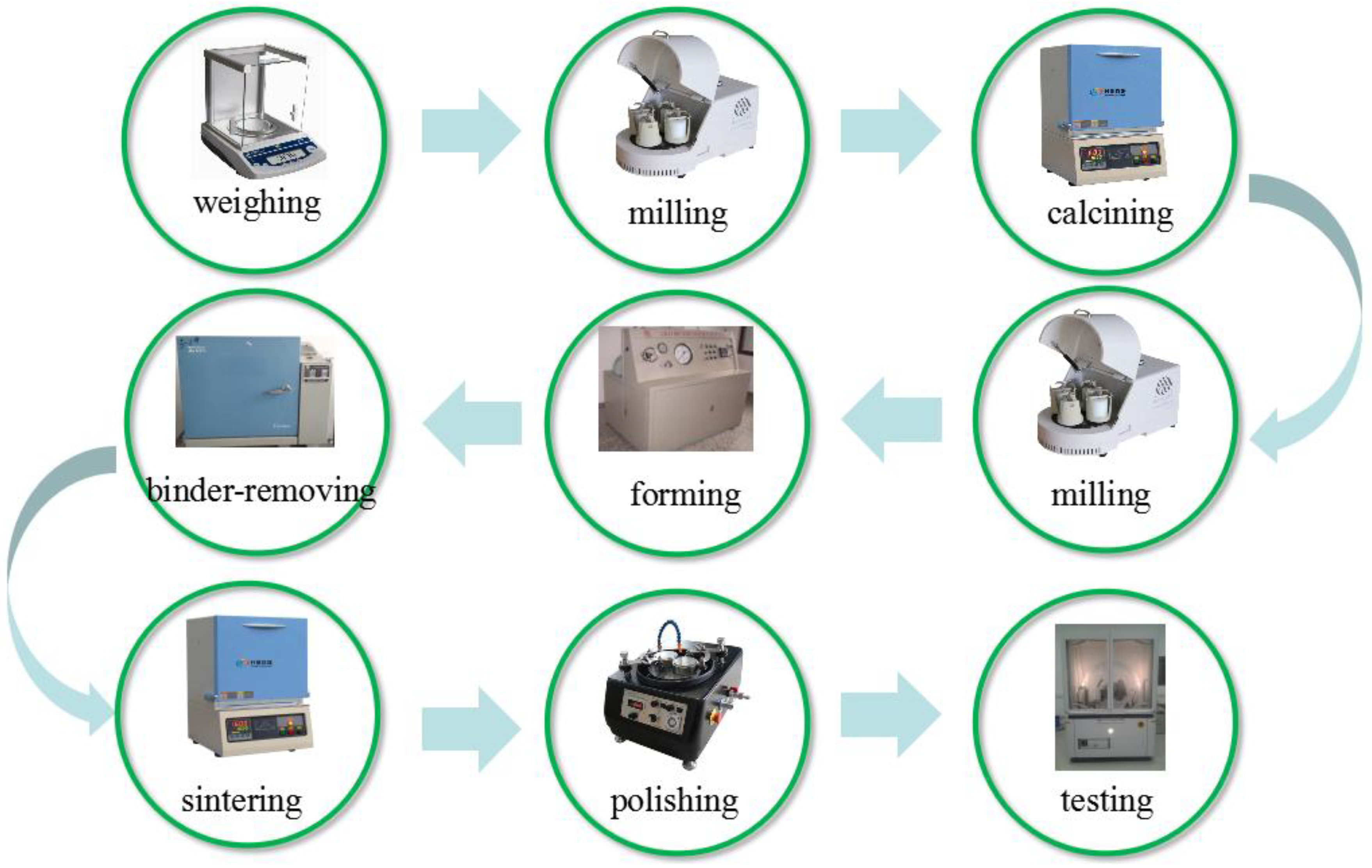
2. Experimental Procedure
2.1. Sample Preparation
2.2. Phase Structure Analysis
3. Results
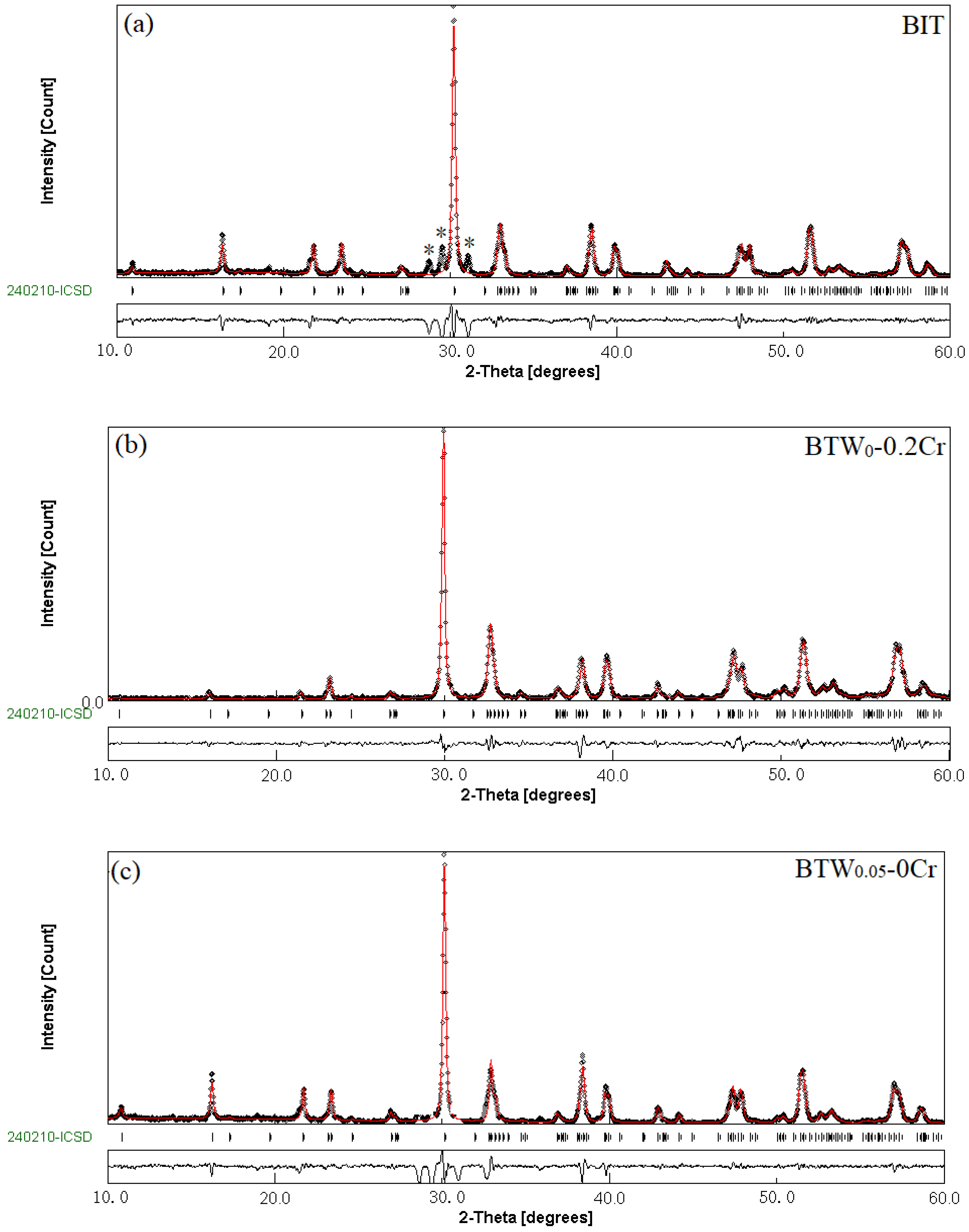
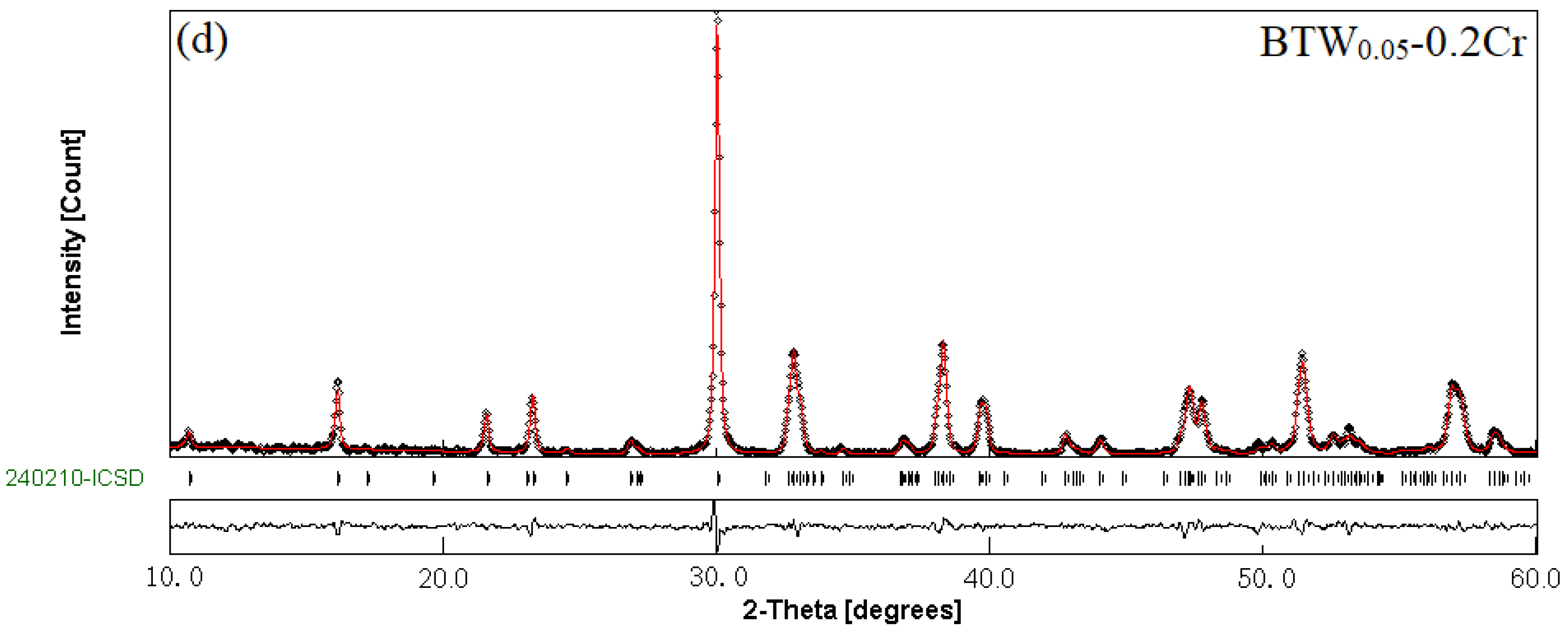
3.1. Phase Structure of BTWC Ceramics
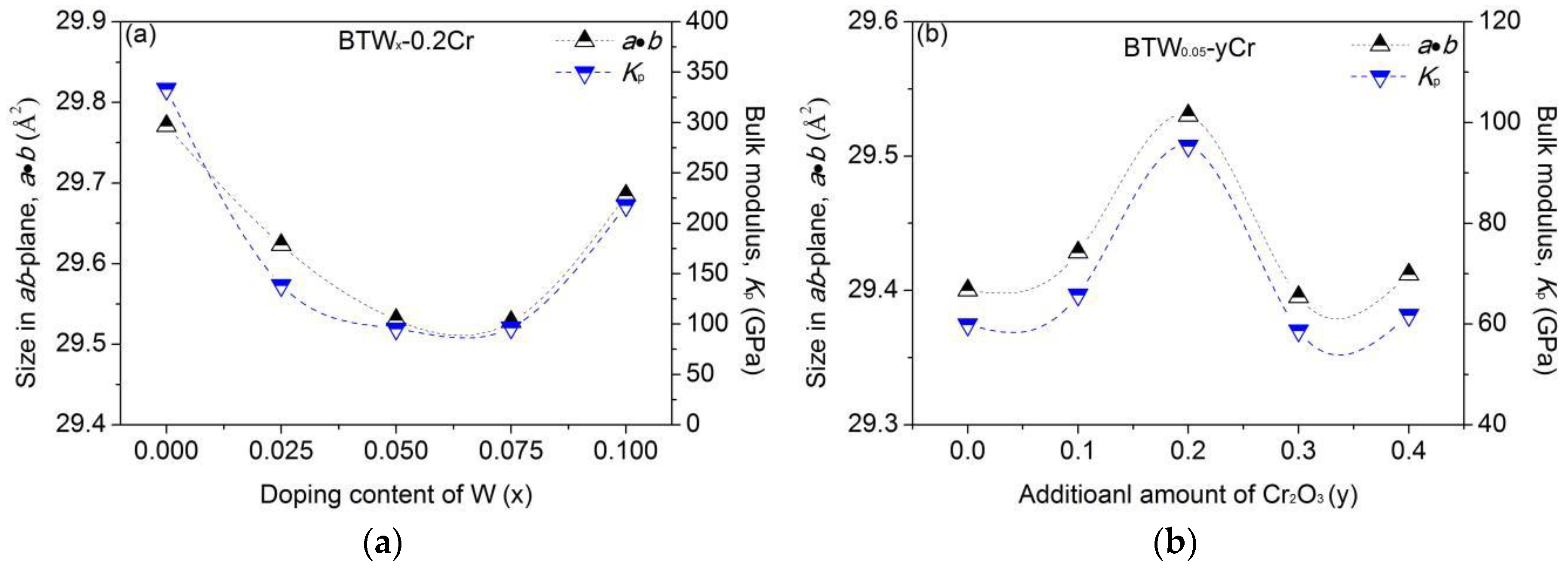
3.2. Cell Parameters of BTWC Ceramics
4. Discussion
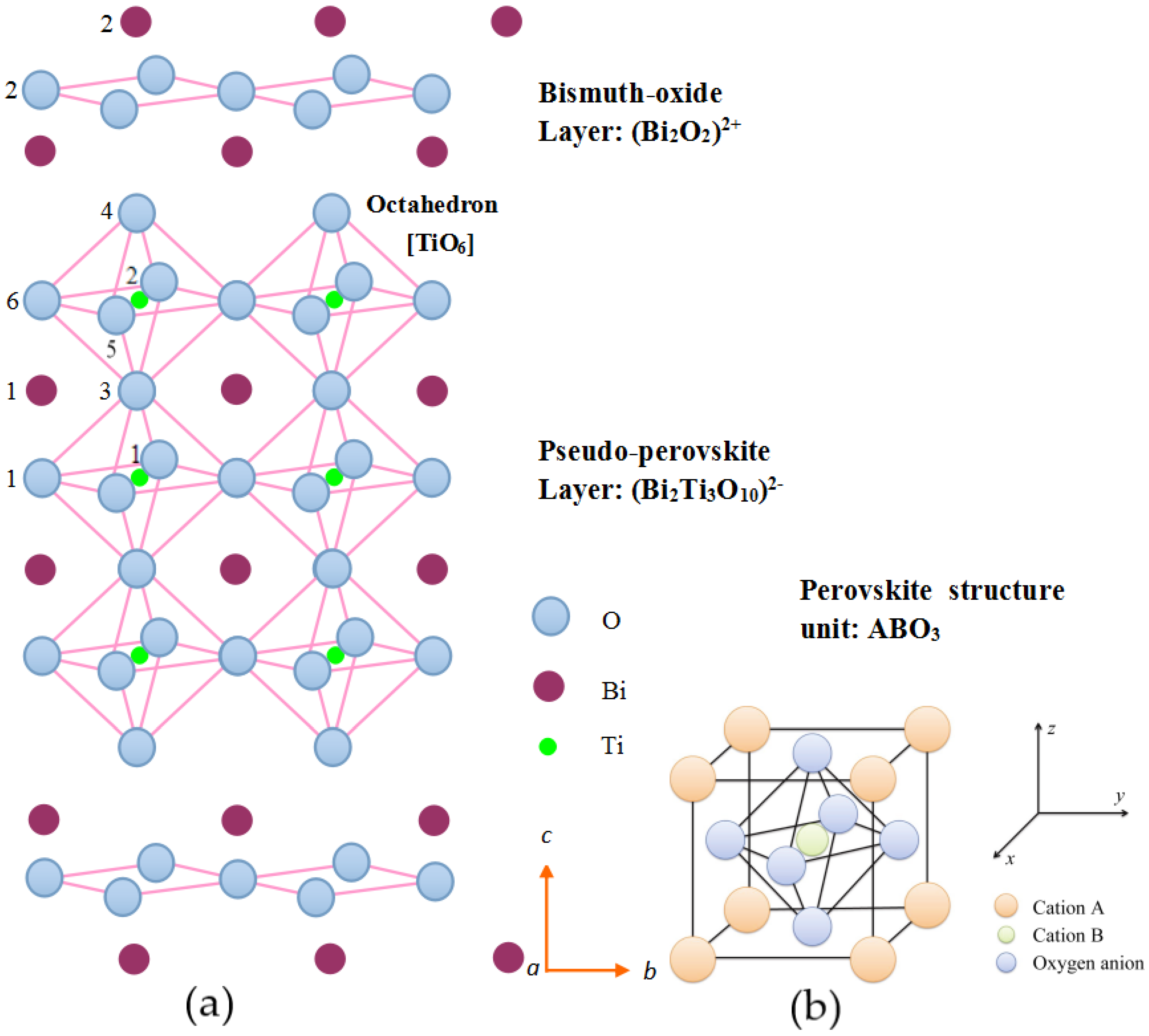
4.1. Lattice Distortion of Bi4Ti3O12 Structure
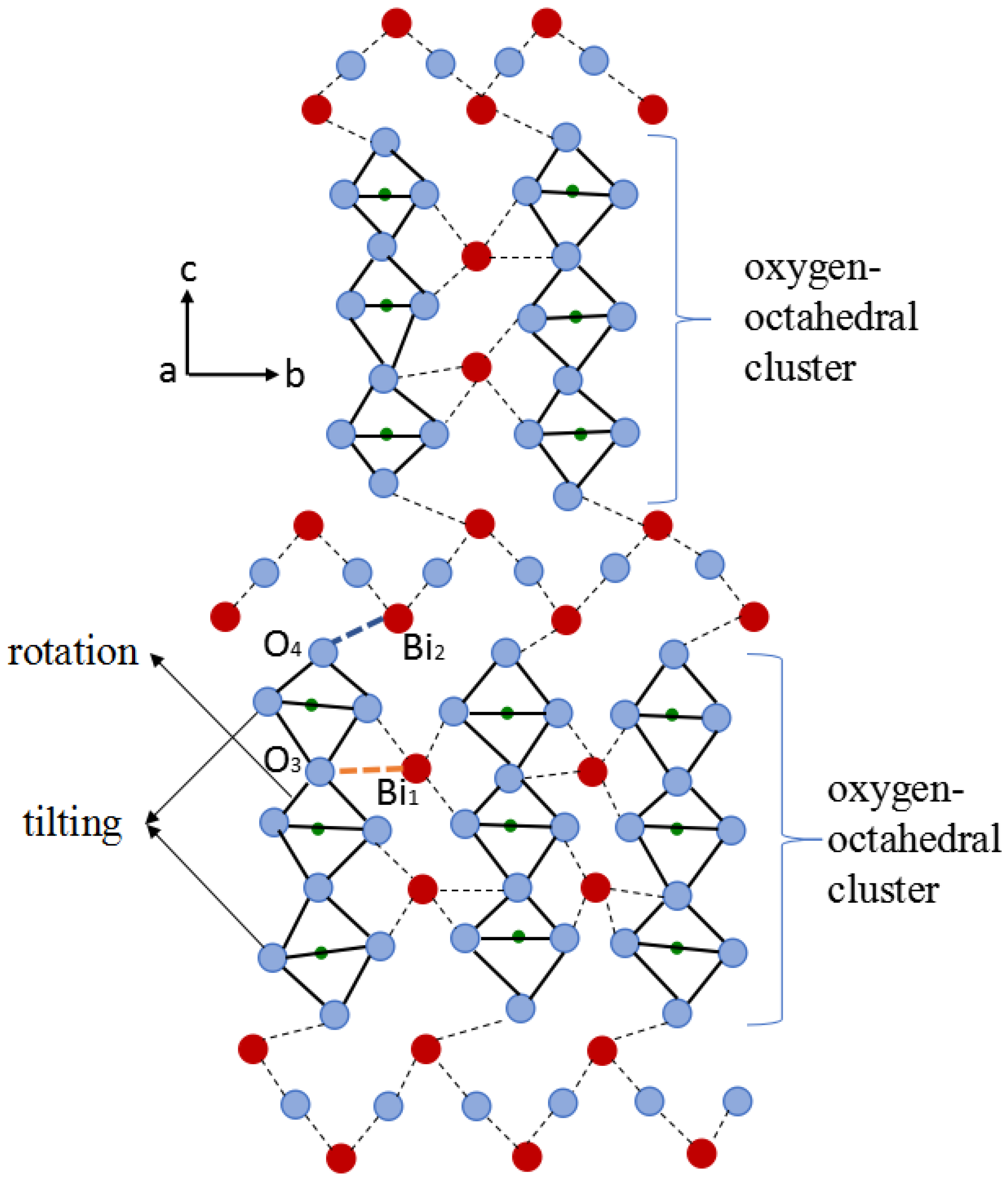
4.1.1. Rotation of Oxygen-Octahedron
4.1.2. Tilting of Oxygen-Octahedron
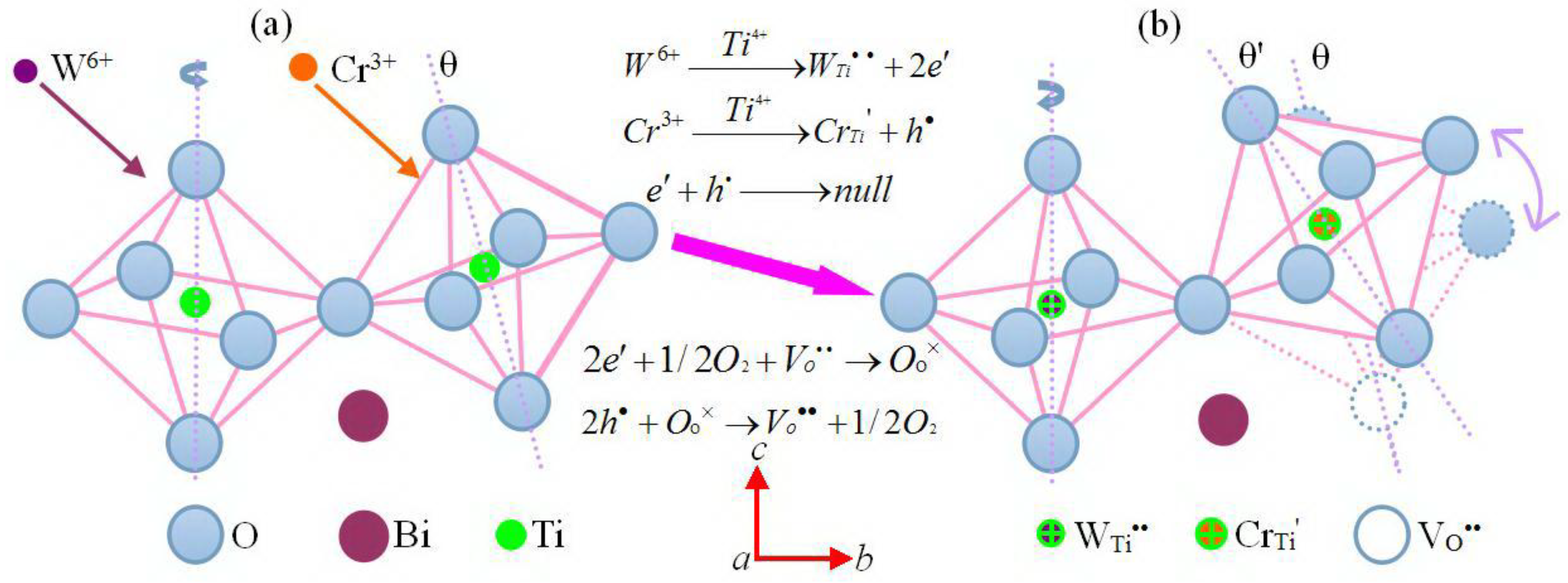
4.1.3. Regulation of Doping-Ions for the Lattice Distortion
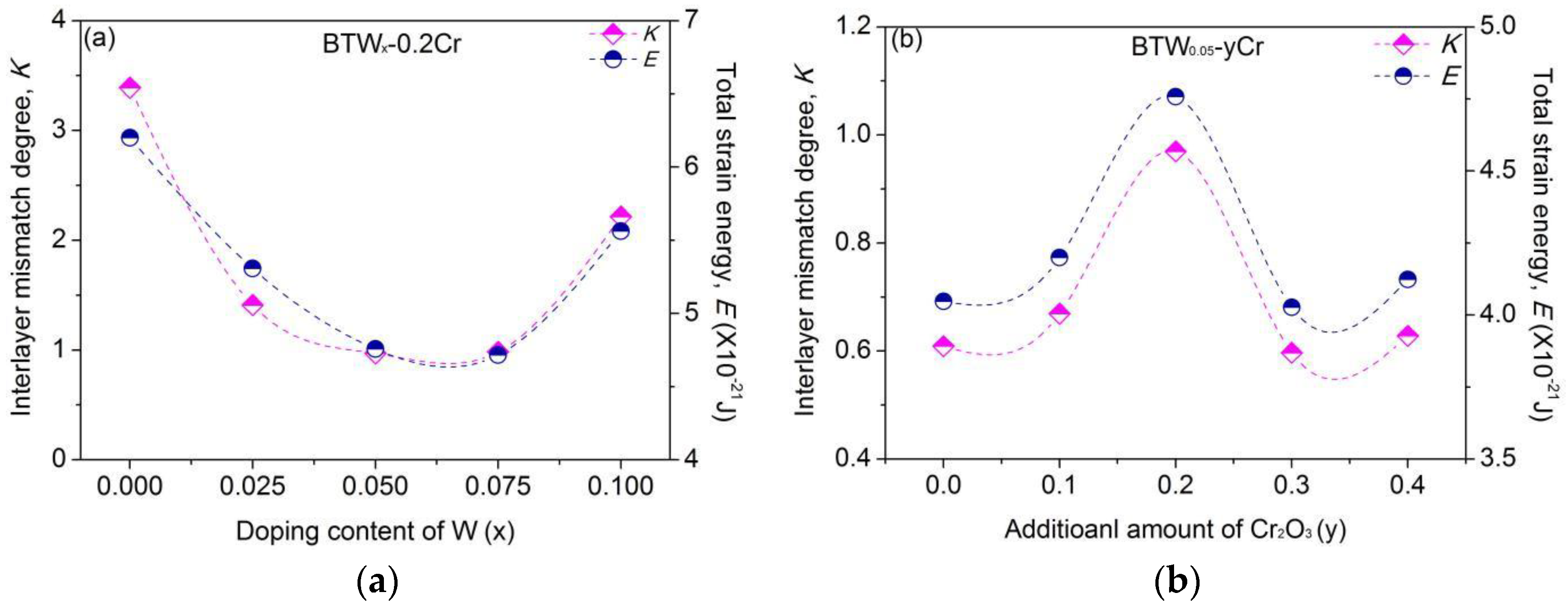
4.2. Interlayer Mismatch of Bi4Ti3O12 Structure
4.2.1. Elastic Model Considering Short Range Forces
4.2.2. Influence of Doping-Ions on the Interlayer Mismatch
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aurivillius, B. Mixed Bismuth Oxides with Layer Lattices I. The Structure Type of CaNb2Bi2O9. Arkiv Kemi 1949, 1, 463–480. [Google Scholar]
- Aurivillius, B. Mixed Bismuth Oxides with Layer Lattices II. Structure of Bi4Ti3O12. Arkiv Kemi 1949, 1, 499–512. [Google Scholar]
- Aurivillius, B. Mixed Bismuth Oxides with Layer Lattices III. Structure of BaBi4Ti4O15. Arkiv Kemi 1950, 2, 519–527. [Google Scholar]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shen, Z.Y.; Zhang, S.; Shrout, T.R. Improved Pyroelectric Properties of CaBi4Ti4O15 Ferroelectrics Ceramics by Nb/Mn Co-Doping for Pyrosensors. J. Am. Ceram. Soc. 2016, 99, 1294–1298. [Google Scholar] [CrossRef]
- Panda, P.K. environmental friendly lead-free piezoelectric materials. J. Mater. Sci. 2009, 44, 5049–5062. [Google Scholar] [CrossRef]
- Axelsson, A.K.; Le Goupil, F.; Valant, M.; Alford, N.M. Electrocaloric effect in lead-free Aurivillius relaxor ferroelectric ceramics. Acta Mater. 2017, 124, 120–126. [Google Scholar] [CrossRef]
- Moure, A. Review and Perspectives of Aurivillius Structures as a Lead-Free Piezoelectric System. Appl. Sci. 2018, 8, 62. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Subbarao, E.C. Crystal chemistry of mixed bismuth oxides with layer-type structure. J. Am. Ceram. Soc. 1962, 45, 166–169. [Google Scholar] [CrossRef]
- Snedden, A.; Lightfoot, P.; Dinges, T.; Islam, M.S. Defect and dopant properties of the Aurivillius phase Bi4Ti3O12. J. Solid State Chem. 2004, 177, 3660–3665. [Google Scholar] [CrossRef]
- Irie, H.; Miyayama, M.; Kudo, T. Structure dependence of ferroelectric properties of bismuth layer-structured ferroelectric single crystals. J. Appl. Phys. 2001, 90, 4089–4094. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Newnham, R.E. Bismuth titanate solid solutions. Mater. Res. Bull. 1972, 7, 1025–1034. [Google Scholar] [CrossRef]
- Chen, Y.; Pen, Z.; Wang, Q.; Zhu, J. Crystalline structure, ferroelectric properties, and electrical conduction characteristics of W/Cr co-doped Bi4Ti3O12 ceramics. J. Alloy. Compd. 2014, 612, 120–125. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.; Wang, H.; Chen, Q.; Wang, Q.; Zhu, J.; Guan, Z. Dielectric abnormality and ferroelectric asymmetry in W/Cr co-doped Bi4Ti3O12 ceramics based on the effect of defect dipoles. J. Alloy. Compd. 2017, 696, 746–753. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, C.; Xie, S.; Xu, L.; Wang, Q.; Zhu, J.; Guan, Z. Microstructural evolutions, elastic properties and mechanical behaviors of W/Cr co-doped Bi4Ti3O12 ceramics. Mater. Des. 2016, 90, 628–634. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, C.; Xie, S.; Xu, L.; Wang, Q.; Zhu, J.; Guan, Z. Fracture Behaviors and Ferroelastic Deformation in W/Cr Co-Doped Bi4Ti3O12 Ceramics. J. Am. Ceram. Soc. 2016, 99, 2103–2109. [Google Scholar] [CrossRef]
- Wiegers, G.A. Misfit layer compounds: Structures and physical properties. Prog. Solid State Chem. 1996, 24, 1–139. [Google Scholar] [CrossRef]
- Kikuchi, T. Stability of layered bismuth compounds in relation to the structural mismatch. Mater. Res. Bull. 1979, 14, 1561–1569. [Google Scholar] [CrossRef]
- Dai, X.; Xu, Z.; Viehland, D. Effect of oxygen octahedron rotations on the phase stability, transformational characteristics, and polarization behavior in the lead zirconate titanate crystalline solution series. J. Am. Ceram. Soc. 1995, 78, 2815–2827. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, R.; Liu, X.; Fang, B.; Yuan, N.; Ding, J.; Luo, H. Large Electrocaloric Effect in Lead-Free (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 Ceramics Prepared via Citrate Route. Materials 2017, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.A.; Sarkar, T.; Fortalnova, E.A.; Politova, E.D.; Stefanovich, S.Y.; Safronenko, M.G.; Nordblad, P.; Mathieu, R. Composition dependence of the multifunctional properties of Nd-doped Bi4Ti3O12 ceramics. J. Mater. Sci. Mater. Electron. 2017, 28, 7692–7707. [Google Scholar] [CrossRef]
- Newnham, R.E.; Wolfe, R.W.; Dorrian, J.F. Structural basis of ferroelectricity in the bismuth titanate family. Mater. Res. Bull. 1971, 6, 1029–1039. [Google Scholar] [CrossRef]
- Kunz, M.; Brown I, D. Out-of-center distortions around octahedrally coordinated d0 transition metals. J. Solid State Chem. 1995, 115, 395–406. [Google Scholar] [CrossRef]
- Hervoches, C.H.; Lightfoot, P. A Variable-Temperature Powder Neutron Diffraction Study of Ferroelectric Bi4Ti3O12. Chem. Mater. 1999, 11, 3359–3364. [Google Scholar] [CrossRef]
- Nichols, E.J.; Shi, J.; Huq, A.; Vogel, S.C.; Misture, S.T. Controlling structure distortions in 3-layer ferroelectric Aurivillius oxides. J. Solid State Chem. 2013, 197, 475–482. [Google Scholar] [CrossRef]
- Thomas, N.W. A re-examination of the relationship between lattice strain, octahedral tilt angle and octahedral strain in rhombohedral perovskites. Acta Crystallogr. 1996, 52, 954–960. [Google Scholar] [CrossRef]
- Magyari-Köpe, B.; Vitos, L.; Johansson, B.; Kollár, J. Origin of octahedral tilting in orthorhombic perovskites. Phys. Rev. B 2002, 66, 092103. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.; Wang, Q.; Zhu, J. Influence of Cr2O3 additive and sintering temperature on the structural characteristics and piezoelectric properties of Bi4Ti2.95W0.05O12.05 Aurivillius ceramics. Prog. Nat. Sci. Mater. Int. 2016, 26, 572–578. [Google Scholar] [CrossRef]
- Levy, R.A.; Collier, R.J. Principles of Solid State Physics. Phys. Today 1969, 22, 75–76. [Google Scholar] [CrossRef]

| x | 0 | 0.025 | 0.05 | 0.075 | 0.1 | |
|---|---|---|---|---|---|---|
| y | ||||||
| 0 | BIT | - | BTW0.05-0Cr | - | - | |
| 0.1 | - | - | BTW0.05-0.1Cr | - | - | |
| 0.2 | BTW0-0.2Cr | BTW0.025-0.2Cr | BTW0.05-0.2Cr | BTW0.075-0.2Cr | BTW0.1-0.2Cr | |
| 0.3 | - | - | BTW0.05-0.3Cr | - | - | |
| 0.4 | - | - | BTW0.05-0.4Cr | - | - | |
| Parameters | Tungsten Content in BTWx-0.2Cr (Space Group of B2cb) | ||||
|---|---|---|---|---|---|
| x = 0 | x = 0.025 | x = 0.05 | x = 0.075 | x = 0.1 | |
| Refinement index | |||||
| Rp | 5.68 | 5.59 | 5.54 | 5.70 | 5.62 |
| Rwp | 7.38 | 7.29 | 7.25 | 6.61 | 7.33 |
| RB | 4.99 | 4.83 | 5.54 | 5.41 | 4.85 |
| λ2 | 1.35 | 1.41 | 1.44 | 1.28 | 1.31 |
| Lattice parameters (Å) | |||||
| a | 5.4699 | 5.4578 | 5.4485 | 5.4464 | 5.4567 |
| b | 5.4427 | 5.4277 | 5.4199 | 5.4215 | 5.4400 |
| c | 32.9354 | 32.9155 | 32.8742 | 32.8320 | 32.9274 |
| V (Å3) | 982 | 975 | 971 | 969 | 979 |
| Orthorhombic distortion (a/b) | 1.0050 | 1.0063 | 1.0053 | 1.0046 | 1.0031 |
| Parameters | Chromium Content in BTW0.05-yCr (Space Group of B2cb) | ||||
|---|---|---|---|---|---|
| y = 0 | y = 0.1 | y = 0.2 | y = 0.3 | y = 0.4 | |
| Refinement index | |||||
| Rp | 5.68 | 5.59 | 5.54 | 5.70 | 5.62 |
| Rwp | 7.38 | 7.29 | 7.25 | 6.61 | 7.33 |
| RB | 4.99 | 4.83 | 5.54 | 5.41 | 4.85 |
| λ2 | 1.35 | 1.41 | 1.44 | 1.28 | 1.31 |
| Lattice parameters (Å) | |||||
| a | 5.4285 | 5.4341 | 5.4485 | 5.4344 | 5.4334 |
| b | 5.4159 | 5.4155 | 5.4199 | 5.4091 | 5.4132 |
| c | 32.8595 | 32.8796 | 32.8742 | 32.8516 | 32.8551 |
| V (Å3) | 966 | 968 | 971 | 967 | 966 |
| Orthorhombic distortion (a/b) | 1.0023 | 1.0034 | 1.0053 | 1.0047 | 1.0037 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xu, J.; Xie, S.; Tan, Z.; Nie, R.; Guan, Z.; Wang, Q.; Zhu, J. Ion Doping Effects on the Lattice Distortion and Interlayer Mismatch of Aurivillius-Type Bismuth Titanate Compounds. Materials 2018, 11, 821. https://doi.org/10.3390/ma11050821
Chen Y, Xu J, Xie S, Tan Z, Nie R, Guan Z, Wang Q, Zhu J. Ion Doping Effects on the Lattice Distortion and Interlayer Mismatch of Aurivillius-Type Bismuth Titanate Compounds. Materials. 2018; 11(5):821. https://doi.org/10.3390/ma11050821
Chicago/Turabian StyleChen, Yu, Jiageng Xu, Shaoxiong Xie, Zhi Tan, Rui Nie, Zhongwei Guan, Qingyuan Wang, and Jianguo Zhu. 2018. "Ion Doping Effects on the Lattice Distortion and Interlayer Mismatch of Aurivillius-Type Bismuth Titanate Compounds" Materials 11, no. 5: 821. https://doi.org/10.3390/ma11050821





