Influence of AlN(0001) Surface Reconstructions on the Wettability of an Al/AlN System: A First-Principle Study
Abstract
:1. Introduction
- (1)
- The AlN ceramics are soaked in an Al melt during a hot dip coating experiment, which means that they are in an Al-rich and N-poor environment. This differs from the contact angle measurement, where the AlN ceramics are typically maintained in a high vacuum environment.
- (2)
- There are dozens of parts per million (ppm) of oxygen in the atmosphere of the dip coting equipment that were used in experiment, which is much higher than the residual oxygen that is found in the contact angle measurement’s high vacuum environment.
2. Method
2.1. Calculation Method
2.2. Work of Adhesion and Contact Angle
3. Results and Discussion
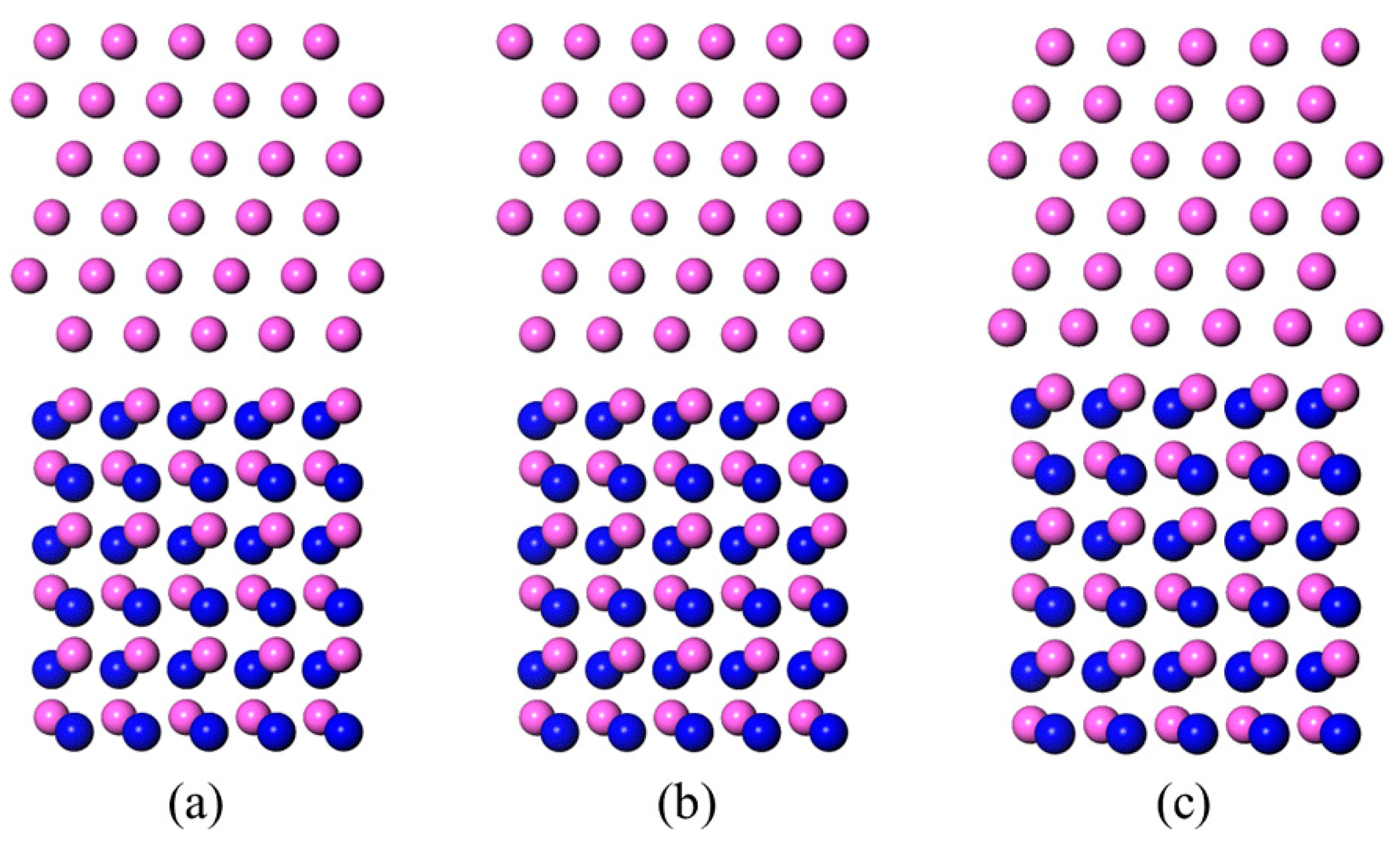
3.1. Al-Terminated AlN(0001)/Al(111) Interface Models
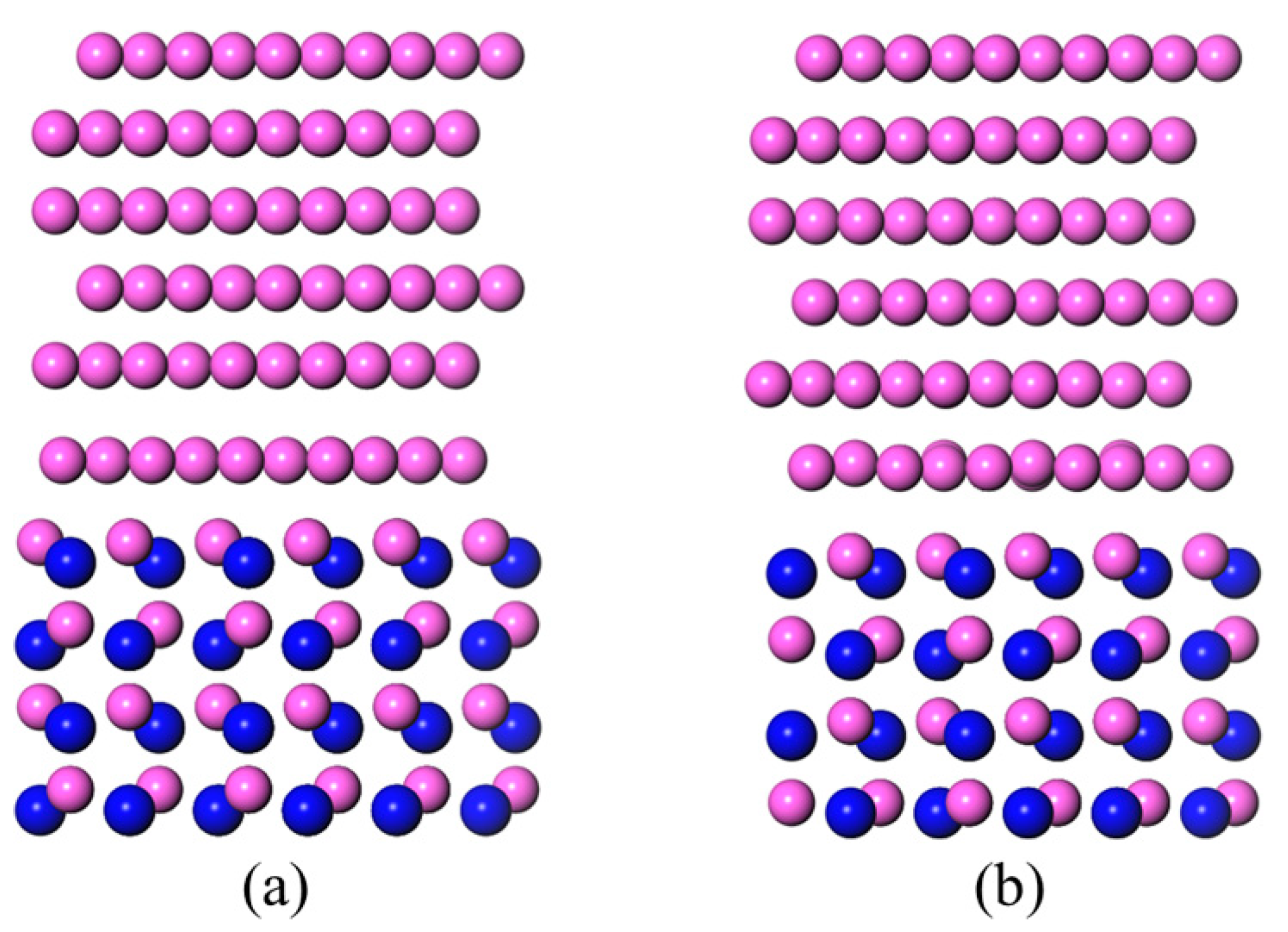
3.2. N-Terminated AlN(0001)/Al(111) Interface Models
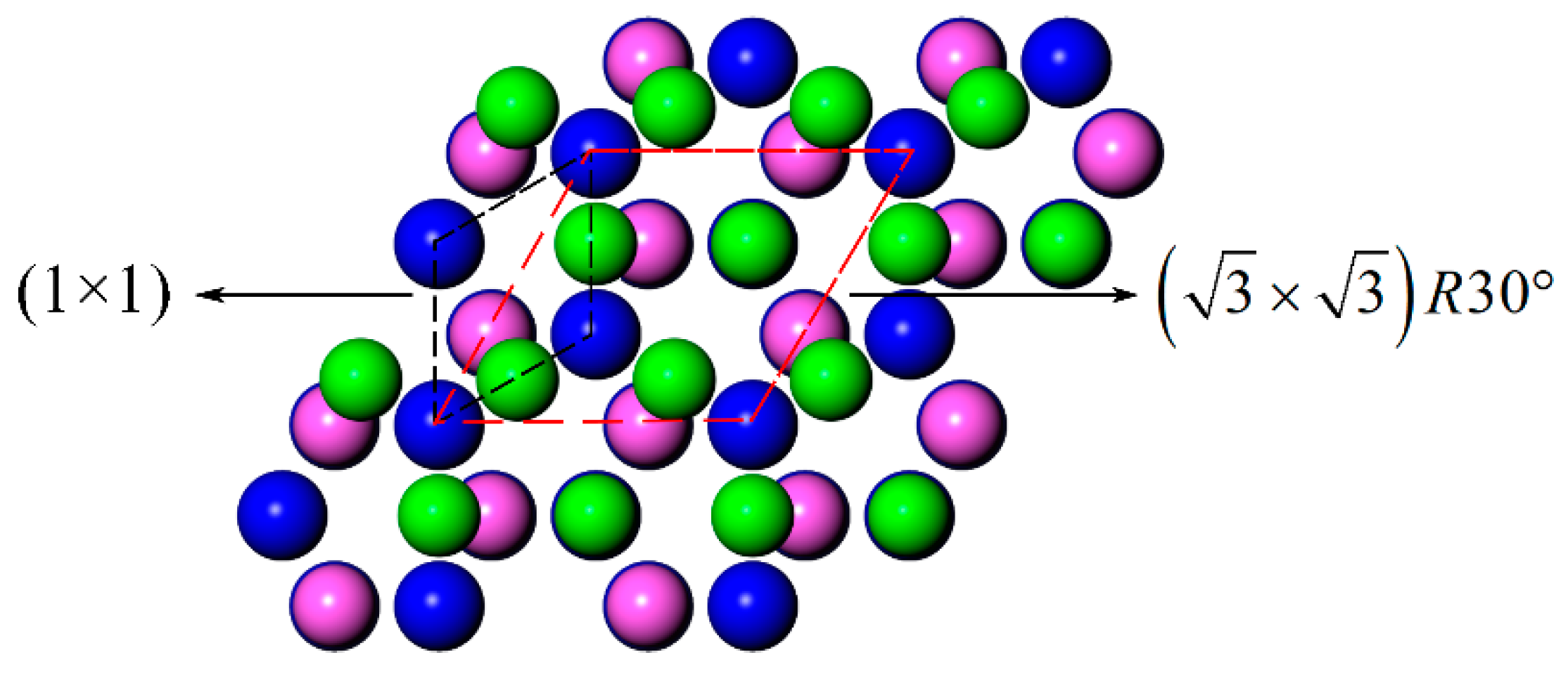
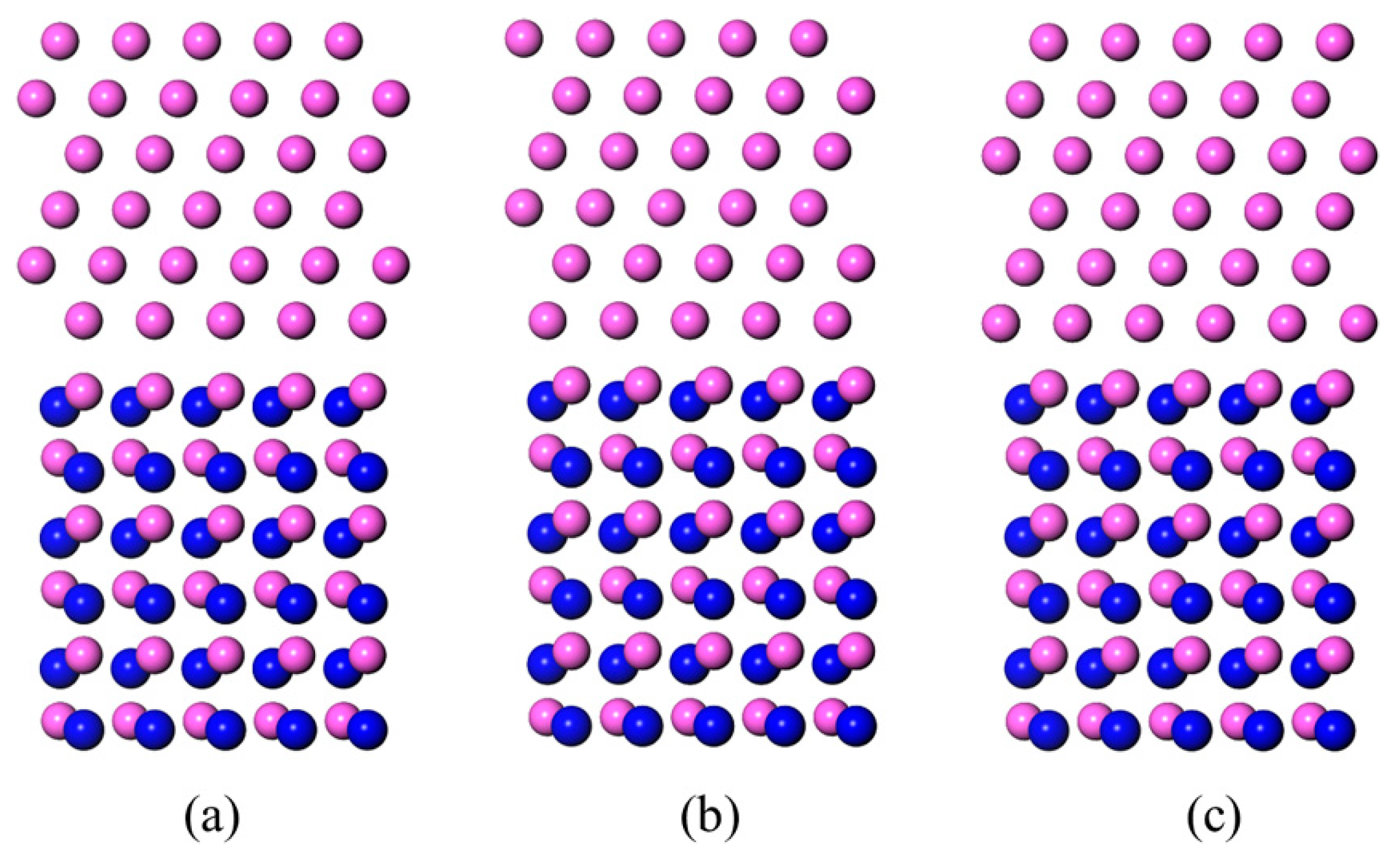
3.3. LCM Reconstructed AlN(0001)/Al(111)
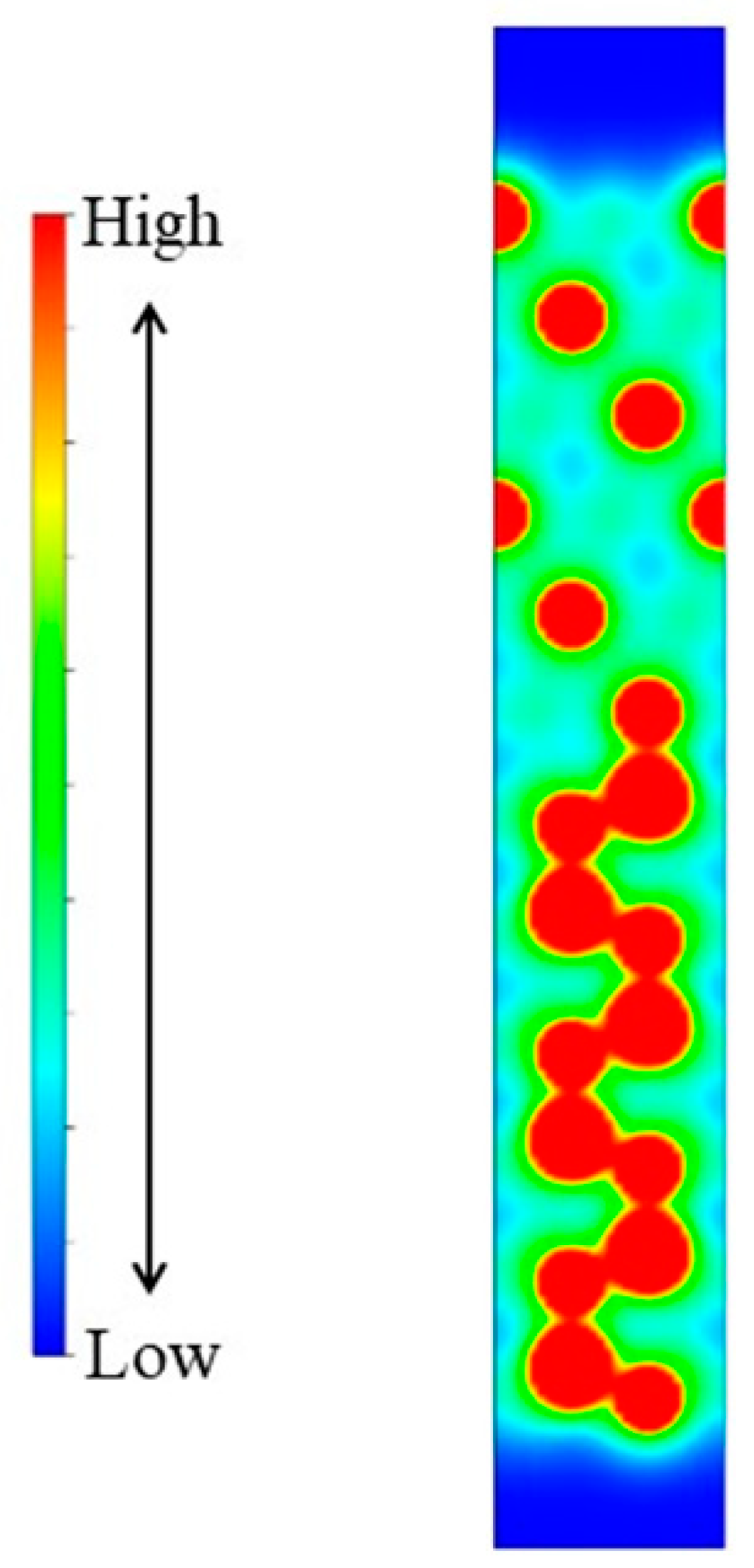
3.4. Influence of Oxygen Adsorption on Free Surface of Al Melt
4. Conclusions
- (1)
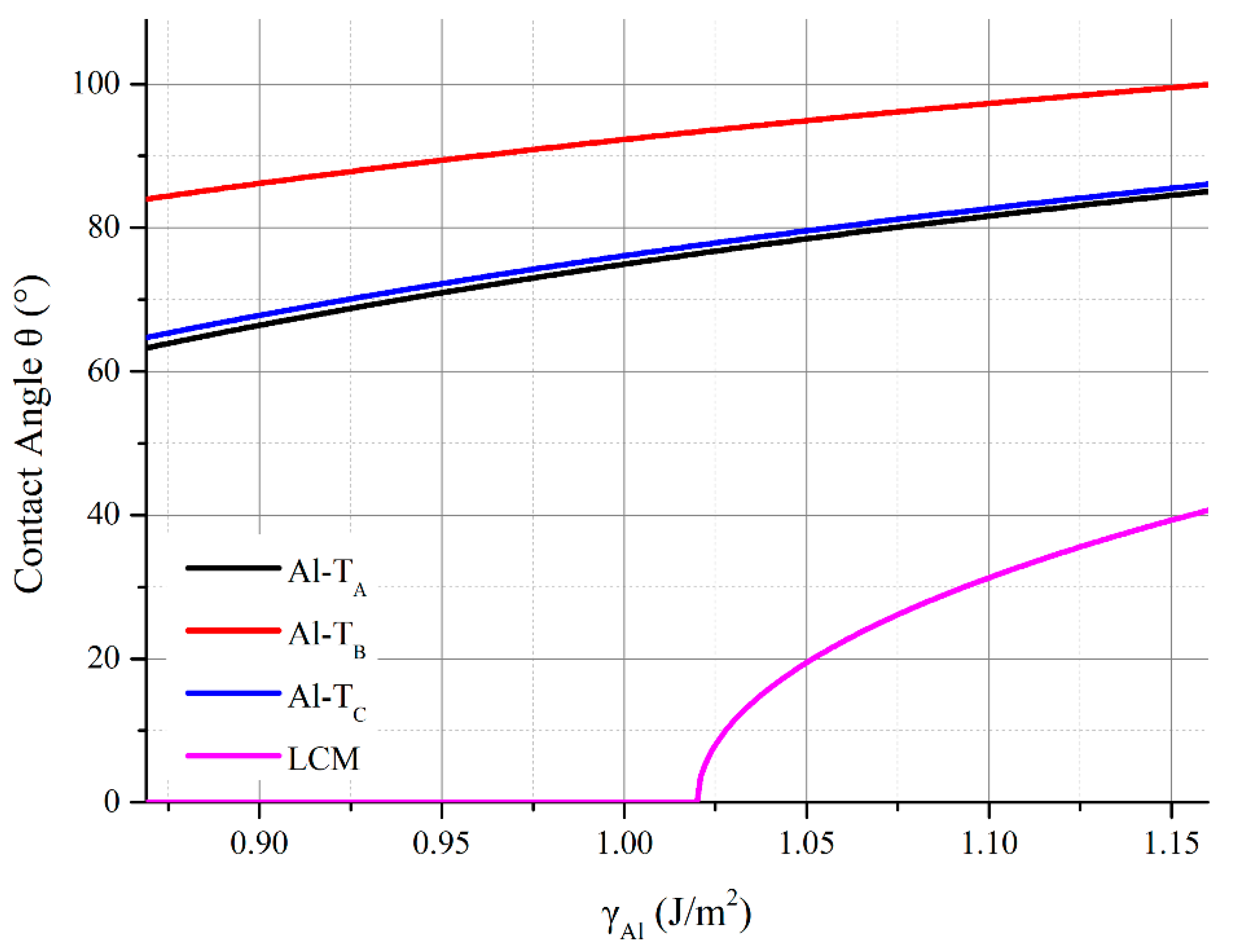
- The Wad of the Al-terminated AlN(0001)/Al(111) was within the limits of 0.96 J/m2~1.26 J/m2, where the calculated contact angle of the Al melt on the Al-terminated AlN(0001) surface was beyond 83°, which agreed well with the sessile drop experiments.
- (2)
- The Wad of the N-TB and N-TC types N-terminated AlN(0001)/Al(111) interface was 0.82 J/m2, which resulted in a 106°~107° calculated contact angle of the Al melt on the N-TB and N-TC surfaces, at 1073 K. The Wad of the N-TA type of the AlN(0001)/Al(111) interface was 2.40 J/m2, which resulted in a 0° calculated contact angle of the Al melt on the N-TA surface at 1073 K. This was caused by the chemical bonds that formed between the terminated N atoms on the AlN(0001) surface and the Al atoms of the Al melt. The results demonstrated that, in an Al-rich environment, the N-terminated AlN surface tended to bond the Al atoms and reconstructed, which resulted in an Al-terminated surface structure.
- (3)
- The LCM reconstruction improved the Al and AlN bonding in the Al-terminated AlN(0001) surface. The Wad of the LCM AlN(0001)/Al(111) interface was 2.04 J/m2, which was higher than the 0.96 J/m2~1.26 J/m2 of the dis-reconstructed interface. The wettability of the Al on the AlN was improved and the contact angle of the Al on the AlN(0001) surface decreased from 83°~100° to 35°~41°.
- (4)
- The adsorption of the oxygen atoms on the free surface of the Al melt further improved the wettability of the Al. The contact angle between the Al melt and the non-reconstructed Al-terminated AlN(0001) surface decreased from 83°~100° to 63°~84°. When one monolayer of oxygen atoms was adsorbed on the surface of the Al melt, the surface tension decreased from 1.122~1.160 J/m2 to 0.869 J/m2. The partial adsorption of the oxygen atoms decreased the contact angle to 0° when the surface tension decreased to 1.02 J/m2 within the LCM reconstruction.
- (5)
- The supernormal wetting phenomenon in the dip coating of the Al on AlN ceramics originated from the surface reconstruction of the AlN ceramics under an Al-excess condition when they were immersed in molten Al. A quick adsorption occurred for the oxygen atoms on the free surface of liquid Al, which were attached on the AlN ceramics, as they withdrew from the Al melt. A thorough immersion and a proper amount of oxygen in the atmosphere were key for the hot dip coating.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werdecker, W.; Aldinger, F. Aluminum nitride—An alternative ceramic substrate for high power applications in microcircuits. IEEE Trans. Compon. Hybrids Manuf. Technol. 1984, 7, 399–404. [Google Scholar] [CrossRef]
- Saiz, E.; Tomsia, A.P.; Suganuma, K. Wetting and strength issues at Al/α–alumina interfaces. J. Eur. Ceram. Soc. 2003, 23, 2787–2796. [Google Scholar] [CrossRef]
- Pan, W.; Okamoto, T.; Ning, X. Joining of Al-plasma-sprayed Si3N4 ceramics. J. Mater. Sci. 1994, 29, 1436–1440. [Google Scholar] [CrossRef]
- Vermeersch, M.; Malengreau, F.; Sporken, R.; Caudano, R. The aluminium/sapphire interface formation at high temperature: An AES and LEED study. Surf. Sci. 1995, 323, 175–187. [Google Scholar] [CrossRef]
- Medlin, D.L.; McCarty, K.F.; Hwang, R.Q.; Guthrie, S.E.; Baskes, M.I. Orientation relationships in heteroepitaxial aluminum films on sapphire. Thin Solid Films 1997, 299, 110–114. [Google Scholar] [CrossRef]
- Dehm, G.; Inkson, B.J.; Wagner, T. Growth and microstructural stability of epitaxial al films on (0001) α-Al2O3 substrates. Acta Mater. 2002, 50, 5021–5032. [Google Scholar] [CrossRef]
- Ning, X.S.; Li, S.; Wang, B.; Li, G.; Bi, N.; Liu, Y. A novel dip coating method for reaction bonding of aluminum on alumina. In Processing and Properties of Advanced Ceramics and Composites VI: Ceramic Transactions; Wiley: Hoboken, NJ, USA, 2013; Volume 249, pp. 93–103. [Google Scholar]
- Li, S. Process Study of Hot-Dipping Aluminum Coating on Ceramics. Master’s Thesis, Tsinghua University, Beijing, China, 2011. [Google Scholar]
- Wang, B. Research on Dip-Coating-Brazing between Ceramics and Aluminum. Master’s Thesis, Tsinghua University, Beijing, China, 2011. [Google Scholar]
- Liu, Y. Experimental and First-Principle Research on Processing and Mechanism of Dip-Coating Aluminum on Al2O3 and AlN. Master’s Thesis, Tsinghua University, Beijing, China, 2015. [Google Scholar]
- Howe, J.M. Interfaces in Materials; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Nicholas, M.G.; Mortimer, D.A.; Jones, L.M.; Crispin, R.M. Some observations on the wetting and bonding of nitride ceramics. J. Mater. Sci. 1990, 25, 2679–2689. [Google Scholar] [CrossRef]
- Fujii, H.; Nakae, H.; Okada, K. 4 wetting phases in ALN AL and ALN composites al systems, models of nonreactive, reactive, and composite systems. Metall. Trans. A 1993, 24, 1391–1397. [Google Scholar] [CrossRef]
- Ho, H.N.; Wu, S.T. The wettability of molten aluminum on sintered aluminum nitride substrate. Mater. Sci Eng. A Struct. 1998, 248, 120–124. [Google Scholar] [CrossRef]
- Nautiyal, P.; Gupta, A.; Seal, S.; Boesl, B.; Agarwal, A. Reactive wetting and filling of boron nitride nanotubes by molten aluminum during equilibrium solidification. Acta Mater. 2017, 126, 124–131. [Google Scholar] [CrossRef]
- Lee, C.D.; Dong, Y.; Feenstra, R.M.; Northrup, J.E.; Neugebauer, J. Reconstructions of the AlN(0001) surface. Phys. Rev. B 2003, 68, 205317. [Google Scholar] [CrossRef]
- Fritsch, J.; Sankey, O.F.; Schmidt, K.E.; Page, J.B. Ab initio calculation of the stoichiometry and structure of the (0001) surfaces of GaN and AlN. Phys. Rev. B 1998, 57, 15360–15371. [Google Scholar] [CrossRef]
- Northrup, J.E.; Di Felice, R.; Neugebauer, J. Atomic structure and stability of AlN (0001) and (000) surfaces. Phys. Rev. B 1997, 55, 13878. [Google Scholar] [CrossRef]
- Miao, M.S.; Janotti, A.; Van de Walle, C.G. Reconstructions and origin of surface states on AlN polar and nonpolar surfaces. Phys. Rev. B 2009, 80, 155319. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, X.-S. Influence of α-Al2O3 (0001) surface reconstruction on wettability of Al/Al2O3 interface: A first-principle study. Comput. Mater. Sci. 2014, 85, 193–199. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for AB initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Touloukian, Y.; Kirby, R.; Taylor, R.; Desai, P. Thermophysical Properties of Matter-the TPRC Data Series. In Thermal Expansion Metallic Elements and Alloys; DTIC Document; IFI/Plenum: New York, NY, USA, 1975; Volume 12. [Google Scholar]
- Siegel, D.J.; Hector Jr, L.G.; Adams, J.B. Adhesion, atomic structure, and bonding at the Al (111)/α-Al2O3 (0001) interface: A first principles study. Phys. Rev. B 2002, 65, 085415. [Google Scholar] [CrossRef]
- Schulz, H.; Thiemann, K. Crystal structure refinement of AlN and GaN. Solid State Commun. 1977, 23, 815–819. [Google Scholar] [CrossRef]
- Montesa, C.M.; Shibata, N.; Tohei, T.; Ikuhara, Y. Tem observation of liquid-phase bonded aluminum–silicon/aluminum nitride hetero interface. J. Mater. Sci. 2011, 46, 4392–4396. [Google Scholar] [CrossRef]
- Lipkin, D.M.; Israelachvili, J.N.; Clarke, D.R. Estimating the metal-ceramic van der Waals adhesion energy. Philos. Mag. A 1997, 76, 715–728. [Google Scholar] [CrossRef]
- Tyson, W.; Miller, W. Surface free energies of solid metals: Estimation from liquid surface tension measurements. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- Niessen, A.D.; De Boer, F.; Boom, R.D.; De Chatel, P.; Mattens, W.; Miedema, A. Model predictions for the enthalpy of formation of transition metal alloys II. Calphad 1983, 7, 51–70. [Google Scholar] [CrossRef]
- Garcia-Cordovilla, C.; Louis, E.; Pamies, A. The surface tension of liquid pure aluminium and aluminium-magnesium alloy. J. Mater. Sci. 1986, 21, 2787–2792. [Google Scholar] [CrossRef]
- Kumamoto, A.; Shibata, N.; Nayuki, K.; Tohei, T.; Terasaki, N.; Nagatomo, Y.; Nagase, T.; Akiyama, K.; Kuromitsu, Y.; Ikuhara, Y. Atomic structures of a liquid-phase bonded metal/nitride heterointerface. Sci. Rep.-UK 2016, 6, 22936. [Google Scholar] [CrossRef] [PubMed]

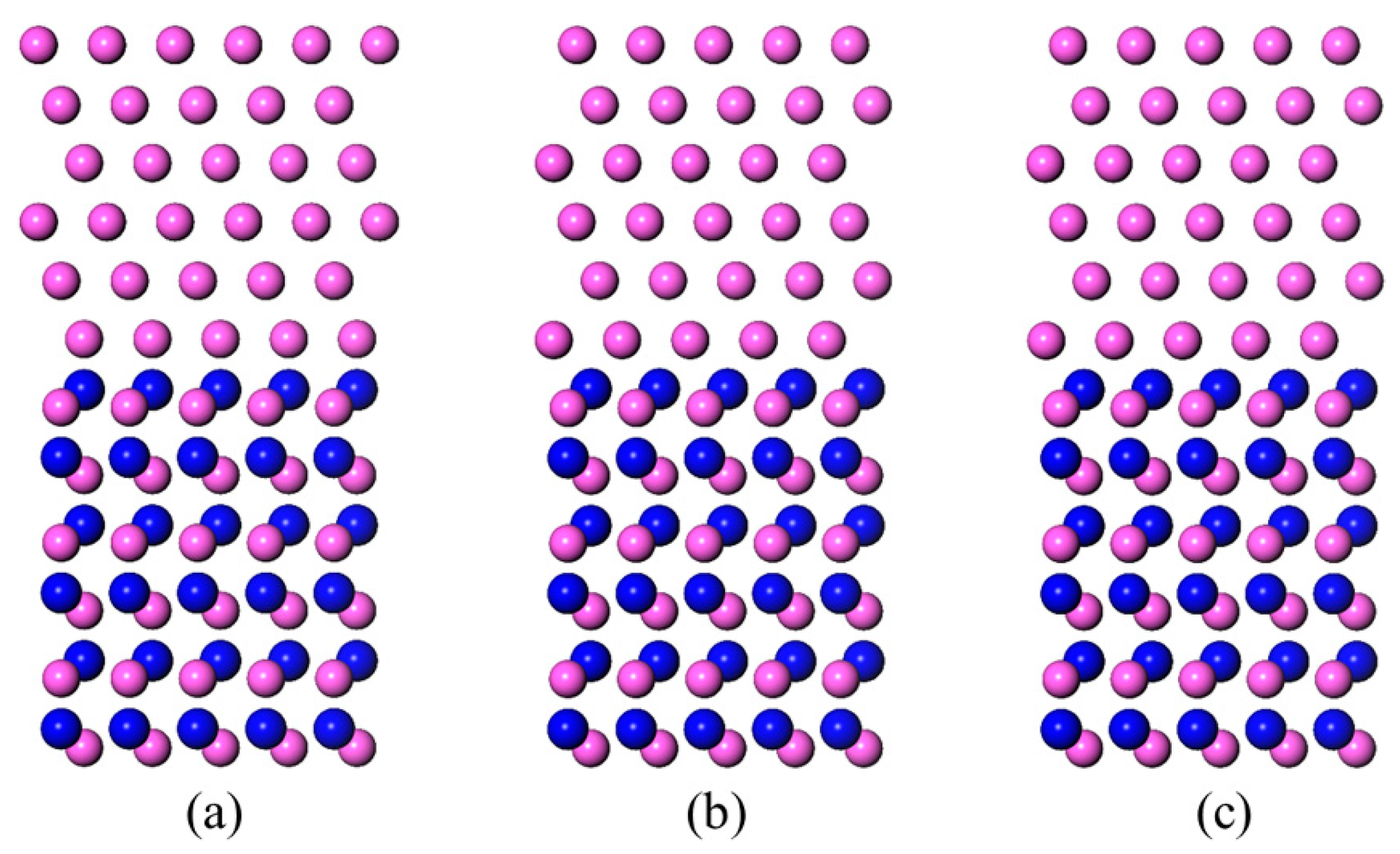
| Interface Model | Work of Adhesion Wad (J/m2) | Contact Angle θ (°) | ||
|---|---|---|---|---|
| γAl = 1.143 J/m2 [29] | γAl = 1.160 J/m2 [30] | γAl = 1.122 J/m2 [31] | ||
| Al-TA | 1.26 | 84 | 85 | 83 |
| Al-TB | 0.96 | 99 | 100 | 98 |
| Al-TC | 1.24 | 85 | 86 | 84 |
| Interface Model | Work of Adhesion Wad (J/m2) | Contact Angle θ (°) | ||
|---|---|---|---|---|
| γAl = 1.143 J/m2 [29] | γAl = 1.160 J/m2 [30] | γAl = 1.122 J/m2 [31] | ||
| N-TA | 2.40 | 0 | 0 | 0 |
| N-TB | 0.82 | 107 | 107 | 106 |
| N-TC | 0.82 | 107 | 107 | 106 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Liu, Y.; Ning, X.-S. Influence of AlN(0001) Surface Reconstructions on the Wettability of an Al/AlN System: A First-Principle Study. Materials 2018, 11, 775. https://doi.org/10.3390/ma11050775
Cao J, Liu Y, Ning X-S. Influence of AlN(0001) Surface Reconstructions on the Wettability of an Al/AlN System: A First-Principle Study. Materials. 2018; 11(5):775. https://doi.org/10.3390/ma11050775
Chicago/Turabian StyleCao, Junhua, Yang Liu, and Xiao-Shan Ning. 2018. "Influence of AlN(0001) Surface Reconstructions on the Wettability of an Al/AlN System: A First-Principle Study" Materials 11, no. 5: 775. https://doi.org/10.3390/ma11050775




