Controllable Fabrication of Fe3O4/ZnO Core–Shell Nanocomposites and Their Electromagnetic Wave Absorption Performance in the 2–18 GHz Frequency Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fe3O4 Nanoparticles
2.3. Synthesis of Fe3O4/ZnO Nanocomposites
2.4. Characterization
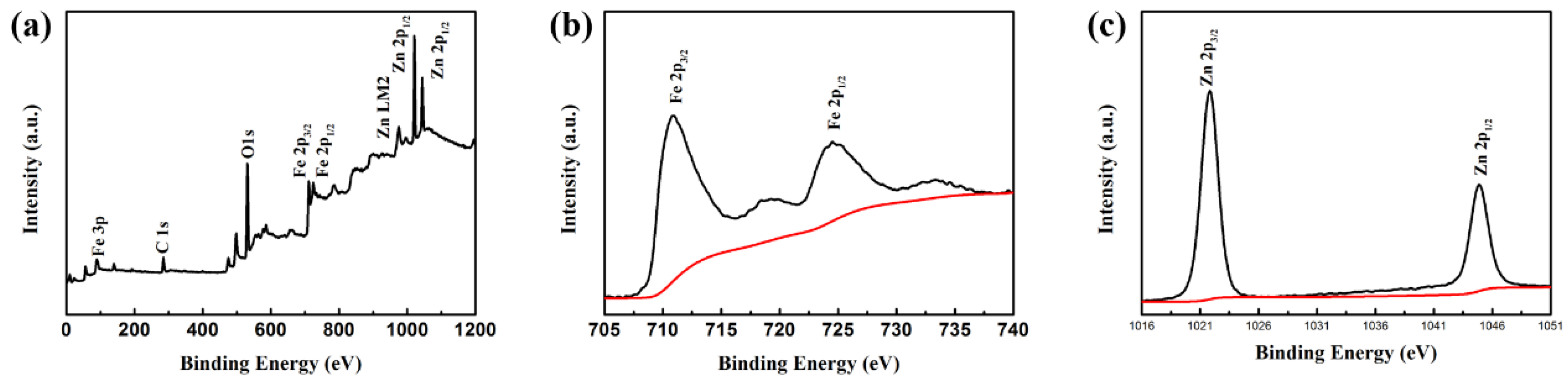
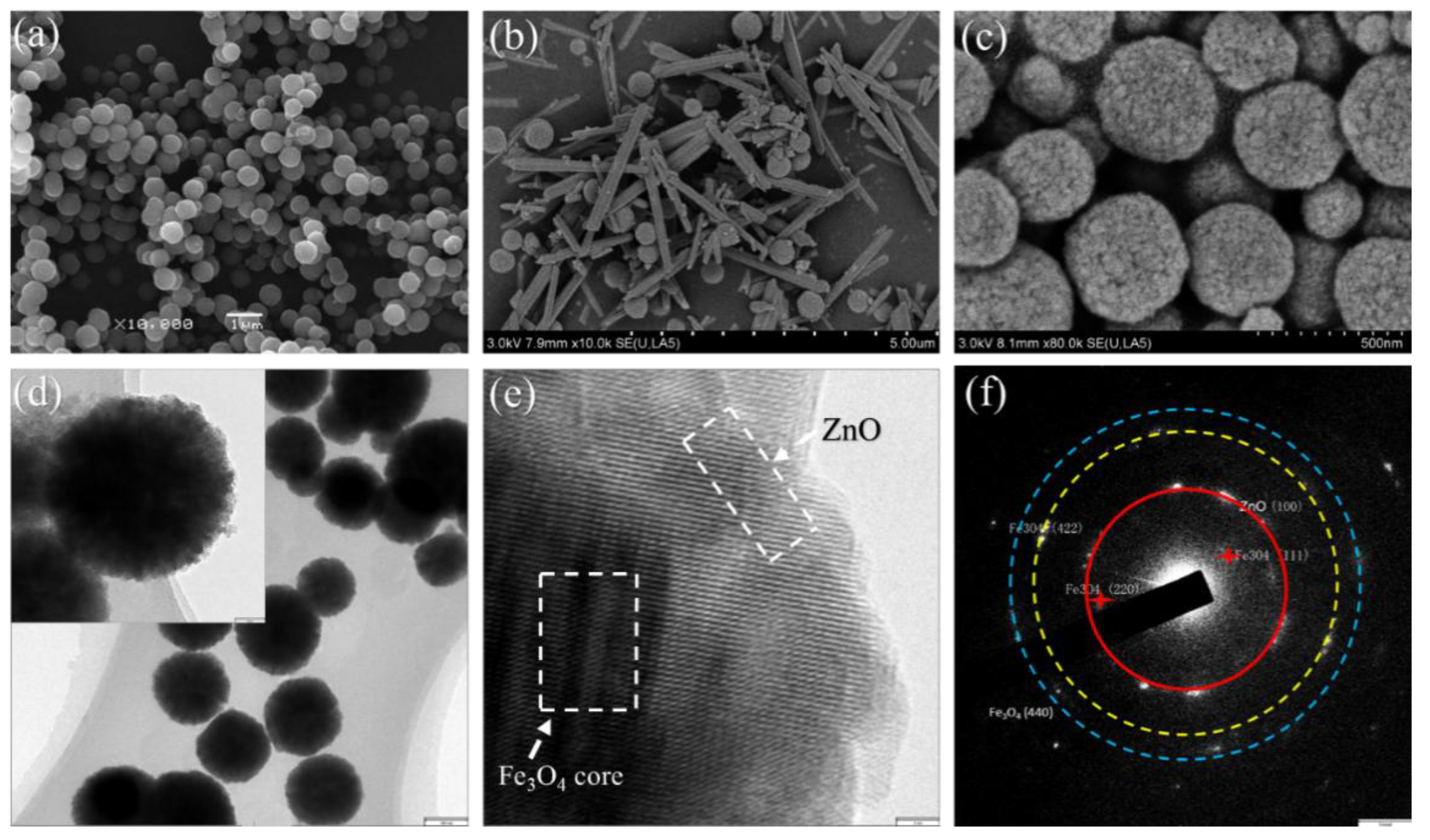
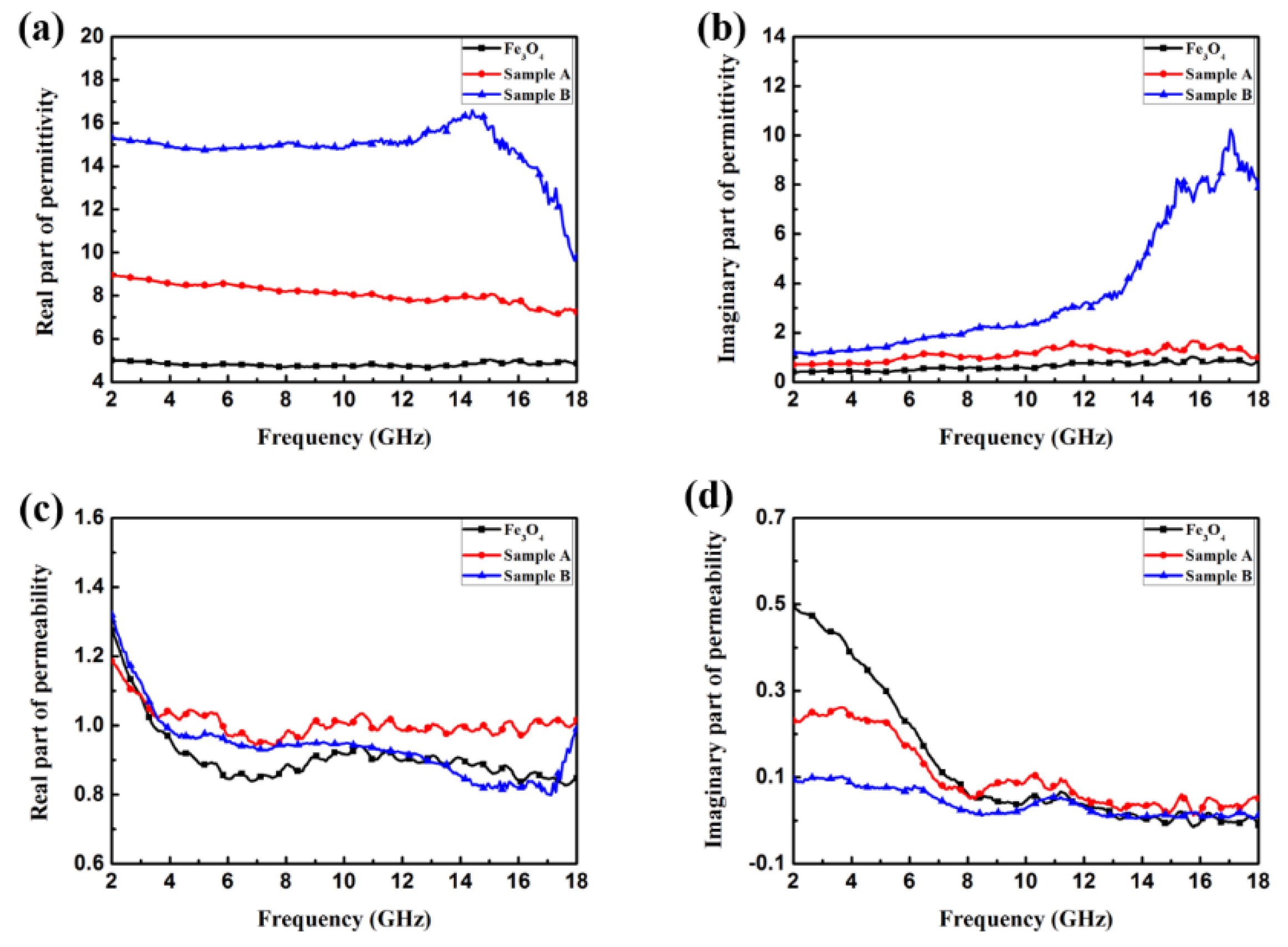
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, J.; Liu, Q.; Li, S.; Lu, Y.; Jin, S.; Li, K.; Chen, H.; Zhang, H. Metal organic framework (MOF)-derived carbonaceous Co3O4/Co microframes anchored on RGO with enhanced electromagnetic wave absorption performances. Synth. Met. 2017, 228, 32–40. [Google Scholar] [CrossRef]
- Hu, Q.; Qi, X.; Cai, H.; Xie, R.; Long, L.; Bai, Z.; Jiang, Y.; Qin, S.; Zhong, W.; Du, Y. Preparation of porous Fe2O3 nanorods-reduced graphene oxide nanohybrids and their excellent microwave absorption properties. Sci. Rep. 2017, 7, 11213. [Google Scholar] [CrossRef] [PubMed]
- Dom, R.; Subasri, R.; Hebalkar, N.Y.; Chary, A.S.; Borse, P.H. Synthesis of a hydrogen producing nanocrystalline ZnFe2O4 visible light photocatalyst using a rapid microwave irradiation method. RSC Adv. 2012, 2, 12782. [Google Scholar] [CrossRef]
- Jian, X.; Chen, X.; Zhou, Z.; Li, G.; Jiang, M.; Xu, X.; Lu, J.; Li, Q.; Wang, Y.; Gou, J.; et al. Remarkable improvement in microwave absorption by cloaking a micro-scaled tetrapod hollow with helical carbon nanofibers. Phys. Chem. Chem. Phys. 2015, 17, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Qiang, R.; Du, Y.; Chen, D.; Ma, W.; Wang, Y.; Xu, P.; Ma, J.; Zhao, H.; Han, X. Electromagnetic functionalized Co/C composites by in situ pyrolysis of metal-organic frameworks (ZIF-67). J. Alloys Compd. 2016, 681, 384–393. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y.; Hou, Y.; Li, L. Tunable design of yolk–shell ZnFe2O4@RGO@TiO2 microspheres for enhanced high-frequency microwave absorption. Inorg. Chem. Front. 2017, 4, 935–945. [Google Scholar] [CrossRef]
- Wan, G.; Wang, G.; Huang, X.; Zhao, H.; Li, X.; Wang, K.; Yu, L.; Peng, X.; Qin, Y. Uniform Fe3O4 coating on flower-like ZnO nanostructures by atomic layer deposition for electromagnetic wave absorption. Dalton Trans. 2015, 44, 18804–18809. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Tong, G.; Wu, W.; Liu, F.; Qian, H.; Hong, D. Selective preparation and enhanced microwave electromagnetic characteristics of polymorphous ZnO architectures made from a facile one-step ethanediamine-assisted hydrothermal approach. CrystEngComm 2013, 15, 1314. [Google Scholar] [CrossRef]
- Sano, E.; Akiba, E. Electromagnetic absorbing materials using nonwoven fabrics coated with multi-walled carbon nanotubes. Carbon 2014, 78, 463–468. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Yan, J.; Yang, Y.; Zhao, Y. Construction of CuS Nanoflakes Vertically Aligned on Magnetically Decorated Graphene and Their Enhanced Microwave Absorption Properties. ACS Appl. Mater. Interfaces 2016, 8, 5536–5546. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, H.; Ji, G.; Xu, Z.J. Interface Strategy to Achieve Tunable High Frequency Attenuation. ACS Appl. Mater. Interfaces 2016, 8, 6529–6538. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Dong, X.L.; Huang, H.; Zhang, X.F.; Zhu, X.G.; Lei, J.P.; Sun, J.P. Microwave absorption properties of the core/shell-type iron and nickel nanoparticles. J. Magn. Magn. Mater. 2008, 320, 1106–1111. [Google Scholar] [CrossRef]
- Qiang, R.; Du, Y.; Zhao, H.; Wang, Y.; Tian, C.; Li, Z.; Han, X.; Xu, P. Metal organic framework-derived Fe/C nanocubes toward efficient microwave absorption. J. Mater. Chem. A 2015, 3, 13426–13434. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Du, Y.; Bai, J.; Fan, H.; Zhang, H.; Peng, Y.; Li, F. One-pot synthesis of CoFe2O4/graphene oxide hybrids and their conversion into FeCo/graphene hybrids for lightweight and highly efficient microwave absorber. J. Mater. Chem. A 2015, 3, 5535–5546. [Google Scholar] [CrossRef]
- Sui, M.; Lü, X.; Xie, A.; Xu, W.; Rong, X.; Wu, G. The synthesis of three-dimensional (3D) polydopamine-functioned carbonyl iron powder@polypyrrole (CIP@PPy) aerogel composites for excellent microwave absorption. Synth. Met. 2015, 210, 156–164. [Google Scholar] [CrossRef]
- Hu, C.; Mou, Z.; Lu, G.; Chen, N.; Dong, Z.; Hu, M.; Qu, L. 3D graphene-Fe3O4 nanocomposites with high-performance microwave absorption. Phys. Chem. Chem. Phys. 2013, 15, 13038–13043. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Weng, X.; Wu, G.; Zhang, Y.; Lv, X.; Gu, G. Synthesis of Fe3O4/polypyrrole/polyaniline nanocomposites by in-situ method and their electromagnetic absorbing properties. J. Saudi Chem. Soc. 2017, 21, 466–472. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.; Xu, G.; Cheng, Y.; Zhang, Y.; Liu, W.; Ji, G.; Du, Y. A permittivity regulating strategy to achieve high-performance electromagnetic wave absorbers with compatibility of impedance matching and energy conservation. New J. Chem. 2017, 41, 1259–1266. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Wang, X.; Wu, Y.; Liu, Y. Electromagnetic and microwave absorption properties of Fe–Sr0.8La0.2Fe11.8Co0.2O19 shell-core composites. J. Magn. Magn. Mater. 2012, 324, 2177–2182. [Google Scholar] [CrossRef]
- Shen, J.; Chen, K.; Li, L.; Wang, W.; Jin, Y. Fabrication and microwave absorbing properties of (Z-type barium ferrite/silica)@polypyrrole composites. J. Alloys Compd. 2014, 615, 488–495. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, C.; Zhang, S.; Li, C.; Chen, Y.; Gao, P.; Yang, P.; Ouyang, Q. Three-dimensional SiO2@Fe3O4 core/shell nanorod array/graphene architecture: Synthesis and electromagnetic absorption properties. Nanoscale 2013, 5, 12296–12303. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, Q.; Zhou, Y.; Zhao, B.; Zhang, R. Facile design of a ZnO nanorod–Ni core–shell composite with dual peaks to tune its microwave absorption properties. RSC Adv. 2017, 7, 9294–9302. [Google Scholar] [CrossRef]
- Najim, M.; Modi, G.; Mishra, Y.K.; Adelung, R.; Singh, D.; Agarwala, V. Ultra-wide bandwidth with enhanced microwave absorption of electroless Ni-P coated tetrapod-shaped ZnO nano- and microstructures. Phys. Chem. Chem. Phys. 2015, 17, 22923–22933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, L.; Hu, Y.; Guo, C.; Zhang, F.; Wen David Lou, X. A magnetically separable photocatalyst based on nest-like gamma-Fe(2)O(3)/ZnO double-shelled hollow structures with enhanced photocatalytic activity. Nanoscale 2012, 4, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-F.; Bi, H.; Wang, C.; Cao, Q.; Jiao, W.; Che, R. Dual-ligand mediated one-pot self-assembly of Cu/ZnO core/shell structures for enhanced microwave absorption. RSC Adv. 2016, 6, 41724–41733. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Y.; Chen, J.; Guo, L.; Ouyang, J.; Jia, D.; Zhou, Y. Microwave absorbing property optimization of starlike ZnO/reduced graphene oxide doped by ZnO nanocrystal composites. Phys. Chem. Chem. Phys. 2017, 19, 14596–14605. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiao, F.; Liu, X.; Feng, C.; Jin, C. Preparation and electromagnetic wave absorption properties of core–shell structured Fe3O4–polyaniline nanoparticles. RSC Adv. 2013, 3, 22554. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, J.; Ding, L.; Zheng, J.; Xu, J.; Xiong, S. Formation of Fe3O4@SiO2@C/Ni hybrids with enhanced catalytic activity and histidine-rich protein separation. Nanoscale 2016, 8, 15978–15988. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liang, C.; Liu, M.; Liu, X.; Yuan, K.; Cao, H.; Che, R. Yolk–shell Fe3O4@ZrO2prepared by a tunable polymer surfactant assisted sol–gel method for high temperature stable microwave absorption. J. Mater. Chem. C 2014, 2, 7275. [Google Scholar] [CrossRef]
- Zhang, Y.; Quan, B.; Liu, W.; Liang, X.; Ji, G.; Du, Y. A facile one-pot strategy for fabrication of carbon-based microwave absorbers: Effects on annealing and paraffin content. Dalton Trans. 2017, 46, 9097–9102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Guo, X.; Zhou, Y.; Su, T.; Ma, C.; Zhang, R. Constructing hierarchical hollow CuS microspheres via a galvanic replacement reaction and their use as wide-band microwave absorbers. CrystEngComm 2017, 19, 2178–2186. [Google Scholar] [CrossRef]
- Xie, A.; Sun, M.; Zhang, K.; Jiang, W.; Wu, F.; He, M. In situ growth of MoS2 nanosheets on reduced graphene oxide (RGO) surfaces: Interfacial enhancement of absorbing performance against electromagnetic pollution. Phys. Chem. Chem. Phys. 2016, 18, 24931–24936. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Sun, X.; Lou, H.; Li, X.; Lv, X.; Li, L.; Gu, G. Synthesis of hollow Fe3O4 particles via one-step solvothermal approach for microwave absorption materials: Effect of reactant concentration, reaction temperature and reaction time. J. Mater. Sci. Mater. Electron. 2018, 29, 7539–7550. [Google Scholar] [CrossRef]
- Liu, T.; Xie, X.; Pang, Y.; Kobayashi, S. Co/C nanoparticles with low graphitization degree: A high performance microwave-absorbing material. J. Mater. Chem. C 2016, 4, 1727–1735. [Google Scholar] [CrossRef]
- Liu, X.; Nie, X.; Yu, R.; Feng, H. Design of dual-frequency electromagnetic wave absorption by interface modulation strategy. Chem. Eng. J. 2018, 334, 153–161. [Google Scholar] [CrossRef]
- Wang, L.; Xing, H.; Liu, Z.; Shen, Z.; Sun, X.; Xu, G. Facile synthesis of net-like Fe3O4/MWCNTs decorated by SnO2nanoparticles as a highly efficient microwave absorber. RSC Adv. 2016, 6, 97142–97151. [Google Scholar] [CrossRef]
- Pan, Y.-F.; Wang, G.-S.; Yue, Y.-H. Fabrication of Fe3O4@SiO2@RGO nanocomposites and their excellent absorption properties with low filler content. RSC Adv. 2015, 5, 71718–71723. [Google Scholar] [CrossRef]
- Liu, X.; Wu, N.; Cui, C.; Bi, N.; Sun, Y. One pot synthesis of Fe3O4/MnO2 core–shell structured nanocomposites and their application as microwave absorbers. RSC Adv. 2015, 5, 24016–24022. [Google Scholar] [CrossRef]
- Li, W.; Lv, B.; Wang, L.; Li, G.; Xu, Y. Fabrication of Fe3O4@C core–shell nanotubes and their application as a lightweight microwave absorbent. RSC Adv. 2014, 4, 55738–55744. [Google Scholar] [CrossRef]
- Guo, H.; Zhan, Y.; Chen, Z.; Meng, F.; Wei, J.; Liu, X. Decoration of basalt fibers with hybrid Fe3O4 microspheres and their microwave absorption application in bisphthalonitrile composites. J. Mater. Chem. A 2013, 1, 2286–2296. [Google Scholar] [CrossRef]


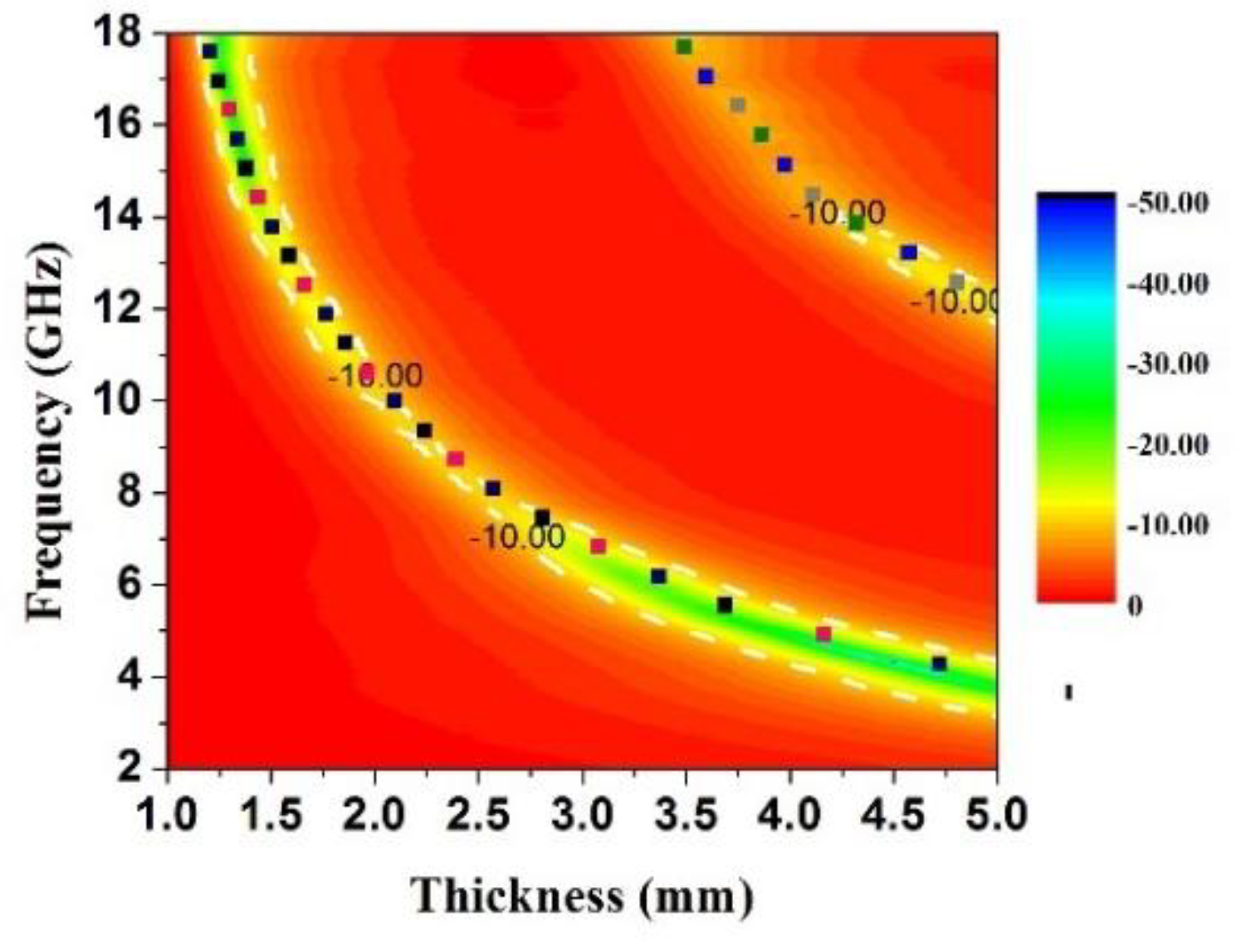
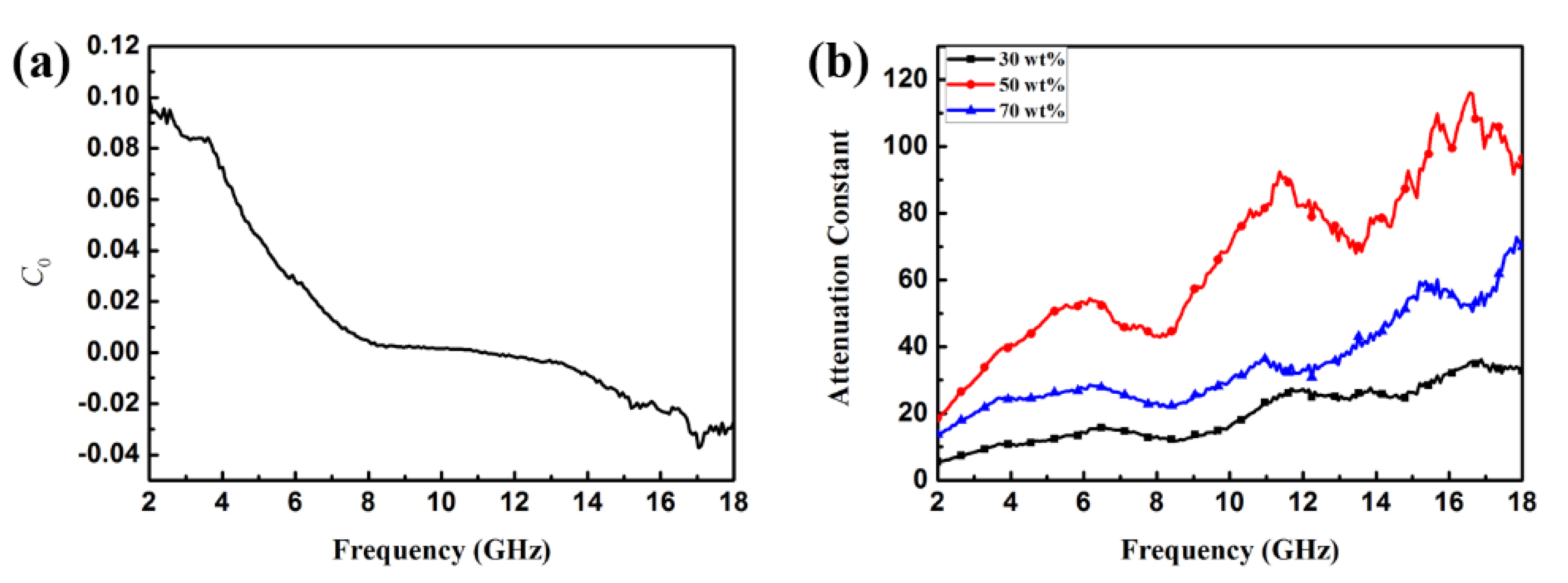
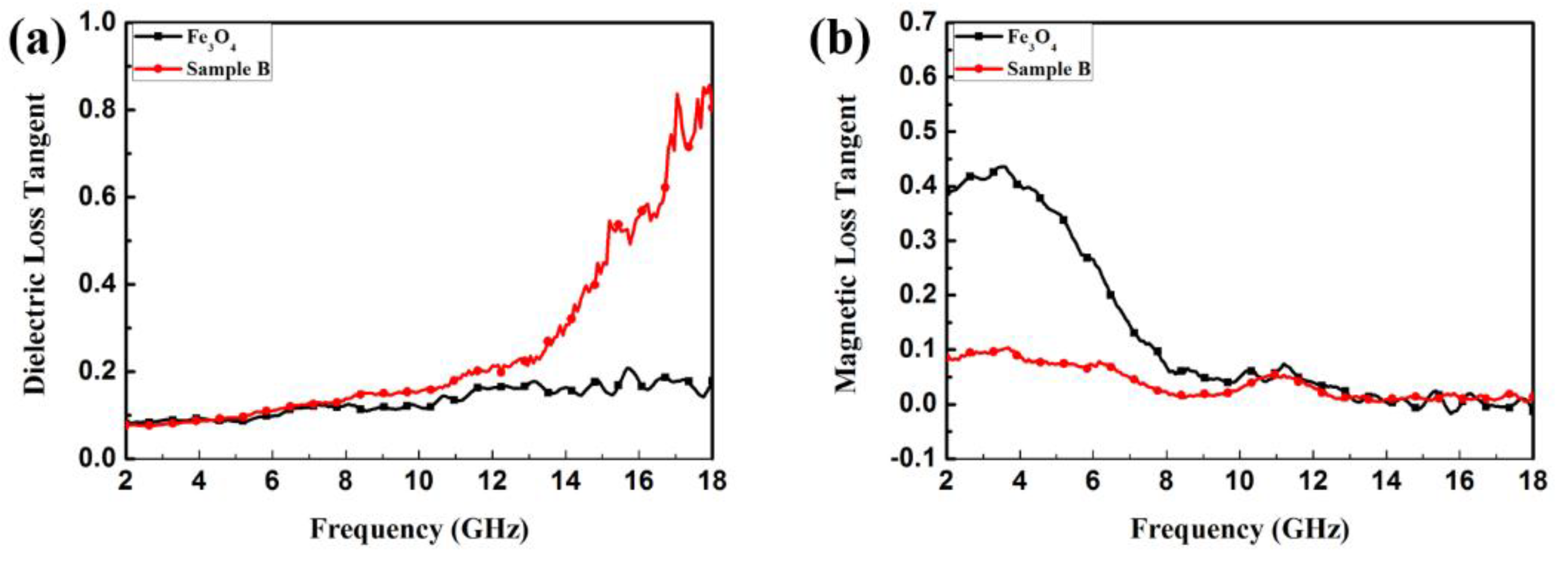
| Sample | wt (%) | Optimum Frequency (GHz) | Minimum RL Value (dB) | Ref. |
|---|---|---|---|---|
| SnO2/Fe3O4/MWCNTs | 70 | 10.90 | −42.00 | [36] |
| Fe3O4/SiO2/rGO | 20 | 9.70 | −26.60 | [37] |
| Fe2O4/MnO2 | 40 | 16.80 | −41.50 | [38] |
| Fe3O4@C | 66.7 | 16.20 | −22.60 | [39] |
| FePc-Fe3O4-BF | 75 | 5.90 | −31.10 | [40] |
| Fe3O4/ZnO | 50 | 4.38 | −50.79 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Ma, G.; Lv, X.; Sui, M.; Li, H.; Wu, F.; Wang, J. Controllable Fabrication of Fe3O4/ZnO Core–Shell Nanocomposites and Their Electromagnetic Wave Absorption Performance in the 2–18 GHz Frequency Range. Materials 2018, 11, 780. https://doi.org/10.3390/ma11050780
Sun X, Ma G, Lv X, Sui M, Li H, Wu F, Wang J. Controllable Fabrication of Fe3O4/ZnO Core–Shell Nanocomposites and Their Electromagnetic Wave Absorption Performance in the 2–18 GHz Frequency Range. Materials. 2018; 11(5):780. https://doi.org/10.3390/ma11050780
Chicago/Turabian StyleSun, Xiaodong, Guangyan Ma, Xuliang Lv, Mingxu Sui, Huabing Li, Fan Wu, and Jijun Wang. 2018. "Controllable Fabrication of Fe3O4/ZnO Core–Shell Nanocomposites and Their Electromagnetic Wave Absorption Performance in the 2–18 GHz Frequency Range" Materials 11, no. 5: 780. https://doi.org/10.3390/ma11050780




