Structure and Electromagnetic Properties of Cellular Glassy Carbon Monoliths with Controlled Cell Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
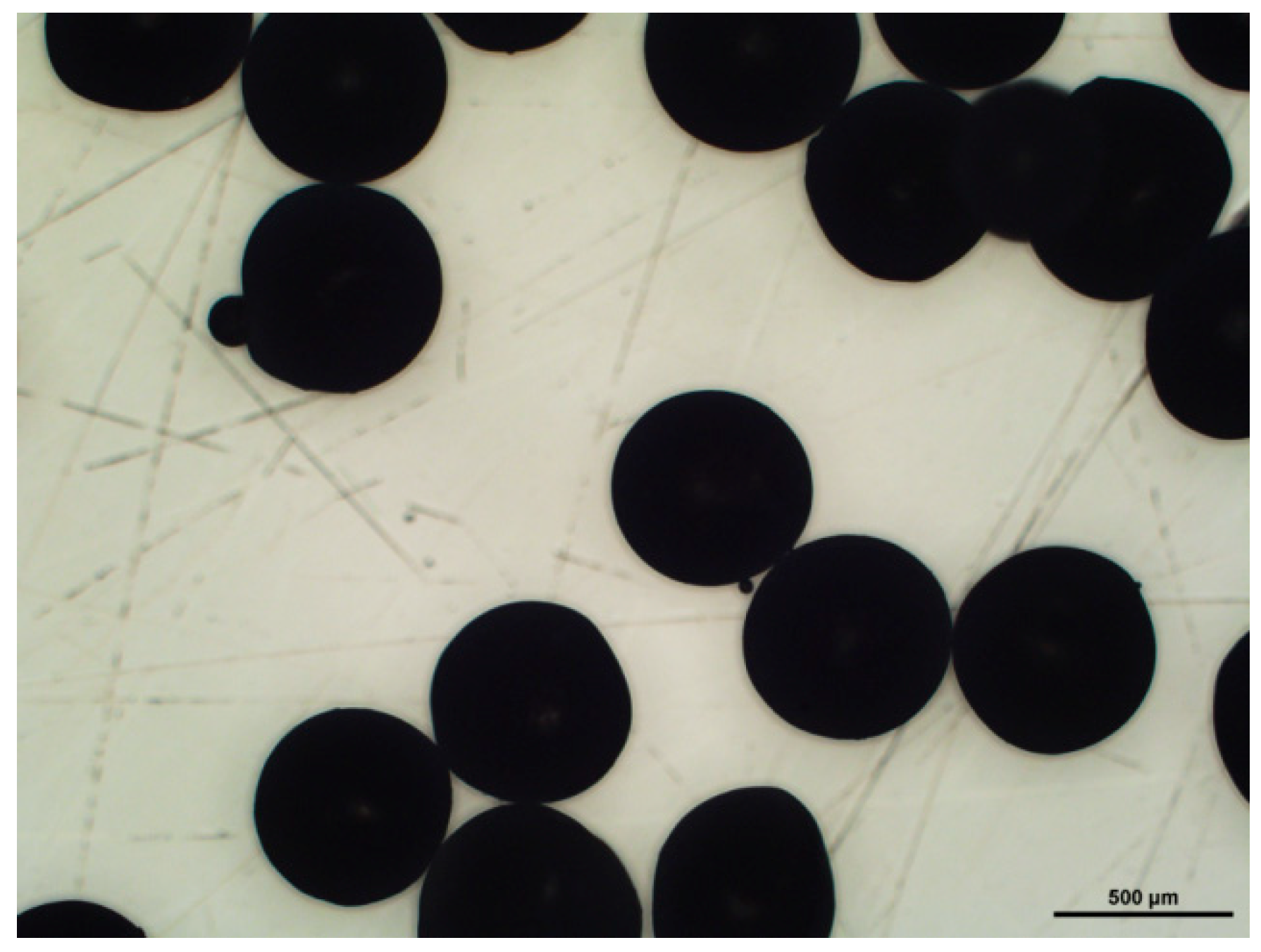
2.1.1. Preparation of Spherical Templates
2.1.2. Preparation of RF Cellular Monoliths and Derived Carbon Monoliths
2.2. Characterisation
2.2.1. Thermogravimetric and Elemental Analyses
2.2.2. Density and Porous Structure
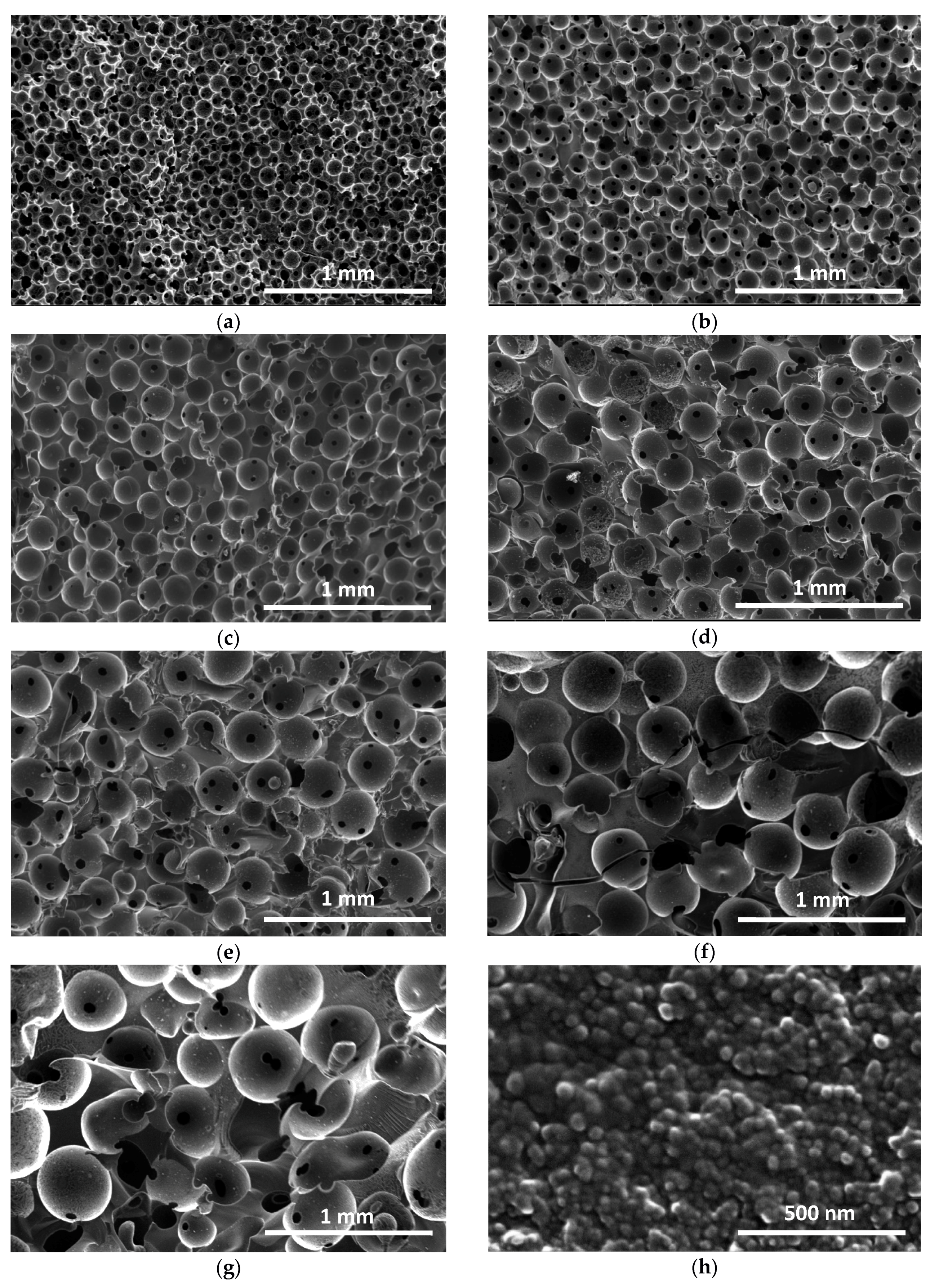
2.2.3. Optical and Electron Microscopy
2.2.4. Electrical and Electromagnetic Measurements
3. Results and Discussion
3.1. Carbonisation Behaviour and Resultant Composition
3.2. Porosity and Pore Texture Parameters
3.2.1. Overall Porosity
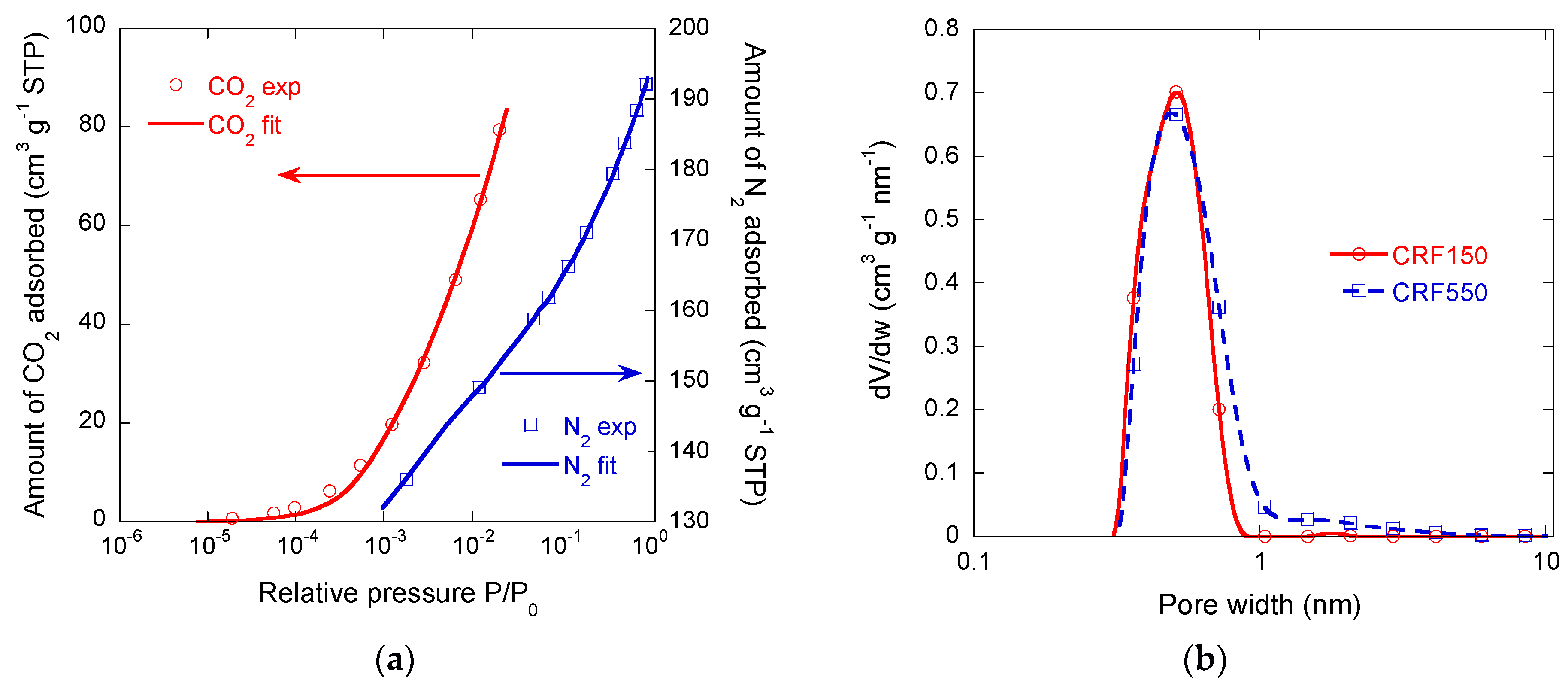
3.2.2. Microporous Structure
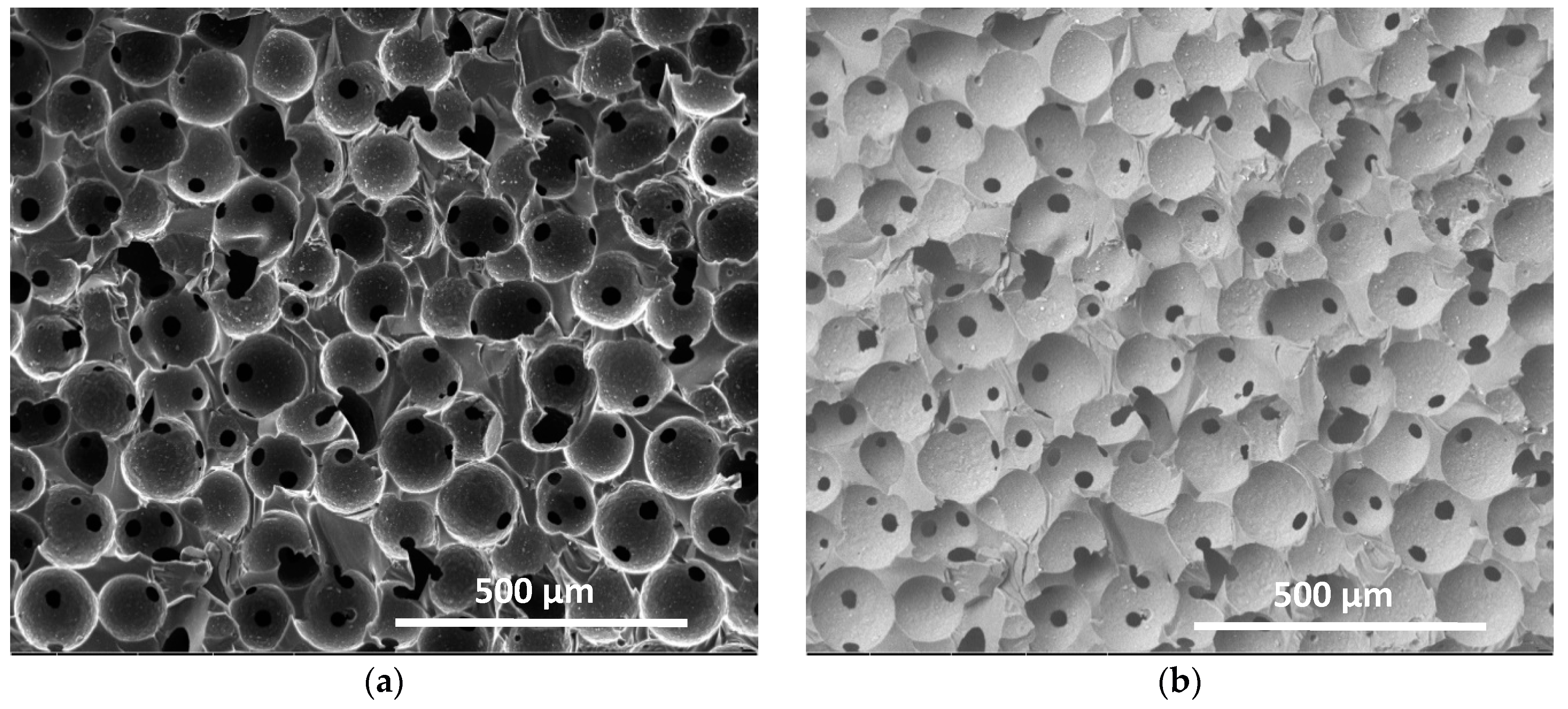
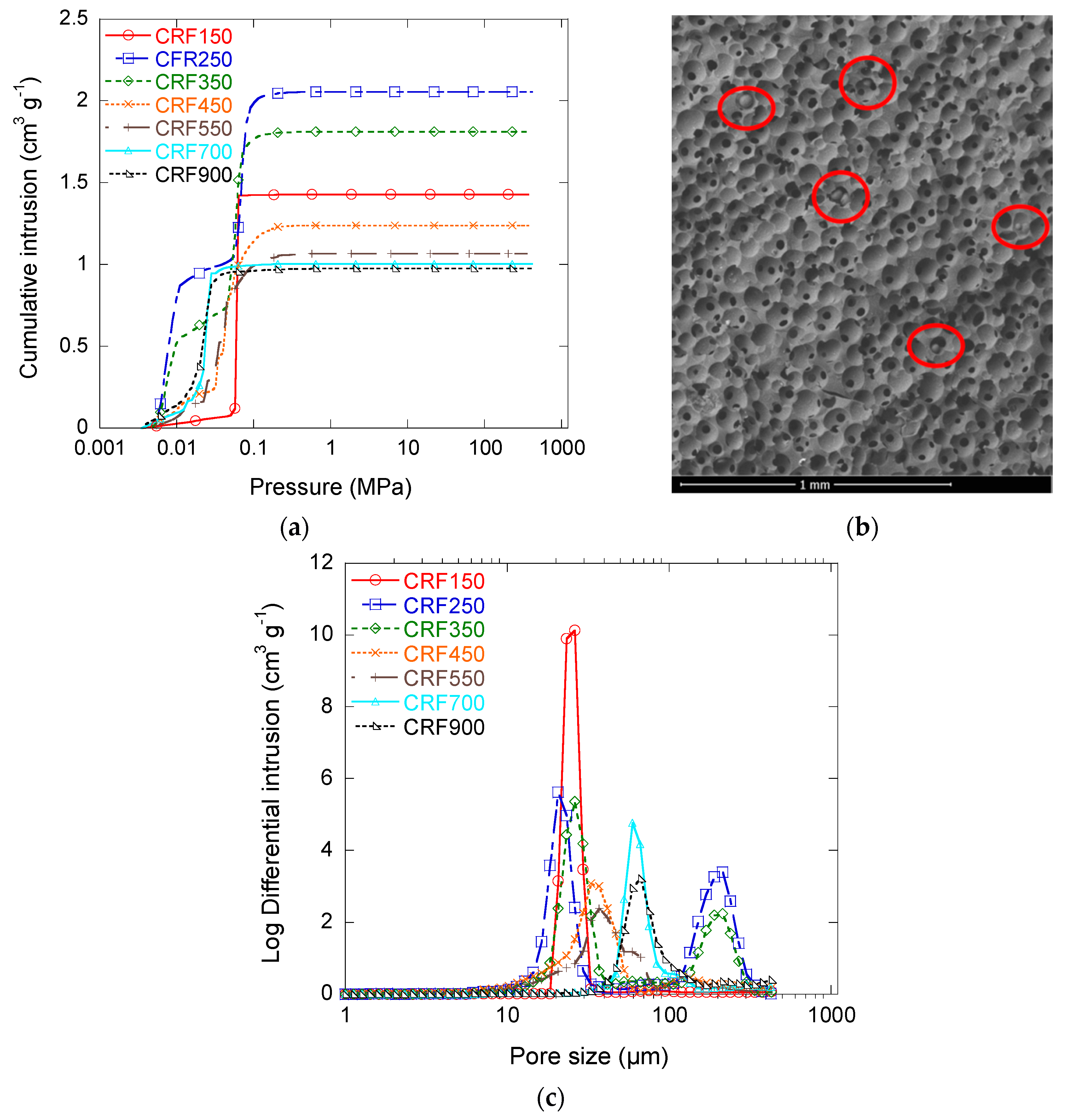
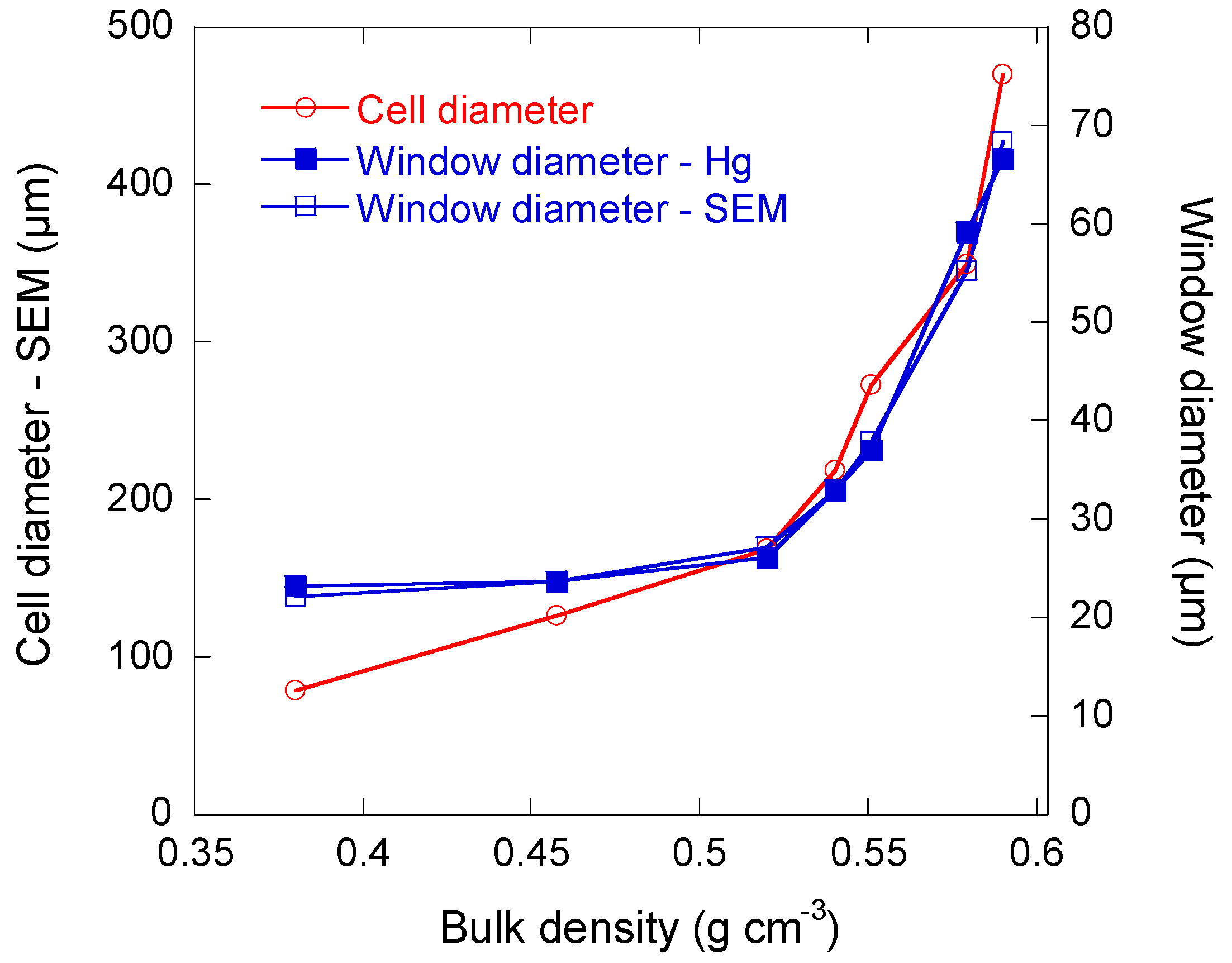
3.2.3. Macroporous Structure
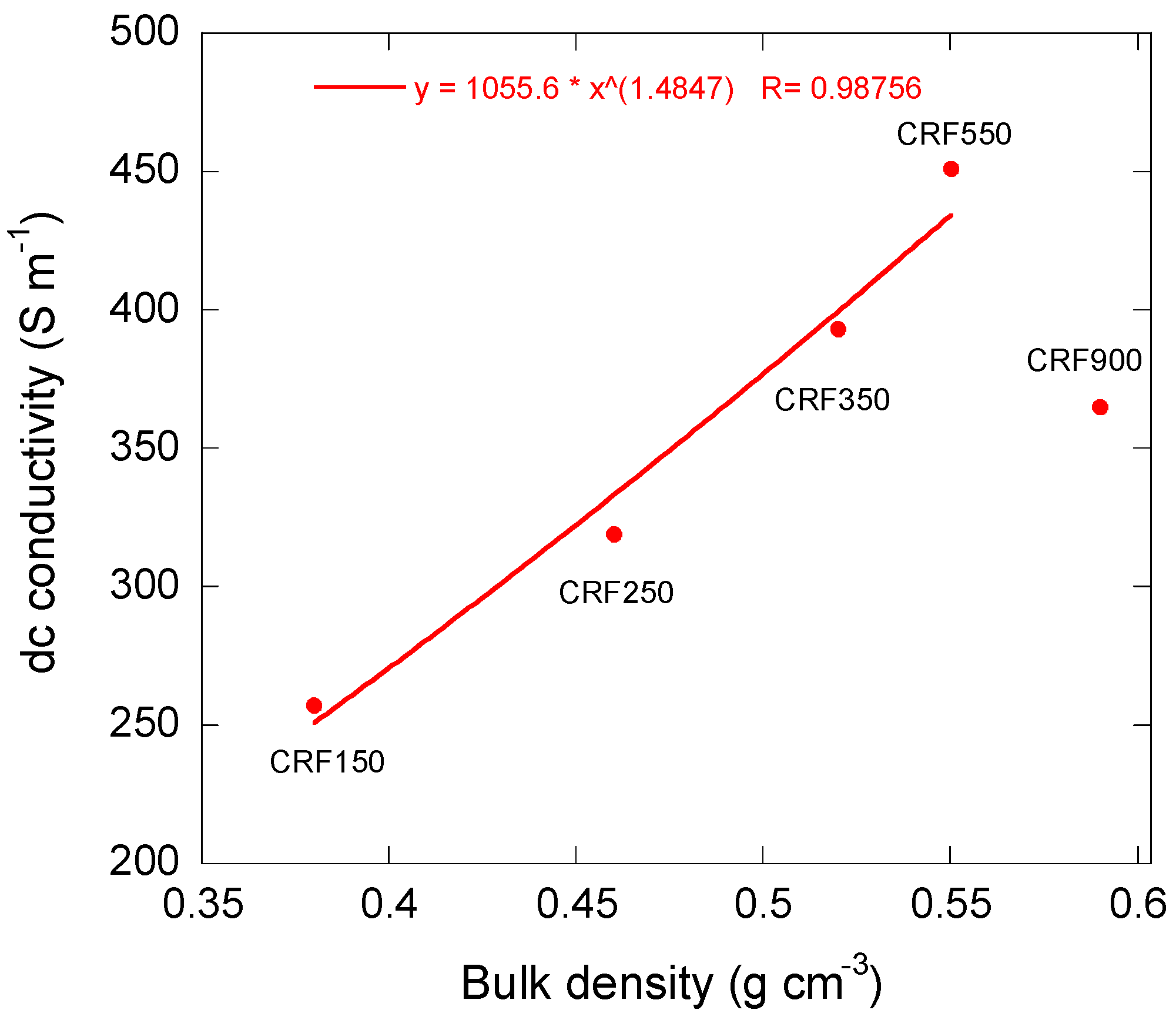
3.3. Electrical Properties
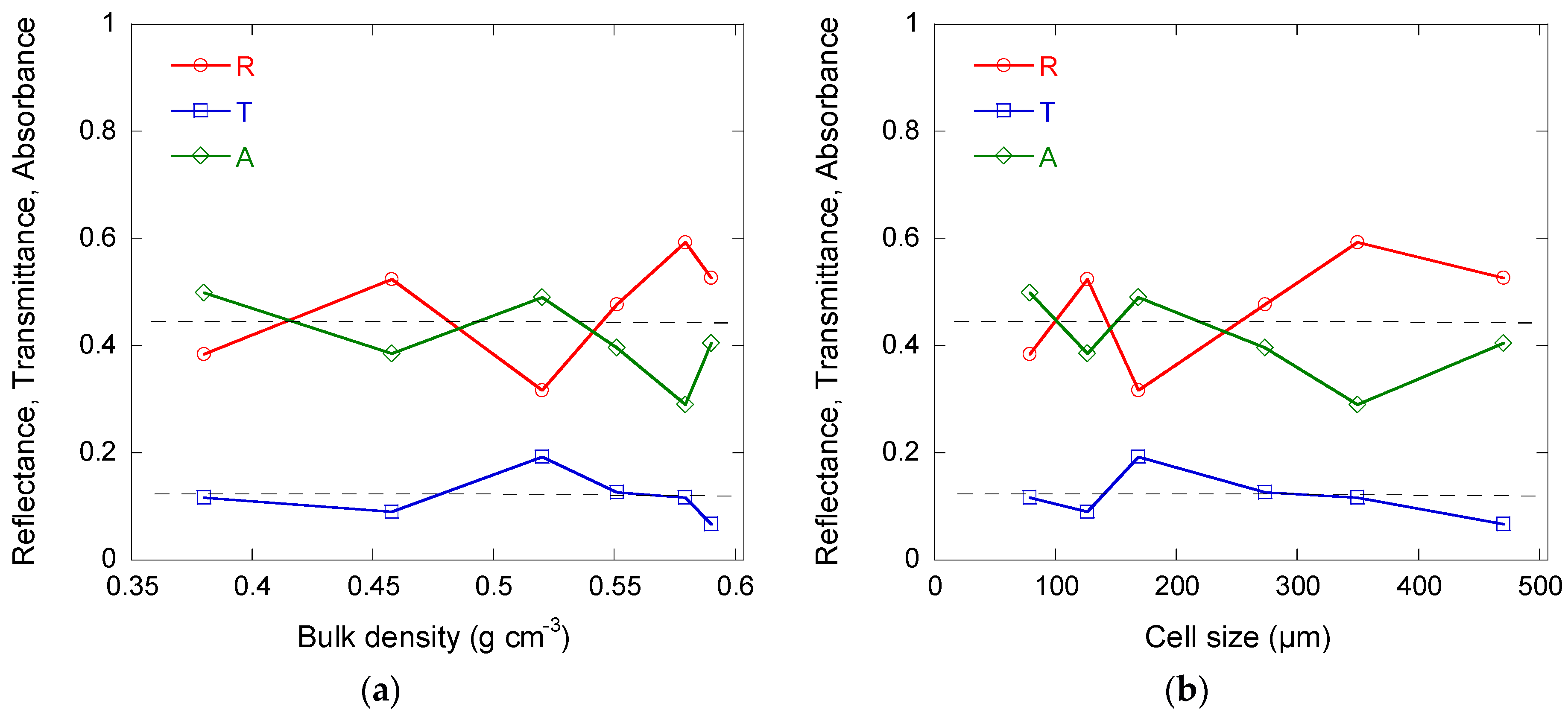
3.4. Electromagnetic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Kennel, E.B.; Stiller, A.H.; Stansberry, P.G.; Zondlo, J.W. Carbon foam derived from various precursors. Carbon 2006, 44, 1535–1543. [Google Scholar] [CrossRef]
- Spradling, D.M.; Guth, R.A. Carbon foams. Adv. Mater. Porcess. 2003, 161, 29–31. [Google Scholar]
- Li, X.; Basso, M.C.; Braghiroli, F.L.; Fierro, V.; Pizzi, A.; Celzard, A. Tailoring the structure of cellular vitreous carbon foams. Carbon 2012, 50, 2026–2036. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Gourdon, E.; Fierro, V.; Pizzi, A.; Celzard, A. Acoustic properties of cellular vitreous carbon foams. Carbon 2013, 58, 76–86. [Google Scholar] [CrossRef]
- Hajiesmaili, S.; Josset, S.; Begin, D.; Pham-Huu, C.; Keller, N.; Keller, V. 3D solid carbon foam-based photocatalytic materials for vapor phase flow-through structured photoreactors. Appl. Catal. A Gen. 2010, 382, 122–130. [Google Scholar] [CrossRef]
- Friedrich, J.M.; Ponce-De-Leon, C.; Reade, G.W.; Walsh, F.C. Reticulated vitreous carbon as an electrode material. J. Electroanal. Chem. 2004, 561, 203–217. [Google Scholar] [CrossRef]
- Lepage, G.; Albernaz, F.O.; Perrier, G.; Merlin, G. Characterization of a microbial fuel cell with reticulated carbon foam electrodes. Bioresour. Technol. 2012, 124, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Celzard, A.; Zhao, W.; Pizzi, A.; Fierro, V. Mechanical properties of tannin-based rigid foams undergoing compression. Mater. Sci. Eng. A Struct. 2010, 527, 4438–4446. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Pizzi, A.; Du, G.; Celzard, A. Effect of composition and processing parameters on the characteristics of tannin-based rigid foams. Part II: Physical properties. Mater. Chem. Phys. 2010, 123, 210–217. [Google Scholar] [CrossRef]
- Moglie, F.; Micheli, D.; Laurenzi, S.; Marchetti, M.; Primiani, V.M. Electromagnetic shielding performance of carbon foams. Carbon 2012, 50, 1972–1980. [Google Scholar] [CrossRef]
- Celzard, A.; Tondi, G.; Lacroix, D.; Jeandel, G.; Monod, B.; Fierro, V.; Pizzi, A. Radiative properties of tannin-based, glasslike, carbon foams. Carbon 2012, 50, 4102–4113. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Stauber, M.; Celzard, A. Carbon meringues derived from flavonoid tannins. Carbon 2013, 65, 214–227. [Google Scholar] [CrossRef]
- Lacoste, C.; Pizzi, A.; Basso, M.C.; Laborie, M.P.; Celzard, A. Pinus pinaster tannin/furanic foams: Part I. Formulation. Ind. Crop Prod. 2014, 52, 450–456. [Google Scholar] [CrossRef]
- Tondi, G.; Fierro, V.; Pizzi, A.; Celzard, A. Tannin-based carbon foams. Carbon 2009, 47, 1480–1492. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Shi, X.C.; Xu, H.; Hu, Y.J.; Yuan, Y.; Shen, N.B. Preparation of pitch-based carbon foam using polyurethane foam template. Carbon 2007, 45, 2132–2134. [Google Scholar] [CrossRef]
- Jana, P.; Fierro, V.; Celzard, A. Ultralow cost reticulated carbon foams from household cleaning pad wastes. Carbon 2013, 62, 517–520. [Google Scholar] [CrossRef]
- Seo, J.; Park, H.; Shin, K.; Baeck, S.H.; Rhym, Y.; Shim, S.E. Lignin-derived macroporous carbon foams prepared by using poly(methyl methacrylate) particles as the template. Carbon 2014, 76, 357–367. [Google Scholar] [CrossRef]
- Cameron, N.R.; Sherrington, D.C. High internal phase emulsions (HIPEs)—Structure, properties and use in polymer preparation. In Biopolymers Liquid Crystalline Polymers Phase Emulsion; Springer: Berlin/Heidelberg, Germany, 1996; Volume 126, pp. 163–214. ISBN 9783540604846. [Google Scholar]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Silverstein, M.S. Emulsion-templated porous polymers: A retrospective perspective. Polymer 2014, 55, 304–320. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Celzard, A. Mayonnaise, whipped cream and meringue, a new carbon cuisine. Carbon 2013, 58, 245–248. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Effect of synthesis pH on the structure of carbon xerogels. Carbon 1997, 35, 1271–1278. [Google Scholar] [CrossRef]
- Moreno, A.H.; Arenillas, A.; Calvo, E.G.; Bermudez, J.M.; Menendez, J.A. Carbonisation of resorcinol-formaldehyde organic xerogels: Effect of temperature, particle size and heating rate on the porosity of carbon xerogels. J. Anal. Appl. Pyrol. 2013, 100, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Rey-Raap, N.; Angel Menéndez, J.; Arenillas, A. Simultaneous adjustment of the main chemical variables to fine-tune the porosity of carbon xerogels. Carbon 2014, 78, 490–499. [Google Scholar] [CrossRef]
- Bychanok, D.; Plyushch, A.; Piasotski, K.; Paddubskaya, A.; Varanovich, S.; Kuzhir, P.; Baturkin, S.; Klochkov, A.; Korovin, E.; Letellier, M.; et al. Electromagnetic properties of polyurethane template-based carbon foams in Ka-band. Phys. Scripta 2015, 90, 094019. [Google Scholar] [CrossRef]
- Szczurek, A.; Ortona, A.; Ferrari, L.; Rezaei, E.; Medjahdi, G.; Fierro, V.; Bychanok, D.; Kuzhir, P.; Celzard, A. Carbon periodic cellular architectures. Carbon 2015, 88, 70–85. [Google Scholar] [CrossRef]
- Bychanok, D.; Plyushch, A.; Kuzhir, P.; Macutkevic, J.; Letellier, M.; Szczurek, A.; Fierro, V.; Celzard, A. Tannin-based carbon foams in microwave frequency range: Toward fully carbon photonic crystal. In Proceedings of the IEEE 2015 Conference on Microwaves, Communications, Antennas and Electronic Systems (COMCAS), Tel Aviv, Israel, 2–4 November 2015. [Google Scholar]
- Ma, P.X.; Choi, J.W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. Fundamentals of theory of adsorption in micropores of carbon adsorbents—Characteristics of their adsorption properties and microporous structures. Carbon 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Jagiello, J.; Ania, C.; Parra, J.B.; Cook, C. Dual gas analysis of microporous carbons using 2D-NLDFT heterogeneous surface model and combined adsorption data of N2 and CO2. Carbon 2015, 91, 330–337. [Google Scholar] [CrossRef]
- Webster, J.G. (Ed.) Electrical Measurement, Signal Processing, and Displays; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780849317330. [Google Scholar]
- Chung, B.K. Dielectric constant measurement for thin material at microwave frequencies. Prog. Electromagn. Res. 2007, 75, 239–252. [Google Scholar] [CrossRef]
- Nielsen, E. Scattering by a cylindrical post of complex permittivity in a waveguide. IEEE Trans. Microw. Theory Tech. 1969, 17, 148–153. [Google Scholar] [CrossRef]
- Zhao, W.; Pizzi, A.; Fierro, V.; Du, G.; Celzard, A. Effect of composition and processing parameters on the characteristics of tannin—Based rigid foams. Part I: Cell structure. Mater. Chem. Phys. 2010, 122, 175–182. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Fierro, V.; Szczurek, A.; Kuznetsov, B.N.; Celzard, A. Biosourced, highly porous, carbon xerogel microspheres. RSC Adv. 2016, 6, 65698–65708. [Google Scholar] [CrossRef]
- Meyer, K.; Klobes, P. Comparison between different presentations of pore size distribution in porous materials. Fresenius J. Anal. Chem. 1999, 363, 174–178. [Google Scholar] [CrossRef]
- Letellier, M.; Ghaffari Mosanenzadeh, S.; Naguib, H.; Fierro, V.; Celzard, A. Acoustic properties of model cellular vitreous carbon foams. Carbon 2017, 119, 241–250. [Google Scholar] [CrossRef]
- Letellier, M.; Szczurek, A.; Basso, M.C.; Pizzi, A.; Fierro, V.; Ferry, O.; Celzard, A. Preparation and structural characterisation of model cellular vitreous carbon foams. Carbon 2017, 112, 208–218. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; ISBN 9781316025420. [Google Scholar]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Celzard, A. Emulsion-templated porous carbon monoliths derived from tannins. Carbon 2014, 74, 352–362. [Google Scholar] [CrossRef]
- Bock, V.; Emmerling, A.; Saliger, R.; Fricke, J. Structural investigation of resorcinol formaldehyde and carbon aerogels using SAXS and BET. J. Porous Mater. 1997, 4, 287–294. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Influence of freeze-drying conditions on the mesoporosity of organic gels as carbon precursors. Carbon 2000, 38, 1099–1105. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Mikami, M.; Okazaki, M. Porous structure of organic and carbon aerogels synthesized by sol–gel polycondensation of resorcinol with formaldehyde. Carbon 1997, 35, 791–796. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. Porosity of resorcinol-formaldehyde organic and carbon aerogels exchanged and dried with supercritical organic solvents. Mater. Chem. Phys. 2011, 129, 1221–1232. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. The use of tannin for preparing carbon gels. Part I. Carbon aerogels. Carbon 2011, 49, 2773–2784. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Celzard, A. The use of tannin for preparing carbon gels. Part II. Carbon cryogels. Carbon 2011, 49, 2785–2794. [Google Scholar] [CrossRef]
- Job, N.; Théry, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.N.; Beguin, F.; Pirard, J.P. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2494. [Google Scholar] [CrossRef]
- Letellier, M.; Macutkevic, J.; Paddubskaya, A.; Plyushch, A.; Kuzhir, P.; Ivanov, M.; Banys, J.; Pizzi, A.; Fierro, V.; Celzard, A. Tannin-Based Carbon Foams for Electromagnetic Applications. IEEE Trans. Electromagn. C 2015, 57, 989–995. [Google Scholar] [CrossRef]
- Cuevas, F.G.; Montes, J.M.; Cintas, J.; Urban, P. Electrical conductivity and porosity relationship in metal foams. J. Porous Mater. 2009, 16, 675–681. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.G.; Gibson, L.J.; Hutchinson, J.W.; Fleck, N.A.; Wadley, H.G.N. Cellular Metals: A Design Guide; Butterworth-Heinemann: Waltham, MA, USA, 2000; p. 192. ISBN 9780080511467. [Google Scholar]
- Letellier, M.; Macutkevic, J.; Kuzhir, P.; Banys, J.; Fierro, V.; Celzard, A. Electromagnetic properties of model vitreous carbon foams. Carbon 2017, 122, 217–227. [Google Scholar] [CrossRef]
- Kuzhir, P.P.; Letellier, M.; Bychanok, D.S.; Paddubskaya, O.G.; Suslyaev, V.I.; Korovin, E.Y.; Baturkin, S.A.; Fierro, V.; Celzard, A. Electrical Properties of Carbon Foam in the Microwave Range. Russ. Phys. J. 2017, 59, 1703–1709. [Google Scholar] [CrossRef]





| Sample | C (wt %) | H (wt %) | O (wt %) | N (wt %) | S (wt %) |
|---|---|---|---|---|---|
| CRF350 | 94.87 | 0.55 | 3.81 | 0.96 | 0.03 |
| Sample | Bulk Density (g cm−3) | Overall Porosity (%) | Open Porosity (%) | Total Specific Pore Volume (cm3 g−1) |
|---|---|---|---|---|
| CRF150 | 0.38 | 83.1 | 89.8 | 2.19 |
| CRF250 | 0.46 | 79.7 | 89.3 | 1.74 |
| CRF350 | 0.52 | 76.9 | 89.3 | 1.48 |
| CRF450 | 0.54 | 76.0 | 90.7 | 1.41 |
| CRF550 | 0.55 | 75.5 | 88.4 | 1.37 |
| CRF700 | 0.58 | 74.3 | 88.9 | 1.28 |
| CRF900 | 0.59 | 73.8 | 88.0 | 1.25 |
| Sample | CRF150 | CRF550 | |
|---|---|---|---|
| N2 adsorption | ABET (m2 g−1) | 482 | 663 |
| VDR,N2 (cm3 g−1) | 0.20 | 0.25 | |
| V0.97 (cm3 g−1) | 0.19 | 0.30 | |
| CO2 adsorption | VDR,CO2 (cm3 g−1) | 0.29 | 0.29 |
| SNLDFT (m2 g−1) | 844 | 976 | |
| Vuµ,NLDFT (cm3 g−1) | 0.199 (93%) | 0.199 (64%) | |
| Vµ,NLDFT (cm3 g−1) | 0.215 (100%) | 0.274 (88%) | |
| VNLDFT (cm3 g−1) | 0.215 | 0.312 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczurek, A.; Fierro, V.; Plyushch, A.; Macutkevic, J.; Kuzhir, P.; Celzard, A. Structure and Electromagnetic Properties of Cellular Glassy Carbon Monoliths with Controlled Cell Size. Materials 2018, 11, 709. https://doi.org/10.3390/ma11050709
Szczurek A, Fierro V, Plyushch A, Macutkevic J, Kuzhir P, Celzard A. Structure and Electromagnetic Properties of Cellular Glassy Carbon Monoliths with Controlled Cell Size. Materials. 2018; 11(5):709. https://doi.org/10.3390/ma11050709
Chicago/Turabian StyleSzczurek, Andrzej, Vanessa Fierro, Artyom Plyushch, Jan Macutkevic, Polina Kuzhir, and Alain Celzard. 2018. "Structure and Electromagnetic Properties of Cellular Glassy Carbon Monoliths with Controlled Cell Size" Materials 11, no. 5: 709. https://doi.org/10.3390/ma11050709






