Improved Optical and Electrochromic Properties of NiOx Films by Low-Temperature Spin-Coating Method Based on NiOx Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
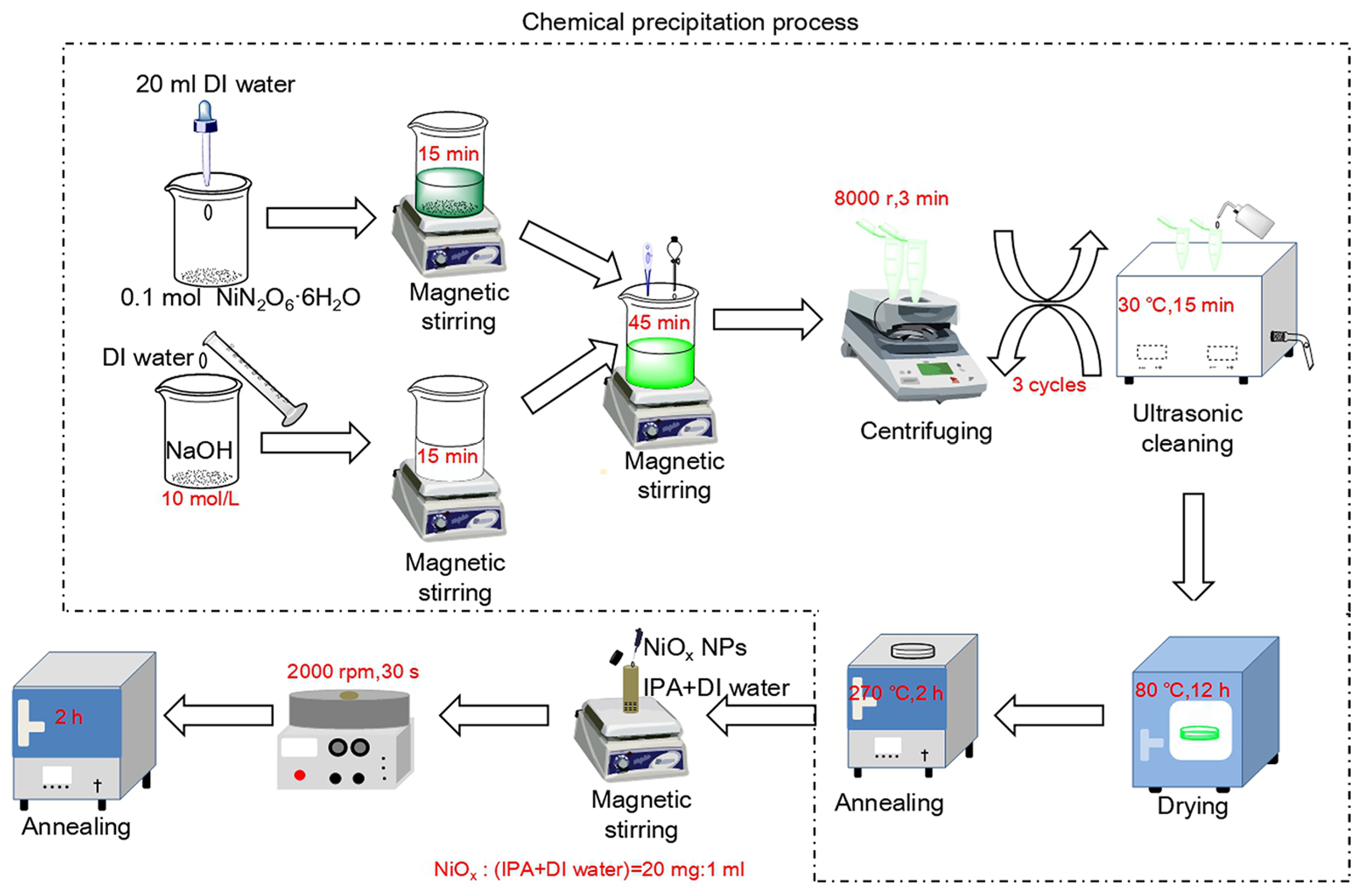
2.1. Fabrication of Samples
2.2. Characterization
3. Results
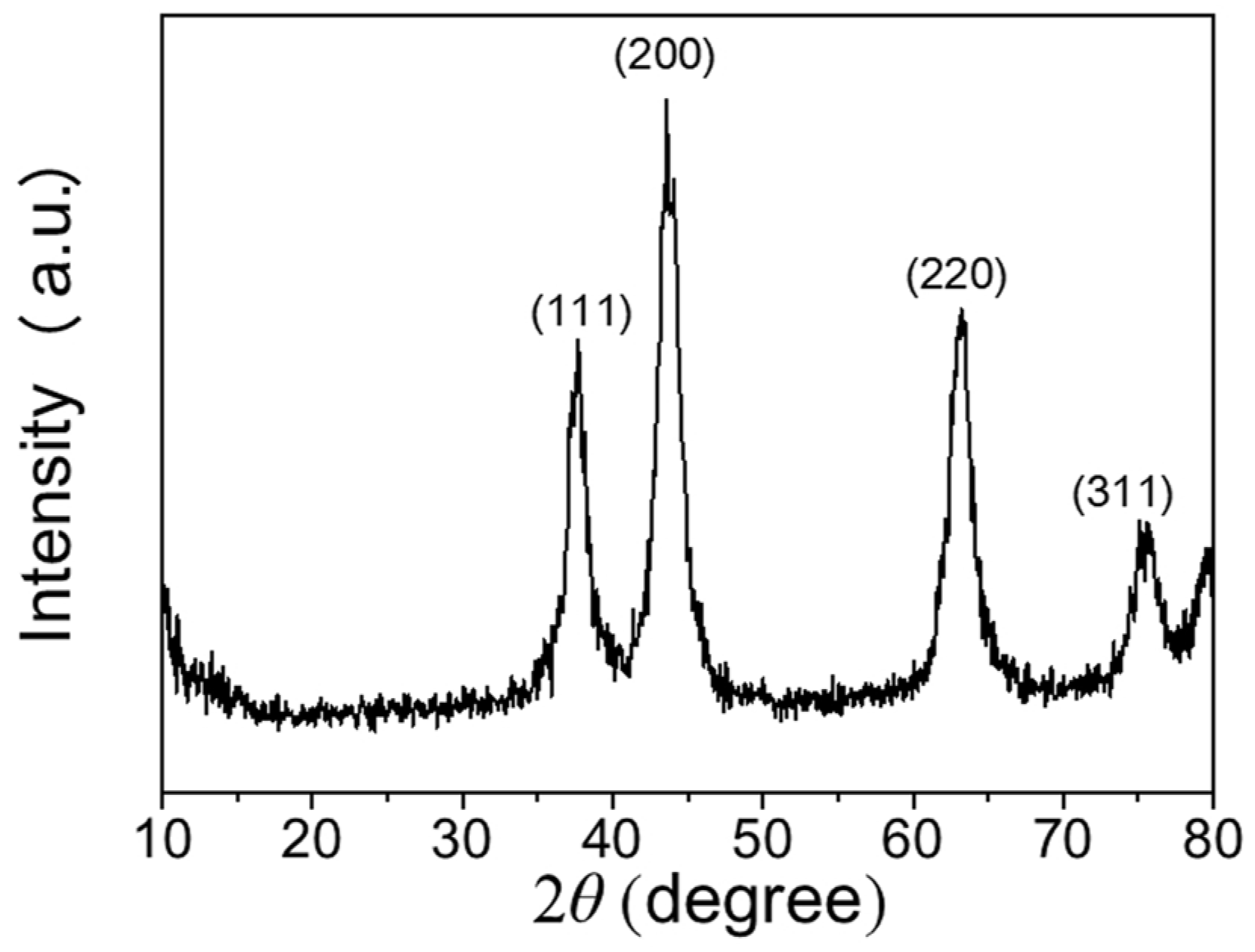
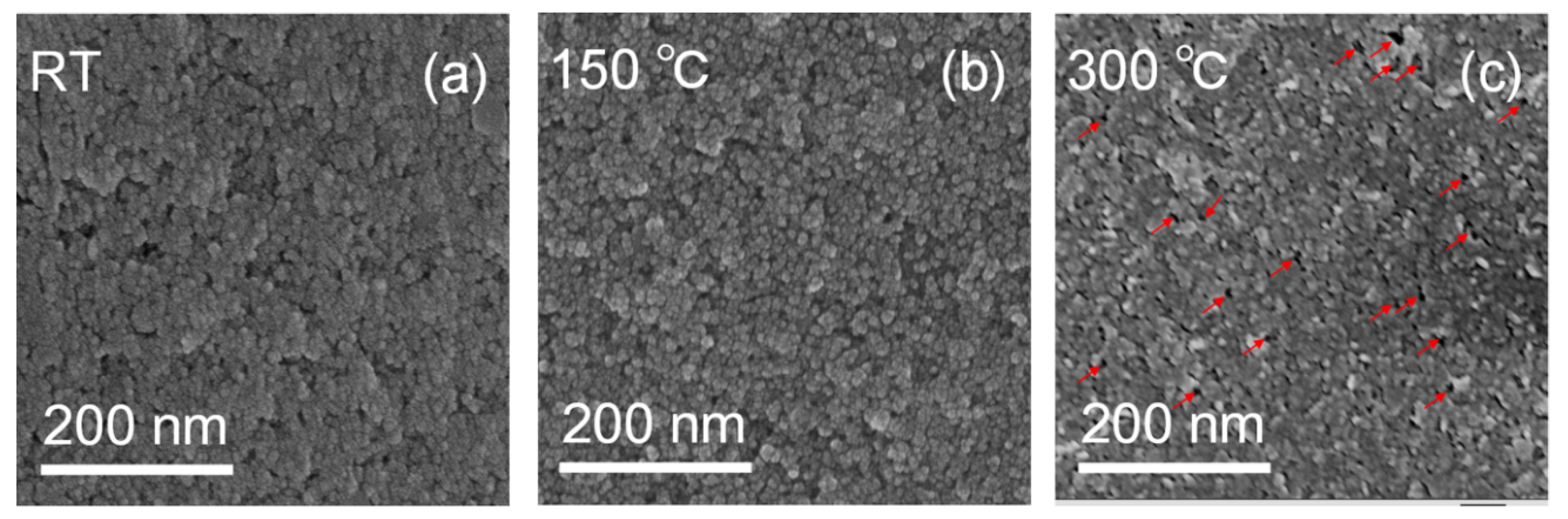
3.1. Microstructure Characteristics of the NiOx NPs
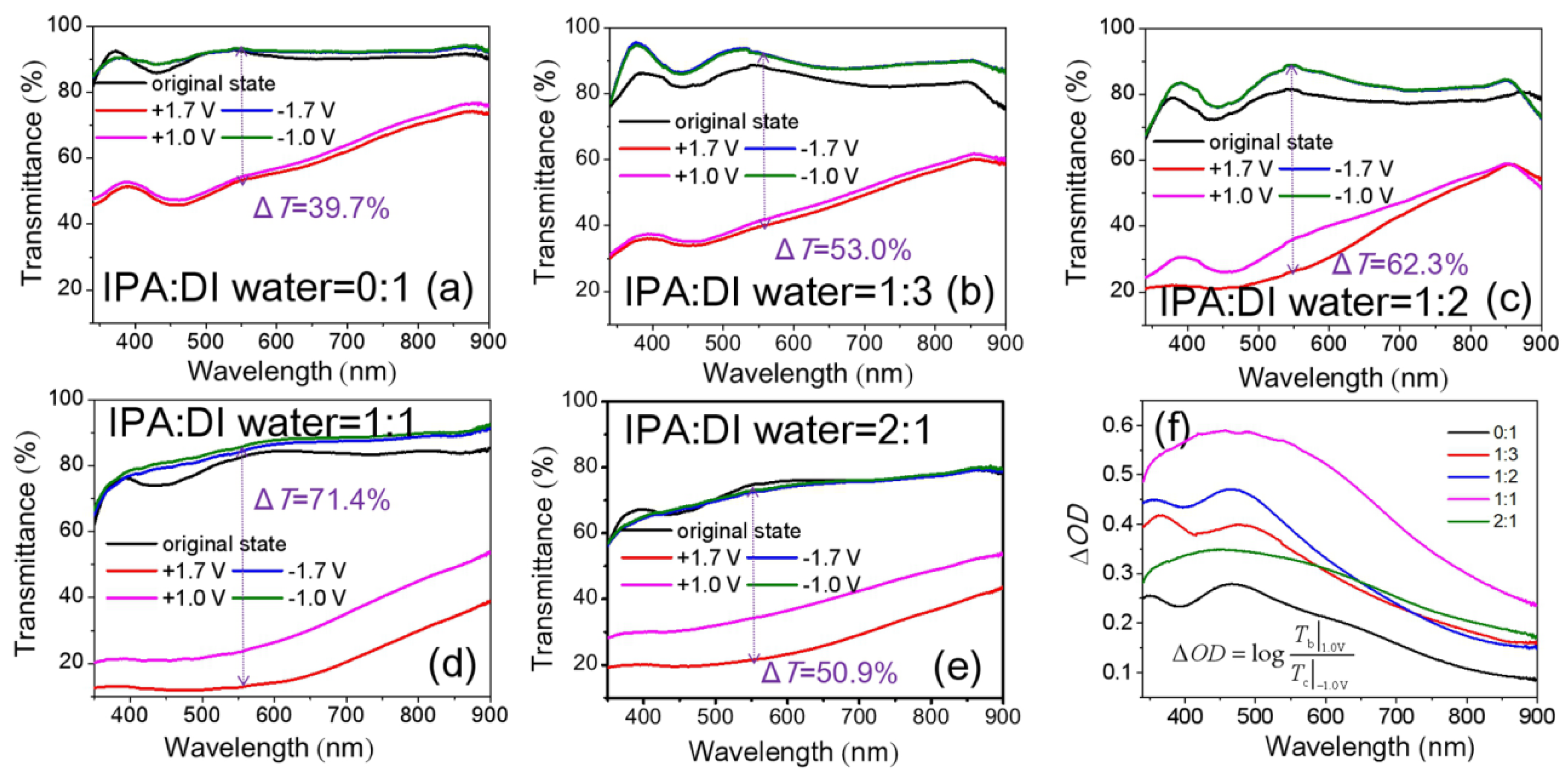
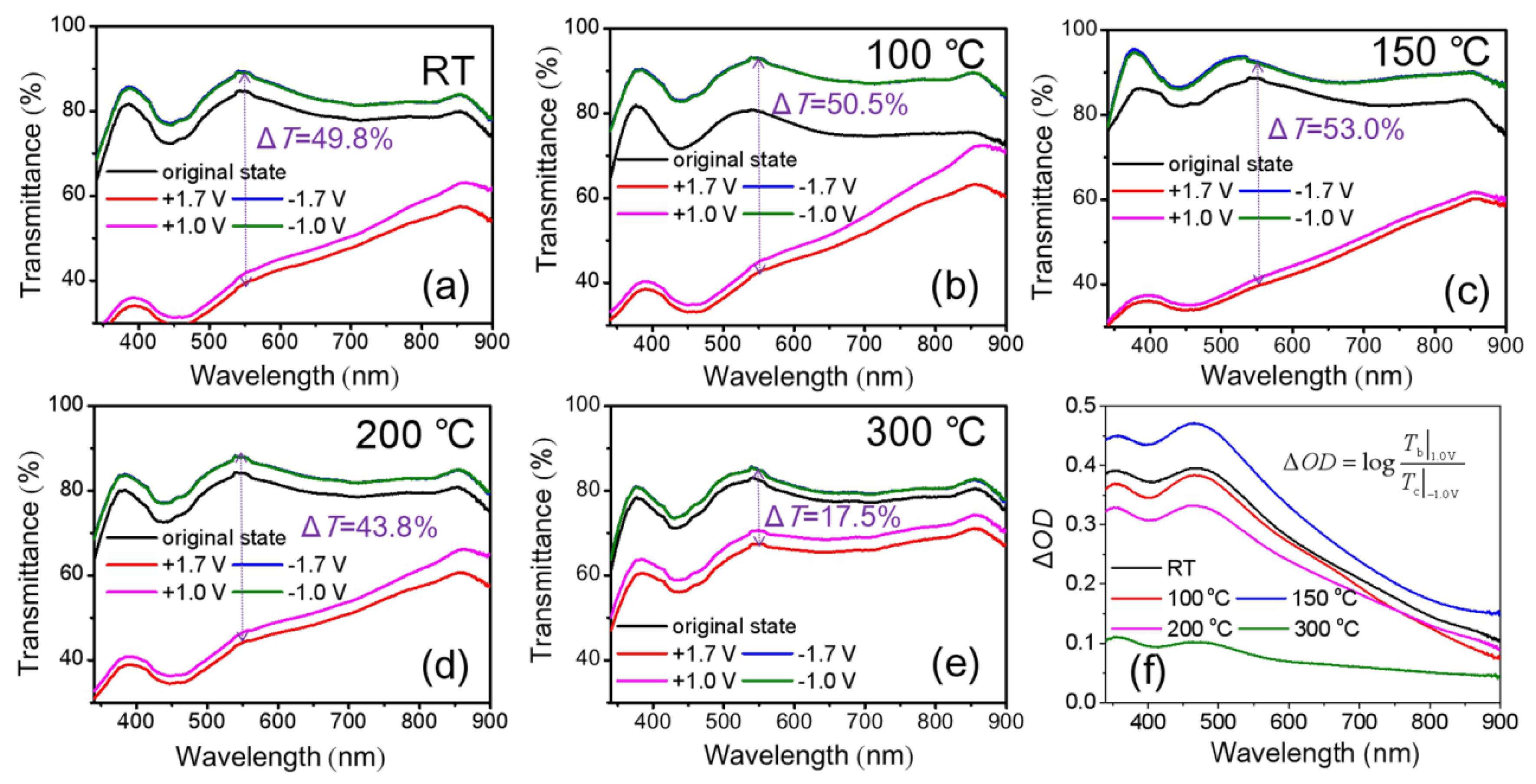
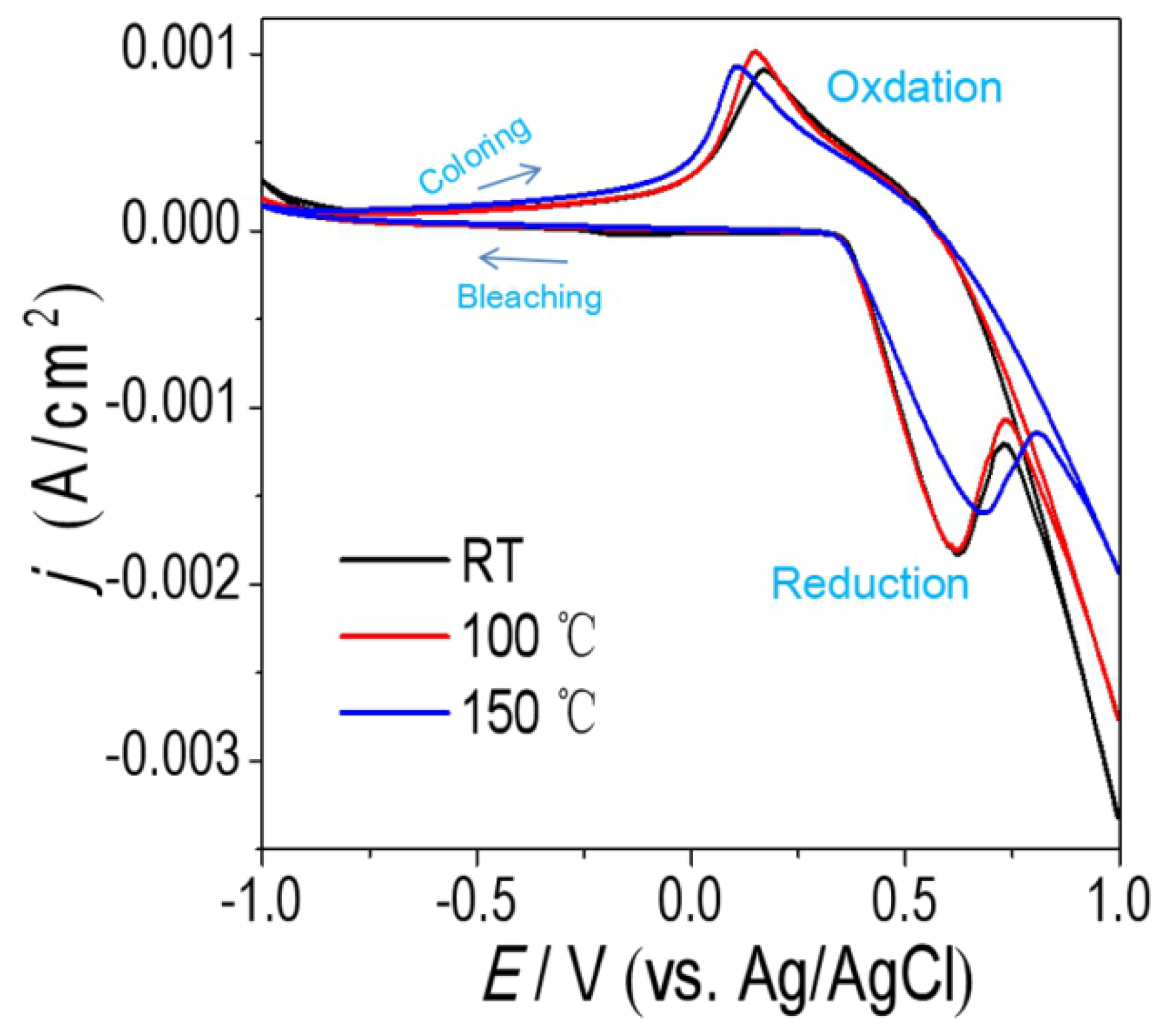
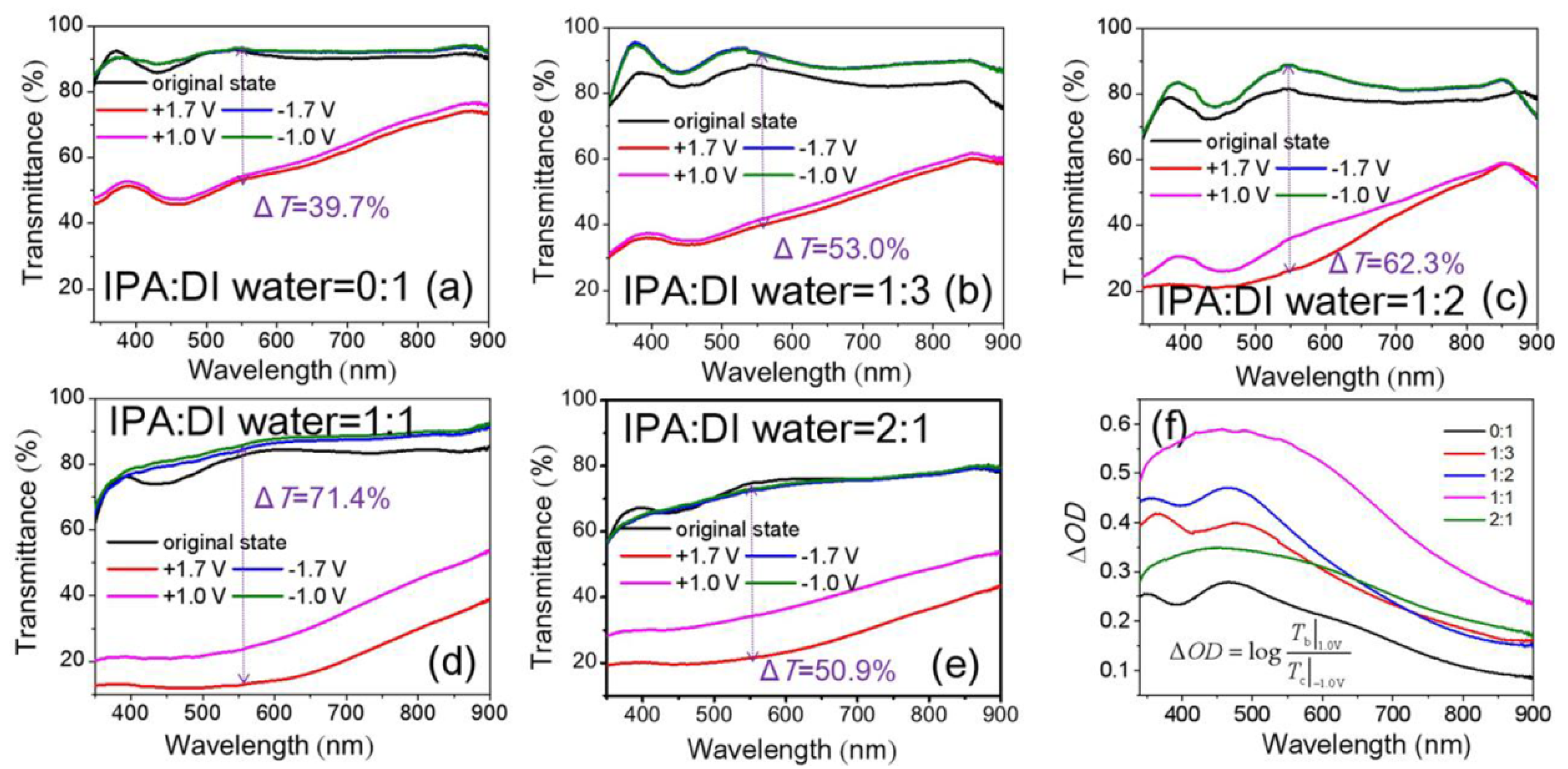
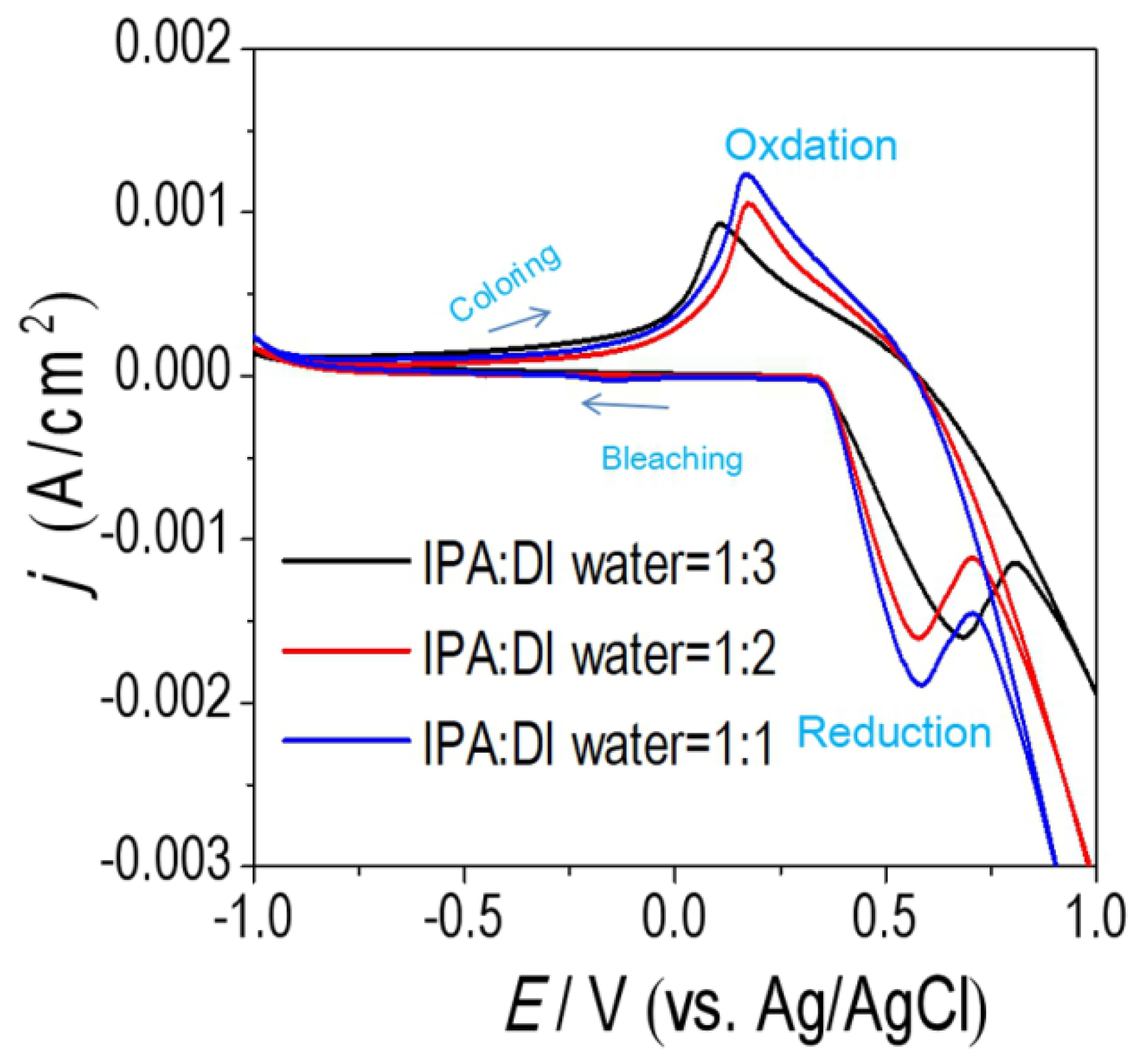
3.2. Optical and EC Properties of NiOx Films
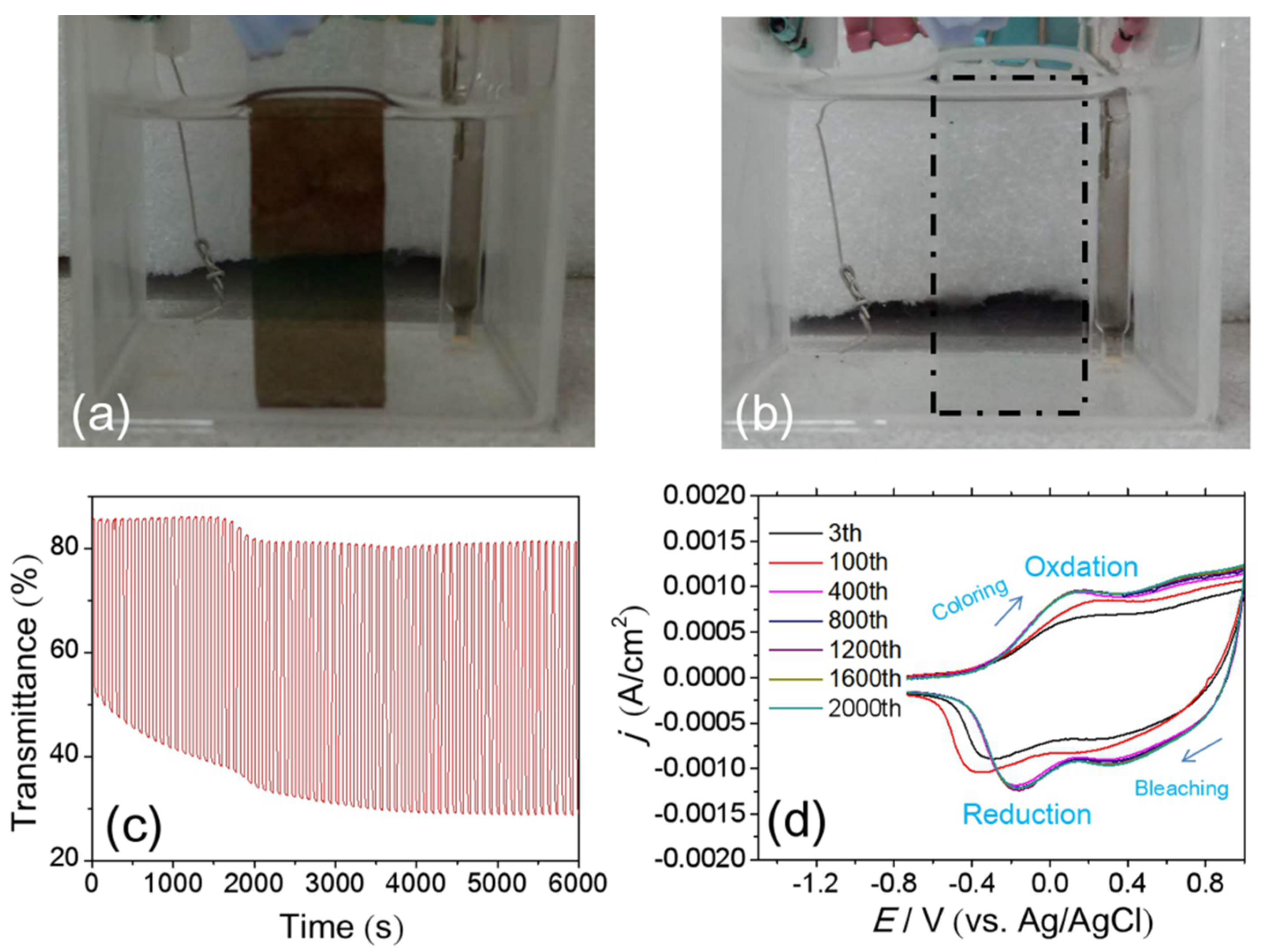
3.3. Cyclic Durability of the NiOx Film with Optimized Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, H.; Du, N.; Yu, S.; Lu, W.; Zhang, Z.; Deng, N.; Li, C. Analysis of typical public building energy consumption in northern China. Energy Build. 2017, 136, 139–150. [Google Scholar] [CrossRef]
- Ma, L.; Shao, N.; Zhang, J.; Zhao, T. The Influence of Doors and Windows on the Indoor Temperature in Rural House. Procedia Eng. 2015, 121, 621–627. [Google Scholar] [CrossRef]
- Somani, P.R.; Radhakrishnan, S. Electrochromic materials and devices: Present and future. Mater. Chem. Phys. 2003, 77, 117–133. [Google Scholar] [CrossRef]
- Drosos, C.; Vernardou, D. Perspectives of energy materials grown by APCVD. Sol. Energy Mater. Sol. Cells 2015, 140, 1–8. [Google Scholar] [CrossRef]
- Vernardou, D.; Psifis, K.; Louloudakis, D.; Papadimitropoulos, G.; Davazoglou, D.; Katsarakis, N.; Koudoumas, E. Low pressure CVD of electrochromic WO3 at 400 °C. J. Electrochem. Soc. 2015, 162, H579–H582. [Google Scholar] [CrossRef]
- Granqvist, C.; Azens, A.; Hjelm, A.; Kullman, L.; Niklasson, G.A.; Rönnow, D.; Mattsson, M.S.; Veszelei, M.; Vaivars, G. Recent advances in electrochromics for smart windows applications. Sol. Energy 1998, 63, 199–216. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, J.; Zhou, Y.; Xie, J.; Zhang, X.; Guan, M.; Pan, B.; Xie, Y. High-performance flexible electrochromic device based on facile semiconductor-to-metal transition realized by WO3·2H2O ultrathin nanosheets. Sci. Rep. 2013, 3, 1936. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; Deng, S.Z.; Tao, Y.T.; Zhao, F.L.; Zhu, L.F.; Gong, L.; Chen, J.; Xu, N.S. Evidence of localized water molecules and their role in the gasochromic effect of WO3 nanowire films. J. Phys. Chem. C 2009, 113, 15877–15881. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, J.; Wang, Y.; Wang, X.; Chen, X.; Liu, P.; Xu, J.; Xie, W.; Chen, H.; Deng, S. Highly Confined and Tunable Hyperbolic Phonon Polaritons in Van Der Waals Semiconducting Transition Metal Oxides. Adv. Mater. 2018, 30, 1705318. [Google Scholar] [CrossRef] [PubMed]
- Paulose, R.; Mohan, R.; Parihar, V. Nanostructured nickel oxide and its electrochemical behaviour—A brief review. Nanostruct. Nano-Obj. 2017, 11, 102–111. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, D.; Xia, X.; Wang, X.; Gu, C.; Tu, J. All-solid-state electrochromic devices based on WO3|| NiO films: Material developments and future applications. Sci. China Chem. 2017, 60, 3–12. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Chen, J.; Wang, X.; Eh, A.L.-S.; Magdassi, S.; Lee, P.S. Inkjet-printed all solid-state electrochromic devices based on NiO/WO3 nanoparticle complementary electrodes. Nanoscale 2016, 8, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dong, G.; Xiao, Y.; Delplancke-Ogletree, M.-P.; Reniers, F.; Diao, X. Electrolytes-relevant cyclic durability of nickel oxide thin films as an ion-storage layer in an all-solid-state complementary electrochromic device. Sol. Energy Mater. Sol. Cells 2016, 157, 844–852. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier: New York, NY, USA, 1995. [Google Scholar]
- Ren, Y.; Zhou, X.; Zhang, H.; Lei, L.; Zhao, G. Preparation of a porous NiO array-patterned film and its enhanced electrochromic performance. J. Mater. Chem. C 2018. [Google Scholar] [CrossRef]
- Zhou, K.; Qi, Z.; Zhao, B.; Lu, S.; Wang, H.; Liu, J.; Yan, H. The influence of crystallinity on the electrochromic properties and durability of NiO thin films. Surf. Interfaces 2017, 6, 91–97. [Google Scholar] [CrossRef]
- Dalavi, D.S.; Devan, R.S.; Patil, R.S.; Ma, Y.-R.; Patil, P.S. Electrochromic performance of sol–gel deposited NiO thin film. Mater. Lett. 2013, 90, 60–63. [Google Scholar] [CrossRef]
- Shi, J.; Lai, L.; Zhang, P.; Li, H.; Qin, Y.; Gao, Y.; Luo, L.; Lu, J. Aluminum doped nickel oxide thin film with improved electrochromic performance from layered double hydroxides precursor in situ pyrolytic route. J. Solid State Chem. 2016, 241, 1–8. [Google Scholar] [CrossRef]
- Jiang, S.; Yuan, G.; Hua, C.; Khan, S.; Wu, Z.; Liu, Y.; Wang, J.; Song, C.; Han, G. Electrochromic Properties of Ni/NiO/rGO Nanocomposite Films Prepared by a Facile Sol-Gel Technique. J. Electrochem. Soc. 2017, 164, H896–H902. [Google Scholar] [CrossRef]
- Zhao, C.; Du, F.; Wang, J. Flower-like nickel oxide micro/nanostructures: Synthesis and enhanced electrochromic properties. RSC Adv. 2015, 5, 38706–38711. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Sun, P.; Yang, P.; Du, L.; Mai, W. Nickel oxide nanoflake-based bifunctional glass electrodes with superior cyclic stability for energy storage and electrochromic applications. J. Mater. Chem. A 2015, 3, 20614–20618. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, A.; Chen, Y.; Zuo, C.; Zhou, S.; Li, L. Highly porous nickel oxide thin films prepared by a hydrothermal synthesis method for electrochromic application. J. Phys. Chem. Solids 2013, 74, 1522–1526. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Cai, G.-F.; Zhou, D.; Tang, H.; Wang, X.-L.; Gu, C.-D.; Tu, J.-P. Co-doped NiO nanoflake array films with enhanced electrochromic properties. J. Mater. Chem. C 2014, 2, 7013–7021. [Google Scholar] [CrossRef]
- Cai, G.; Tu, J.; Zhou, D.; Li, L.; Zhang, J.; Wang, X.; Gu, C. Constructed TiO2/NiO core/shell nanorod array for efficient electrochromic application. J. Phys. Chem. C 2014, 118, 6690–6696. [Google Scholar] [CrossRef]
- Lou, X.; Zhao, X.; He, X. Boron doping effects in electrochromic properties of NiO films prepared by sol–gel. Sol. Energy 2009, 83, 2103–2108. [Google Scholar] [CrossRef]
- Tenent, R.C.; Gillaspie, D.T.; Miedaner, A.; Parilla, P.A.; Curtis, C.J.; Dillon, A.C. Fast-switching electrochromic Li+-doped NiO films by ultrasonic spray deposition. J. Electrochem. Soc. 2010, 157, H318–H322. [Google Scholar] [CrossRef]
- Lang, F.; Liu, J.; Wang, H.; Yan, H. NiO Nanocrystalline/Reduced Graphene Oxide Composite Film with Enhanced Electrochromic Properties. Nano 2017, 12, 1750058. [Google Scholar] [CrossRef]
- Cai, G.-F.; Tu, J.-P.; Zhang, J.; Mai, Y.-J.; Lu, Y.; Gu, C.-D.; Wang, X.-L. An efficient route to a porous NiO/reduced graphene oxide hybrid film with highly improved electrochromic properties. Nanoscale 2012, 4, 5724–5730. [Google Scholar] [CrossRef] [PubMed]
- Talebian, N.; Kheiri, M. Sol–gel derived nanostructured nickel oxide films: effect of solvent on crystallographic orientations. Solid State Sci. 2014, 27, 79–83. [Google Scholar] [CrossRef]
- Jiang, F.; Choy, W.C.; Li, X.; Zhang, D.; Cheng, J. Post-treatment-Free Solution-Processed Non-stoichiometric NiOx Nanoparticles for Efficient Hole-Transport Layers of Organic Optoelectronic Devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Birks, L.; Friedman, H. Particle size determination from X-ray line broadening. J. Appl. Phys. 1946, 17, 687–692. [Google Scholar] [CrossRef]
- Sun, D.-L.; Zhao, B.-W.; Liu, J.-B.; Wang, H.; Yan, H. Application of nickel oxide nanoparticles in electrochromic materials. Ionics 2017, 23, 1509–1515. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J. Inorganic electrochromic materials based on tungsten oxide and nickel oxide nanostructures. Sci. China Chem. 2017, 60, 54–62. [Google Scholar] [CrossRef]
- Sahu, D.; Wu, T.-J.; Wang, S.-C.; Huang, J.-L. Electrochromic behavior of NiO film prepared by e-beam evaporation. J. Sci. Adv. Mater. Devices 2017, 2, 225–232. [Google Scholar] [CrossRef]
- Atak, G.; Coşkun, Ö.D. Annealing effects of NiO thin films for all-solid-state electrochromic devices. Solid State Ionics 2017, 305, 43–51. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.; Zhang, Y.; Liu, J.; Yan, H. An advanced technique to evaluate the electrochromic performances of NiO films by multi-cycle double-step potential chronocoulometry. J. Electrochem. Soc. 2016, 163, H1033–H1040. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, X.; Tian, Y.; Zhao, J.; Geng, H.; Qu, H.; Zhang, H.; Zhang, K.; Wang, B.; Gavrilyuk, A. Improved Electrochemical Cycling Durability in a Nickel Oxide Double-Layered Film. Chem. Asian J. 2017, 12, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bhatt, G.; Ray, J.; Suryavanshi, P.; Panchal, C. All-inorganic solid-state electrochromic devices: A review. J. Solid State Electrochem. 2017, 21, 337–347. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, G.; Wei, Y.; Zheng, J.; Xu, C. Enhanced electrochromic performances and cycle stability of NiO-based thin films via Li–Ti co-doping prepared by sol–gel method. Electrochim. Acta 2015, 186, 182–191. [Google Scholar] [CrossRef]


| No. | Methods | Films | Annealing Condition | ΔT (%) | CE (cm2/C) | Tc/Tb (s) | Ref. | Year |
|---|---|---|---|---|---|---|---|---|
| 1 | Dip-coating | NiO film | 500 °C | 51 | 40 | 7/5 | [15] | 2017 |
| 2 | Dip-coating | NiO film | 350 °C | 50.7 | 71.4 | - | [16] | 2017 |
| 3 | Inkjet printing | NiO film | 200 °C | 64.2 | 136.7 | 9/6 | [12] | 2016 |
| 4 | Hydrothermal | NiO film | 300 °C | 35.8 | 49.8 | 1.3/3.2 | [20] | 2015 |
| 5 | Hydrothermal | NiO film | 400 °C/Ar | 40 | 63.2 | 2.7/1.8 | [21] | 2015 |
| 6 | Hydrothermal | NiO film | 300 °C/Ar | 77 | 49 | 3/4 | [22] | 2013 |
| 7 | Spin-coating | Al-doped NiO film | 400 °C | 58.4 | 54.2 | 4.2/1.8 | [18] | 2016 |
| 8 | CBD | Co-doped NiO film | 300 °C | 88.3 | 47.7 | 5.4/3.4 | [23] | 2014 |
| 9 | Dip-coating | NiO/GO film | 350 °C | 40.7 | 12.85 | 4.3/3.9 | [27] | 2017 |
| 10 | Spin-coating | Ni/NiO/rGO film | 350 °C/N2 and air | 51.6 | 48.15 | 4.2/2.4 | [19] | 2017 |
| 11 | CBD | TiO2/NiO film | 300 °C/Ar | 83 | 60.6 | 6.8/14.8 | [24] | 2014 |
| 12 | Spin-coating | NiOx film | 150 °C/air | 62.3 | 42.8 | 4/3 | This work | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Gao, C.; Du, X.; Zhu, G.; Xie, W.; Liu, P.; Tang, Z. Improved Optical and Electrochromic Properties of NiOx Films by Low-Temperature Spin-Coating Method Based on NiOx Nanoparticles. Materials 2018, 11, 760. https://doi.org/10.3390/ma11050760
Xie X, Gao C, Du X, Zhu G, Xie W, Liu P, Tang Z. Improved Optical and Electrochromic Properties of NiOx Films by Low-Temperature Spin-Coating Method Based on NiOx Nanoparticles. Materials. 2018; 11(5):760. https://doi.org/10.3390/ma11050760
Chicago/Turabian StyleXie, Xiaohong, Changkang Gao, Xiang Du, Gangyi Zhu, Weiguang Xie, Pengyi Liu, and Zhenfang Tang. 2018. "Improved Optical and Electrochromic Properties of NiOx Films by Low-Temperature Spin-Coating Method Based on NiOx Nanoparticles" Materials 11, no. 5: 760. https://doi.org/10.3390/ma11050760




