Influence of Manufacturing Parameters on Microstructure and Hydrogen Sorption Behavior of Electron Beam Melted Titanium Ti-6Al-4V Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Experimental Procedure
3. Results
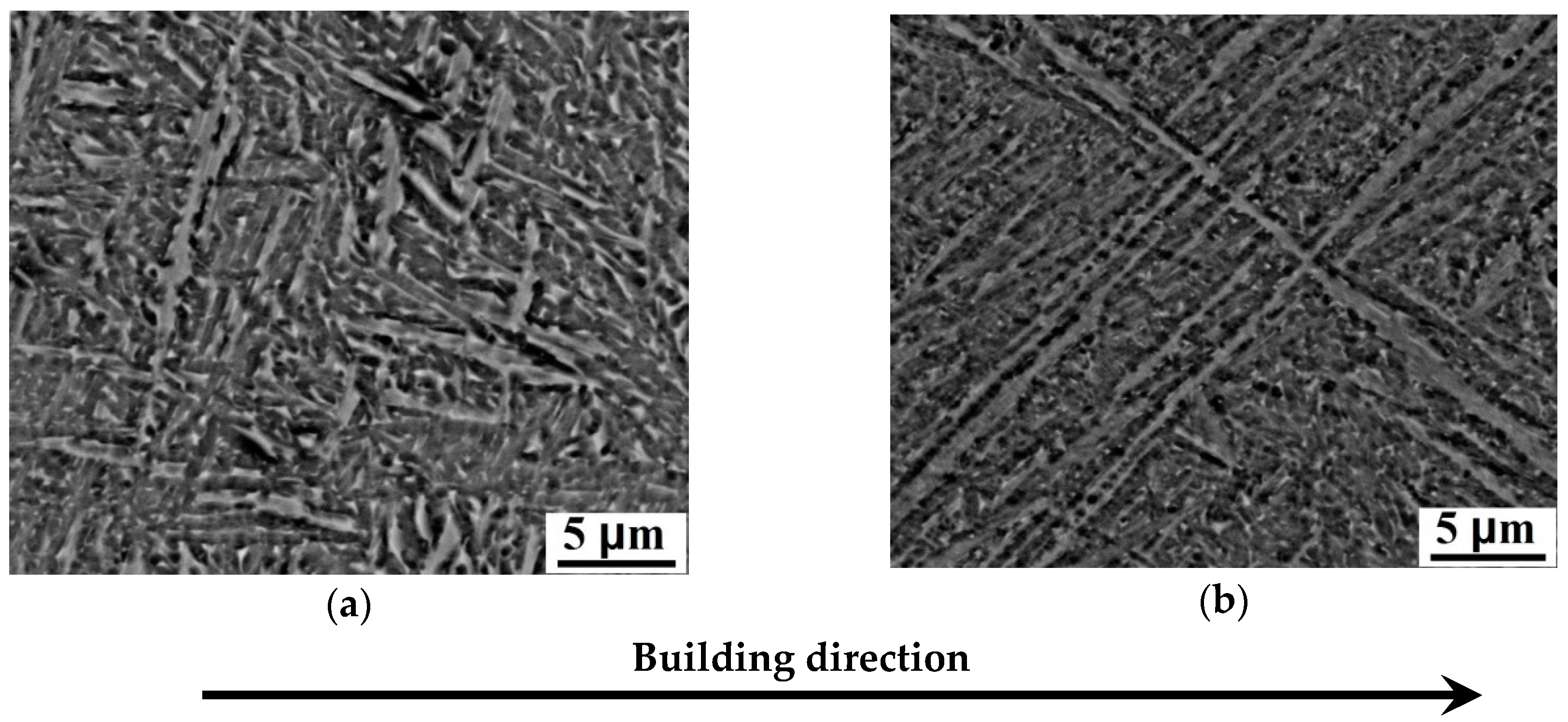
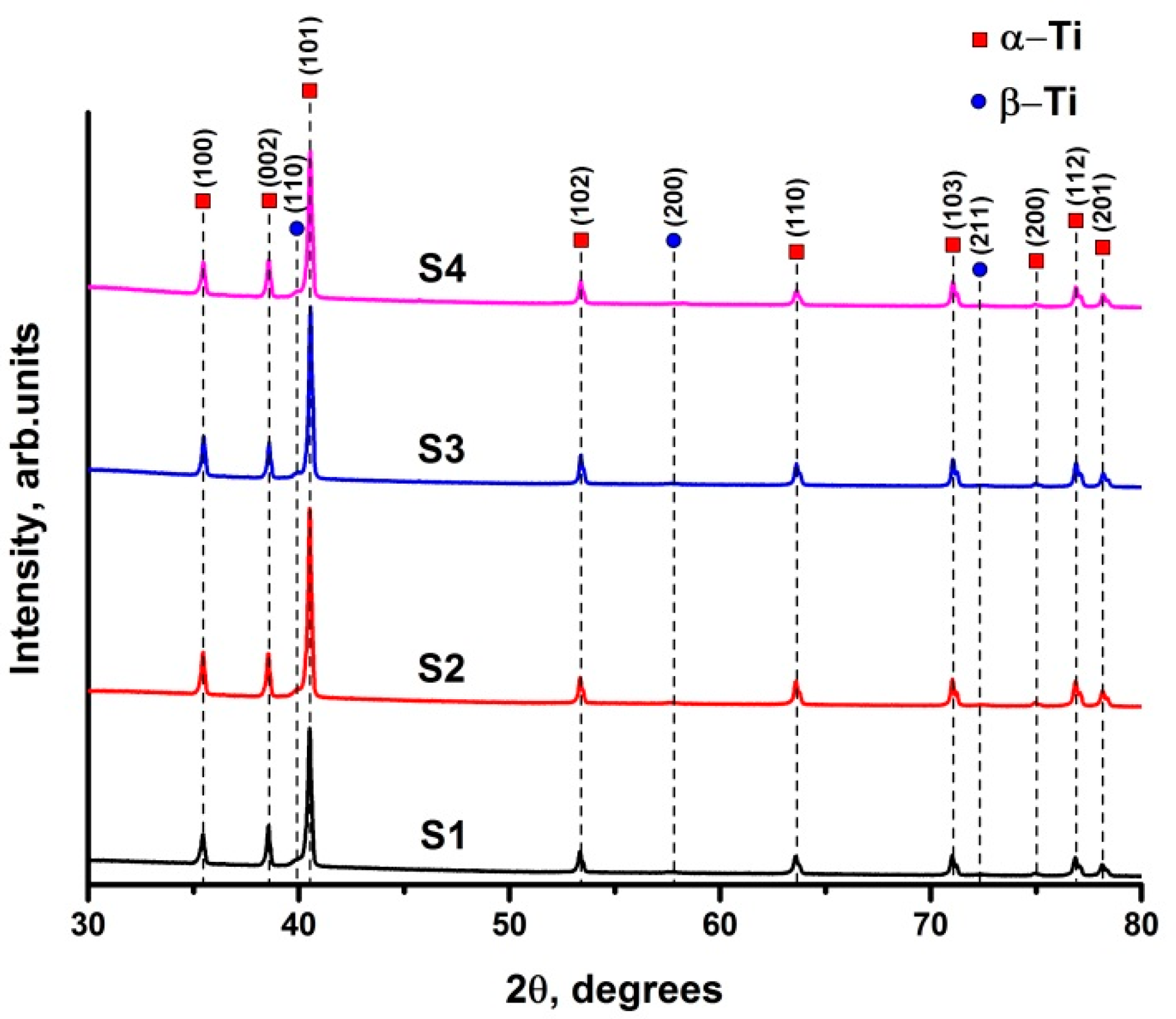
3.1. Microstructure of EBM Ti-6Al-4V Samples
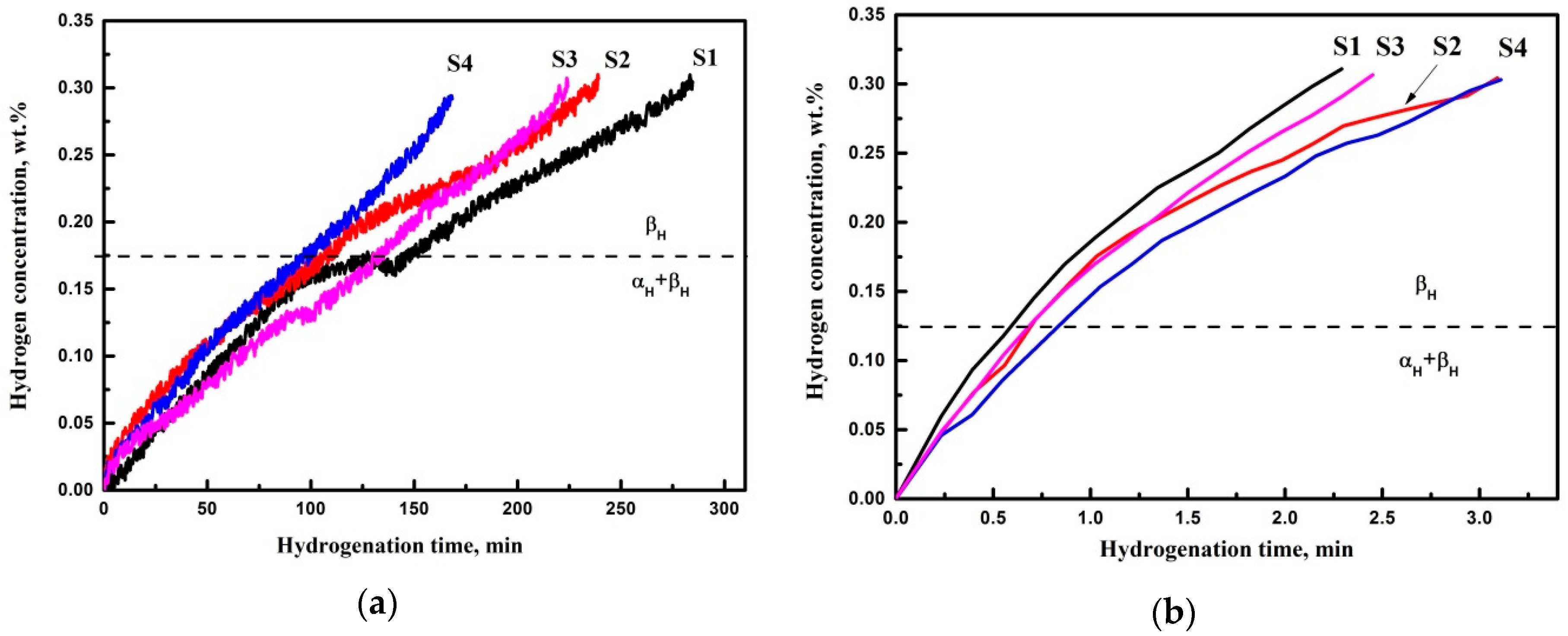
3.2. Hydrogen Sorption
4. Discussion
5. Conclusions
- The average α lath width decreases with the increase of the speed function at the fixed beam current (17 mA). At the fixed speed function, the decrease of the α lath width also occurs when changing the beam current from 17 to 13 mA. Finer microstructure has been formed at the beam current (BC) 17 mA and speed function 98, while coarser microstructure has been formed at 17 mA and 85, respectively. The bcc β phase in EBM Ti-6Al-4V has been formed as discrete flat rods embedded in the continuous hcp α phase. The phase composition of the samples changes insignificantly at the varied parameters. The content of β phase varies from 2.4 to 3.1 vol %.
- Microstructure has significantly affected hydrogen sorption kinetics of EBM Ti-6Al-4V parts during the gas-phase hydrogenation at 500 °C. The average hydrogen sorption rate was higher in the sample manufactured at BC 17 mA and SF 98 due to finer microstructure (finer α lath) and distribution of β phase. Lower hydrogen sorption was demonstrated in the sample with BC 17 mA and SF 85. The shape of the kinetics curves indicates the phase transition αH + βH→βH, which depends on the dimensions of α plates and β phase content and distribution.
- Hydrogen sorption kinetics at 650 °C has not significantly changed at the indicated manufacturing parameters due to the increase of hydrogen diffusion in α phase. Thus, the transition αH + βH→βH proceeds rapidly as compared to hydrogenation at 500 °C. The hydrogenation time to 0.3 wt % is about 2–3 min.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brewer, W.D.; Bird, R.K.; Wallace, T.A. Titanium alloys and processing for high speed aircraft. Mater. Sci. Eng. A 1998, 243, 299–304. [Google Scholar] [CrossRef]
- Immarigeon, J.-P.; Holt, R.T.; Koul, A.K.; Zhao, L.; Wallace, W.; Beddoes, J.C. Lightweight materials for aircraft applications. Mater. Charact. 1995, 35, 41–67. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Liu, Y.; Cao, G.; Zhang, L.C. Phase transition, microstructural evolution and mechanical properties of Ti-Nb-Fe alloys induced by Fe addition. Mater. Des. 2016, 97, 279–286. [Google Scholar] [CrossRef]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Zeng, W.D.; Yu, H.Q. An investigation of a new near-beta forging process for titanium alloys and its application in aviation components. Mater. Sci. Eng. A 2005, 393, 204–212. [Google Scholar] [CrossRef]
- Eliezer, D.; Böllinghaus, T.H. Hydrogen effects in titanium alloys. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Gangloff, R.P., Somerday, B.P., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2012; pp. 668–706. [Google Scholar]
- Tal-Gutelmacher, E.; Eliezer, D. Hydrogen cracking in titanium-based alloys. J. Alloy. Compd. 2005, 404–406, 621–625. [Google Scholar] [CrossRef]
- Yuan, B.G.; Yu, H.P.; Li, C.F.; Sun, D.L. Effect of hydrogen on fracture behavior of Ti-6Al-4V alloy by in-situ tensile test. Int. J. Hydrog. Energy 2010, 35, 1829–1838. [Google Scholar] [CrossRef]
- Madina, V.; Azkarate, I. Compatibility of materials with hydrogen. Particular case: Hydrogen embrittlement of titanium alloys. Int. J. Hydrog. Energy 2009, 34, 5976–5980. [Google Scholar] [CrossRef]
- Tao, J.; Hu, S.; Ji, L. Effect of trace solute hydrogen on the fatigue life of electron beam welded Ti-6Al-4V alloy joints. Mater. Sci. Eng. A 2017, 684, 542–551. [Google Scholar] [CrossRef]
- Bilgin, G.M.; Esen, Z.; Akın, Ş.K.; Dericioglu, A.F. Optimization of the mechanical properties of Ti-6Al-4V alloy fabricated by selective laser melting using thermohydrogen processes. Mater. Sci. Eng. A 2017, 700, 574–582. [Google Scholar] [CrossRef]
- Zong, Y.; Wu, K. Thermo hydrogen treatment for microstructure refinement and mechanical properties improvement of Ti-6Al-4V alloy. Mater. Sci. Eng. A 2017, 703, 430–437. [Google Scholar] [CrossRef]
- Shen, C.-C.; Perng, T.-P. Pressure–composition isotherms and reversible hydrogen-induced phase transformations in Ti-6Al-4V. Acta Mater. 2007, 55, 1053–1058. [Google Scholar] [CrossRef]
- Shen, C.-C.; Wang, C.-M. Effects of hydrogen loading and type of titanium hydride on grain refinement and mechanical properties of Ti-6Al-4V. J. Alloy. Compd. 2014, 601, 274–279. [Google Scholar] [CrossRef]
- Sun, P.; Fang, Z.Z.; Koopman, M.; Paramore, J.; Chandran, K.S.R.; Ren, Y.; Lu, J. An experimental study of the (Ti-6Al-4V)-xH phase diagram using in situ synchrotron XRD and TGA/DSC techniques. Acta Mater. 2015, 84, 29–41. [Google Scholar] [CrossRef]
- Qazi, J.I.; Rahim, J.; Fores, F.H.; Senkov, O.N.; Genc, A. Phase transformations in Ti-6Al-4V-xH alloys. Metall. Mater. Trans. A 2001, 32, 2453–2463. [Google Scholar] [CrossRef]
- Rännar, L.E.; Koptyug, A.; Olsén, J.; Saeidi, K.; Shen, Z. Hierarchical structures of stainless steel 316L manufactured by Electron Beam Melting. Addit. Manuf. 2017, 17, 106–112. [Google Scholar] [CrossRef]
- Rao, H.; Giet, S.; Yang, K.; Wu, X.; Davies, C.H. The influence of processing parameters on aluminium alloy A357 manufactured by selective laser melting. Mater. Des. 2016, 109, 334–346. [Google Scholar] [CrossRef]
- Deng, D.; Moverare, J.; Peng, R.L.; Söderberg, H. Microstructure and anisotropic mechanical properties of EBM manufactured Inconel 718 and effects of post heat treatments. Mater. Sci. Eng. A 2017, 693, 151–163. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Wu, X.; Dargusch, M.S. Comparative study of commercially pure titanium produced by laser engineered net shaping, selective laser melting and casting processes. Mater. Sci. Eng. A 2017, 705, 385–393. [Google Scholar] [CrossRef]
- Murr, L.E. Metallurgy of additive manufacturing: Examples from electron beam melting. Addit. Manuf. 2015, 5, 40–53. [Google Scholar] [CrossRef]
- Koptioug, A.; Rännar, L.E.; Bäckström, M.; Shen, Z.J. New metallurgy of additive manufacturing in metal: experiences from the material and process development with electron beam melting technology (EBM). Mater. Sci. Forum 2016, 879, 996–1001. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Okulov, I.V.; Wendrock, H.; Bӧnisch, M.; Volegov, A.S.; Calin, M.; Eckert, J.; Dargusch, M.S. Nanoindentation and wear properties of Ti and Ti-TiB composite materials produced by selective laser melting. Mater. Sci. Eng. A 2017, 688, 20–26. [Google Scholar] [CrossRef]
- Antonysamy, A.A.; Meyer, J.; Prangnell, P.B. Effect of build geometry on the β-grain structure and texture in additive manufacture of Ti6Al4V by selective electron beam melting. Mater. Charact. 2013, 84, 153–168. [Google Scholar] [CrossRef]
- Safdar, A.; Wei, L.-Y.; Snis, A.; Lai, Z. Evaluation of microstructural development in electron beam melted Ti-6Al-4V. Mater. Charact. 2012, 65, 8–15. [Google Scholar] [CrossRef]
- Puebla, K.; Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Effect of Melt Scan Rate on Microstructure and Macrostructure for Electron Beam Melting of Ti-6Al-4V. Mater. Sci. Appl. 2012, 3, 259–264. [Google Scholar] [CrossRef]
- Al-Bermani, S.S.; Blackmore, M.L.; Zhang, W.; Todd, I. The origin of microstructural diversity, texture, and mechanical properties in electron beam melted Ti-6Al-4V. Metall. Mater. Trans. A 2010, 41, 3422–3434. [Google Scholar] [CrossRef]
- Gil Mur, F.X.; Rodríguez, D.; Planell, J.A. Influence of tempering temperature and time on the α′-Ti-6Al-4V martensite. J. Alloy. Compd. 1996, 234, 287–289. [Google Scholar] [CrossRef]
- Juechter, V.; Scharowsky, T.; Singer, R.F.; Körner, C. Processing window and evaporation phenomena for Ti-6Al-4V produced by selective electron beam melting. Acta Mater. 2014, 76, 252–258. [Google Scholar] [CrossRef]
- Hrabe, N.; Quinn, T. Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti-6Al-4V) fabricated using electron beam melting (EBM), part 1: Distance from build plate and part size. Mater. Sci. Eng. A 2013, 573, 264–270. [Google Scholar] [CrossRef]
- Guo, C.; Ge, W.; Lin, F. Effects of scanning parameters on material deposition during Electron Beam Selective Melting of Ti-6Al-4V powder. J. Mater. Process. Technol. 2015, 217, 148–157. [Google Scholar] [CrossRef]
- Tammas-Williams, S.; Zhao, H.; Léonard, F.; Derguti, F.; Todd, I.; Prangnell, P.B. XCT analysis of the influence of melt strategies on defect population in Ti-6Al-4V components manufactured by Selective Electron Beam Melting. Mater. Charact. 2015, 102, 47–61. [Google Scholar] [CrossRef]
- Wang, X.; Chou, K. EBSD study of beam speed effects on Ti-6Al-4V alloy by powder bed electron beam additive manufacturing. J. Alloy. Compd. 2018, 748, 236–244. [Google Scholar] [CrossRef]
- American Society for Testing Materials. Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium ELI (Extra Low Interstitial) with Powder Bed Fusion; ASTM F2924–14; American Society for Testing Materials: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Kudiiarov, V.N.; Syrtanov, M.S.; Bordulev, Y.S.; Babikhina, M.N.; Lider, A.M.; Gubin, V.E.; Murashkina, T.L. The hydrogen sorption and desorption behavior in spherical powder of pure titanium used for additive manufacturing. Int. J. Hydrog. Energy 2017, 42, 15283–15289. [Google Scholar] [CrossRef]
- Bai, Y.; Gai, X.; Li, S.; Zhang, L.-C.; Liu, Y.; Hao, Y.; Zhang, X.; Yang, R.; Gao, Y. Improved corrosion behaviour of electron beam melted Ti-6Al–4V alloy in phosphate buffered saline. Corros. Sci. 2017, 123, 289–296. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Zhang, M.; Liu, Y.; Sercombe, T.B.; Wang, S.; Hao, Y.; Yang, R.; Murr, L.E. Comparison of the microstructures and mechanical properties of Ti-6Al-4V fabricated by selective laser melting and electron beam melting. Mater. Des. 2016, 95, 21–31. [Google Scholar] [CrossRef]
- Chen, S.Y.; Kuo, C.N.; Su, Y.L.; Huang, J.C.; Wu, Y.C.; Lin, Y.H.; Chung, Y.C.; Ng, C.H. Microstructure and fracture properties of open-cell porous Ti-6Al-4V with high porosity fabricated by electron beam melting. Mater. Charact. 2018, 138, 255–262. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Phase transformations during cooling in α + β titanium alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- Cheng, B.; Price, S.; Gong, X.; Lydon, J.; Cooper, K.; Chou, K. Speed Function Effects in Electron Beam Additive Manufacturing. In Advanced Manufacturing; ASME: New York, NY, USA, 2014; Volume 2A, p. V02AT02A003. [Google Scholar]
- Jamshidinia, M.; Kong, F.; Kovacevic, R. Numerical modeling of heat distribution in the electron beam melting of Ti-6Al-4V. J. Manuf. Sci. Eng. 2013, 135, 061010. [Google Scholar] [CrossRef]
- Stepanova, E.N.; Kudiiarov, V.N.; Sypchenko, V.S.; Lider, A.M.; Liu, G. Research of hydrogenation and dehydrogenation effect on the structural and phase state of the titanium alloy. Key Eng. Mater. 2016, 683, 187–192. [Google Scholar] [CrossRef]
- Kolachev, B.A.; Mamonova, F.S.; Lyasotskaya, V.S. Characteristics of the structure and properties of quenched titanium alloys. Met. Sci. Heat Treat. 1975, 17, 695–697. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D. Hydrogen-assisted degradation of titanium based alloys. Mater. Trans. 2004, 45, 1594–1600. [Google Scholar] [CrossRef]
- Abdul-Hamid, O.S.; Latanision, R.M. Diffusion of Hydrogen in Titanium. In Hydrogen Effects in Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 205–214. [Google Scholar]
- Gaddam, R.; Akerfeldt, P.; Pederson, R.; Antti, M.-L. Influence of hydrogen environment on the mechanical properties of cast and electron beam melted Ti-6Al-4 V. In 12th World Conference on Titanium; Zhou, L., Chang, H., Lu, Y., Eds.; Science Press: Beijing, China, 2012. [Google Scholar]
- Papazoglou, T.P.; Hepworth, M.T. The diffusion of hydrogen in titanium. Trans. Metall. Soc. AIME 1968, 242, 682. [Google Scholar]
- Wasilewski, R.J.; Kehl, G.L. Diffusion of hydrogen in titanium. Metallurgia 1954, 50, 225–230. [Google Scholar]
- Hirohata, Y.; Aihara, Y.; Hino, T.; Miki, N.; Nakagawa, S. Evaluation of hydrogen sorption and desorption for Ti-6A1-4V alloy as a vacuum vessel material. In Fusion Technology 1996; Elsevier: New York, NY, USA, 1997; pp. 363–366. [Google Scholar]



| Sample | Beam Current (BC), mA | Speed Function (SF) | Beam Speed, mm/s |
|---|---|---|---|
| S1 | 17 | 85 | 3227.7 |
| S2 | 15 | 85 | 2797.6 |
| S3 | 13 | 85 | 2797.6 |
| S4 | 17 | 98 | 3218.8 |
| Sample | Phase | Phase Content, vol % | Lattice Parameters, Å | c/a |
|---|---|---|---|---|
| S1 | Ti_hexagonal | 96.9 | A = 2.9253 c = 4.6709 | 1.597 |
| Ti_cubic | 3.1 | A = 3.1954 | - | |
| S2 | Ti_hexagonal | 97.1 | A = 2.9248 c = 4.6717 | 1.597 |
| Ti_cubic | 2.9 | A = 3.1905 | - | |
| S3 | Ti_hexagonal | 97.6 | a = 2.9253 c = 4.6720 | 1.597 |
| Ti_cubic | 2.4 | a = 3.1926 | - | |
| S4 | Ti_hexagonal | 97.1 | a = 2.9245 c = 4.6710 | 1.597 |
| Ti_cubic | 2.9 | a = 3.1911 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushilina, N.; Syrtanov, M.; Kashkarov, E.; Murashkina, T.; Kudiiarov, V.; Laptev, R.; Lider, A.; Koptyug, A. Influence of Manufacturing Parameters on Microstructure and Hydrogen Sorption Behavior of Electron Beam Melted Titanium Ti-6Al-4V Alloy. Materials 2018, 11, 763. https://doi.org/10.3390/ma11050763
Pushilina N, Syrtanov M, Kashkarov E, Murashkina T, Kudiiarov V, Laptev R, Lider A, Koptyug A. Influence of Manufacturing Parameters on Microstructure and Hydrogen Sorption Behavior of Electron Beam Melted Titanium Ti-6Al-4V Alloy. Materials. 2018; 11(5):763. https://doi.org/10.3390/ma11050763
Chicago/Turabian StylePushilina, Natalia, Maxim Syrtanov, Egor Kashkarov, Tatyana Murashkina, Viktor Kudiiarov, Roman Laptev, Andrey Lider, and Andrey Koptyug. 2018. "Influence of Manufacturing Parameters on Microstructure and Hydrogen Sorption Behavior of Electron Beam Melted Titanium Ti-6Al-4V Alloy" Materials 11, no. 5: 763. https://doi.org/10.3390/ma11050763






