In Vitro Comparative Study of Oxygen Plasma Treated Poly(Lactic–Co–Glycolic) (PLGA) Membranes and Supported Nanostructured Oxides for Guided Bone Regeneration Processes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
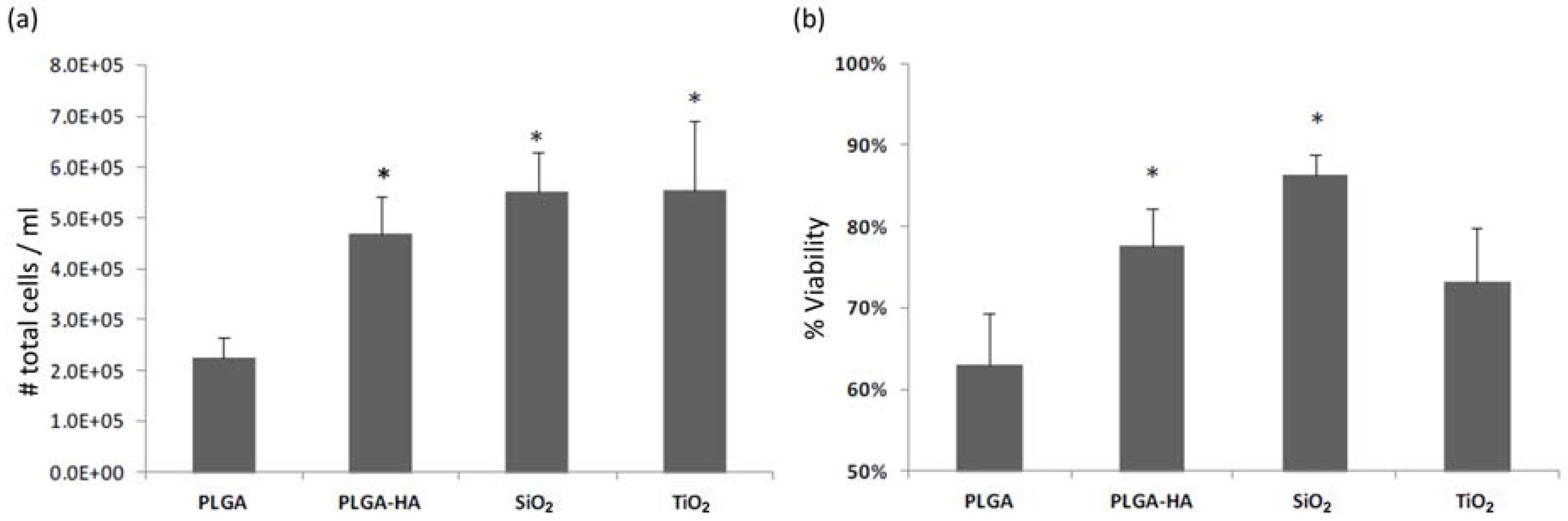
3.1. Test 1
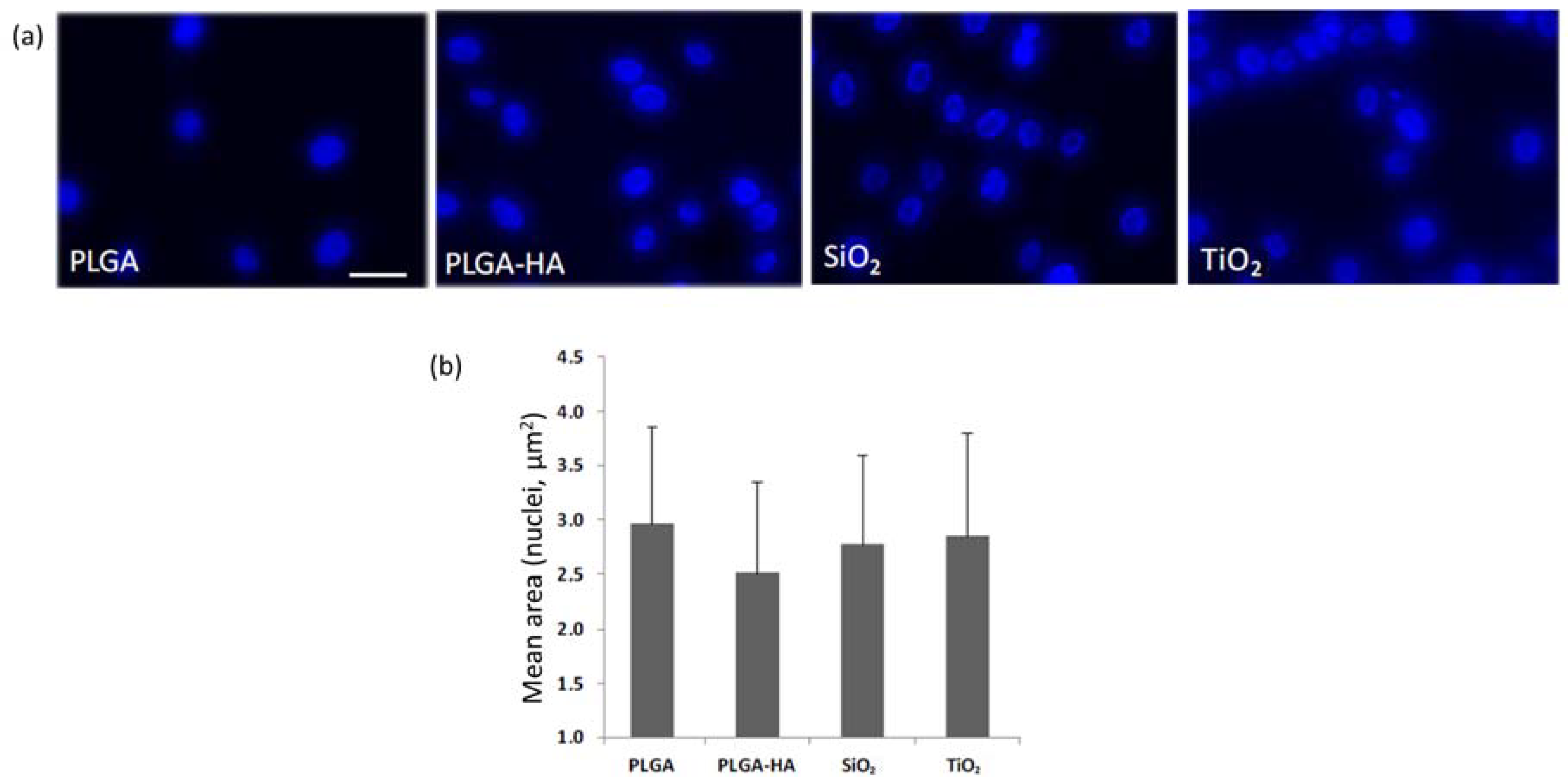
3.2. Test 2
3.3. Test 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hild, N.; Schneider, O.D.; Mohn, D.; Luechinger, N.A.; Koehler, F.M.; Hofmann, S.; Vetsch, J.R.; Thimm, B.W.; Müller, R.; Stark, W.J. Two-layer membranes of calcium phosphate/collagen/PLGA nanofibres: In vitro biomineralisation and osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2011, 3, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; He, Y.; Bi, L.; Qu, Z.H.; Zou, J.W.; Pan, Z.; Fan, J.J.; Chen, L.; Dong, X.; Liu, X.N.; et al. Enhancing the bioactivity of Poly(lactic-co-glycolic acid) scaffold with a nano-hydroxyapatite coating for the treatment of segmental bone defect in a rabbit model. Int. J. Nanomed. 2013, 8, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Dalí, G.; Velázquez-Cayón, R.; Serrera-Figallo, M.A.; Rodríguez-González-Elipe, A.; Gutierrez-Pérez, J.L.; Torres-Lagares, D. Importance of poly(lactic-co-glycolic acid) in scaffolds for guided bone regeneration: A focused review. J. Oral Implantol. 2015, 41, e152–e157. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lagares, D.; Castellanos-Cosano, L.; Serrera-Figallo, M.Á.; García-García, F.J.; López-Santos, C.; Barranco, A.; Gonzalez-Elipe, A.R.; Rivera-Jiménez, C.; Gutiérrez-Pérez, J.L. In vitro and in vivo study of poly(lactic–co–glycolic) (plga) membranes treated with oxygen plasma and coated with nanostructured hydroxyapatite ultrathin films for guided bone regeneration processes. Polymers 2017, 9, 410. [Google Scholar] [CrossRef]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.L.; Batista, A.; Barranco, A.; Cabezas-Talavero, J.; Lynch, C.D.; Barouk, B.; Llorens, A.; et al. In vivo comparative model of oxygen plasma and nanocomposite particles on PLGA membranes for guided bone regeneration processes to be applied in pre-prosthetic surgery: a pilot study. J. Dent. 2014, 42, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Batista-Cruzado, A.; López-Santos, C.; Rodríguez-González-Elipe, A.; Saffar, J.L.; Lynch, C.D.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. Reliability of new poly (lactic-co-glycolic acid) membranes treated with oxygen plasma plus silicon dioxide layers for pre-prosthetic guided bone regeneration processes. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e242–e250. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.L.; Batista-Cruzado, A.; Lynch, C.D.; Sloan, A.J.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. Pre-prosthetic use of poly(lactic-co-glycolic acid) membranes treated with oxygen plasma and TiO2 nanocomposite particles for guided bone regeneration processes. J. Dent. 2016, 47, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wong-Lee, J.G.; Lovett, M. Rapid and sensitive PCR method for identification of Mycoplasma species in tissue culture. In Diagnostic Molecular Microbiology: Principles and Applications; Persing, D.H., Smith, T.F., Tenover, F.C., White, T.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1993; pp. 257–260. [Google Scholar]
- Paz-Pumpido, F. Biocompatibilidad de los adhesivos dentinarios. Av. Odontoestomatol. 2005, 21, 339–345. [Google Scholar] [CrossRef]
- Lopez Santos, C.; Terriza, A.; Portoles, J.; Yubero, F.; Gonzalez-Elipe, A.R. Physiological Degradation Mechanisms of PLGA Membrane Films under Oxygen Plasma Treatment. J. Phys. Chem. C 2015, 119, 20446–20452. [Google Scholar] [CrossRef]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R.; Dubruel, P.; Leys, C.; Beaurain, A.; Payen, E.; Morent, R. Plasma surface modification of polylactic acid to promote interaction with fibroblasts. J. Mater. Sci. Mater. Med. 2013, 24, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Borrás, A.; Barranco, A.; González-Elipe, A.R. Design and control of porosity in oxide thin films grown by PECVD. J. Mater. Sci. 2006, 41, 5220–5226. [Google Scholar] [CrossRef]
- Sánchez-Valencia, J.R.; Borrás, A.; Barranco, A.; Rico, V.J.; Espinós, J.P.; González-Elipe, A.R. Preillumination of TiO2 and Ta2O5 photoactive thin films as a tool to tailor the synthesis of composite materials. Langmuir 2008, 24, 9460–9469. [Google Scholar] [CrossRef] [PubMed]
- Borrás, A.; Yanguas-Gil, A.; Barranco, A.; Cotrino, J.; González-Elipe, A.R. Relationship between scaling behavior and porosity of plasma-deposited TiO2 thin films. Phys. Rev. B 2007, 76, 235303. [Google Scholar] [CrossRef]
- Nieh, T.G.; Jankowski, A.F.; Koike, J. Processing and characterization of hydroxyapatite coatings on titanium produced by magnetron sputtering. J. Mater. Res. 2001, 16, 3238–3245. [Google Scholar] [CrossRef]
- Ruiz-Gaspa, S.; Nogues, X.; Enjuanes, A.; Monllau, J.C.; Blanch, J.; Carreras, R.; Mellibovsky, L.; Grinberg, D.; Balcells, S.; Díez-Perez, A.; et al. Simvastatin and atorvastatin enhance gene expression of collagen type 1 and osteocalcin in primary human osteoblasts and mg-63 cultures. J. Cell. Biochem. 2007, 101, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, J.B. Enhanced calcium ion mobilization in osteoblasts on amino group containing plasma polymer nanolayer. Cell Biosci. 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Di Toro, R.; Betti, V.; Spampinato, S. Biocompatibility and integrin-mediated adhesion of human osteoblasts to poly(dl-lactide-co-glycolide) copolymers. Eur. J. Pharm. Sci. 2014, 21, 161–169. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Lv, W.; Zhang, M.; Yang, S.; Yin, L.; Hong, J.; Han, D.; Chen, C.; Swarts, S.; et al. Interleukin 11 protects bone marrow mitochondria from radiation damage. Adv. Exp. Med. Biol. 2013, 789, 257–264. [Google Scholar] [PubMed]
- Waggoner, A.; DeBiasio, R.; Conrad, P.; Bright, G.R.; Ernst, L.; Ryan, K.; Nederlof, M.; Taylor, D. Multiple spectral parameter imaging. Methods Cell Biol. 1989, 30, 449–478. [Google Scholar] [PubMed]
- Kubista, M.; Akerman, B.; Nordén, B. Characterization of interaction between DNA and 4′,6-diamidino-2-phenylindole by optical spectroscopy. Biochemistry 1987, 26, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Kokovic, V.; Jurisic, M.; Yaman, D.; Subramani, K.F.; Weber, E. Guided bone regeneration with a synthetic biodegradable membrane: a comparative study in dogs. Clin. Oral. Implants Res. 2011, 22, 802–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide-co-glycolide). Biomaterials 2007, 28, 4219–4230. [Google Scholar] [CrossRef] [PubMed]
- Khang, G.; Jeon, J.H.; Lee, J.W.; Cho, S.C.; Lee, H.B. Cell and platelet adhesions on plasma glow discharge-treated poly(lactide-co-glycolide). Biomed. Mater. Eng. 1997, 7, 357–368. [Google Scholar] [PubMed]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Botelho, C.M.; Brooks, R.A.; Best, S.M.; Lopes, M.A.; Santos, J.D.; Rushton, N.; Bonfield, W. Human osteoblast response to silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2006, 79, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.; Bose, S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013, 9, 9137–9148. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Tokuda, S.; Kasuga, T. Enhanced in vitro cell activity on silicon-doped vaterite/poly(lactic acid) composites. Acta Biomater. 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Wieland, M. Experimental Determination and Quantitative Evaluation of the Surface Composition and Topography of Medical Implant Surfaces and Their Influence on Osteoblastic Cell Surface Interactions. Ph.D. Thesis, Swiss Federal Institute of Technology, Zurich, Switzerland, 1999. [Google Scholar]
- Tiainen, H.; Wohlfahrt, J.C.; Verket, A.; Lyngstadaas, S.P.; Haugen, H.J. Bone formation in TiO2 bone scaffolds in extraction sockets of minipigs. Acta Biomater. 2012, 8, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.E.; Thomsen, P.; Lyngstadaas, S.P. Advances in dental implant materials and tissue regeneration. Periodontol. 2000 2006, 41, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Länge, K.; Herold, M.; Scheideler, L.; Geis-Gerstorfer, J.; Wendel, H.P.; Gauglitz, G. Investigation of initial pellicle formation on modified titanium dioxide (TiO2) surfaces by reflectometric interference spectroscopy (RIfS) in a model system. Dent. Mater. 2004, 20, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Terriza, A.; Vilches-Pérez, J.I.; de la Orden, E.; Yubero, F.; González-Caballero, J.L.; González-Elipe, A.R.; Vilches, J.; Salido, M. Osteoconductive Potential of Barrier NanoSiO2 PLGA Membranes Functionalized by Plasma Enhanced Chemical Vapour Deposition. BioMed. Res. Int. 2014, 10, 253590. [Google Scholar]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]



| Ratio Red/Green | Measure 1 | Measure 2 | Measure 3 | Mean | SD 1 |
|---|---|---|---|---|---|
| PLGA | 1.44 | 2.16 | 1.46 | 1.69 | 0.41 |
| PLGA-HA | 2.59 | 2.66 | 2.55 | 2.57 | 0.09 |
| PLGA-SiO2 | 2.57 | 2.18 | 2.39 | 2.38 | 0.19 |
| PLGA-TiO2 | 1.34 | 1.43 | 1.71 | 1.49 | 0.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Lagares, D.; Castellanos-Cosano, L.; Serrera-Figallo, M.-A.; López-Santos, C.; Barranco, A.; Rodríguez-González-Elipe, A.; Gutierrez-Perez, J.-L. In Vitro Comparative Study of Oxygen Plasma Treated Poly(Lactic–Co–Glycolic) (PLGA) Membranes and Supported Nanostructured Oxides for Guided Bone Regeneration Processes. Materials 2018, 11, 752. https://doi.org/10.3390/ma11050752
Torres-Lagares D, Castellanos-Cosano L, Serrera-Figallo M-A, López-Santos C, Barranco A, Rodríguez-González-Elipe A, Gutierrez-Perez J-L. In Vitro Comparative Study of Oxygen Plasma Treated Poly(Lactic–Co–Glycolic) (PLGA) Membranes and Supported Nanostructured Oxides for Guided Bone Regeneration Processes. Materials. 2018; 11(5):752. https://doi.org/10.3390/ma11050752
Chicago/Turabian StyleTorres-Lagares, Daniel, Lizett Castellanos-Cosano, Maria-Angeles Serrera-Figallo, Carmen López-Santos, Angel Barranco, Agustín Rodríguez-González-Elipe, and Jose-Luis Gutierrez-Perez. 2018. "In Vitro Comparative Study of Oxygen Plasma Treated Poly(Lactic–Co–Glycolic) (PLGA) Membranes and Supported Nanostructured Oxides for Guided Bone Regeneration Processes" Materials 11, no. 5: 752. https://doi.org/10.3390/ma11050752
APA StyleTorres-Lagares, D., Castellanos-Cosano, L., Serrera-Figallo, M.-A., López-Santos, C., Barranco, A., Rodríguez-González-Elipe, A., & Gutierrez-Perez, J.-L. (2018). In Vitro Comparative Study of Oxygen Plasma Treated Poly(Lactic–Co–Glycolic) (PLGA) Membranes and Supported Nanostructured Oxides for Guided Bone Regeneration Processes. Materials, 11(5), 752. https://doi.org/10.3390/ma11050752









