The Effect of Different Porogens on Porous PMMA Microspheres by Seed Swelling Polymerization and Its Application in High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the polymethyl methacrylate seed particles
2.3. Preparation of Monodispersed Porous PMMA Microspheres
2.4. Characterization
2.5. Chromatography
3. Results and Discussion
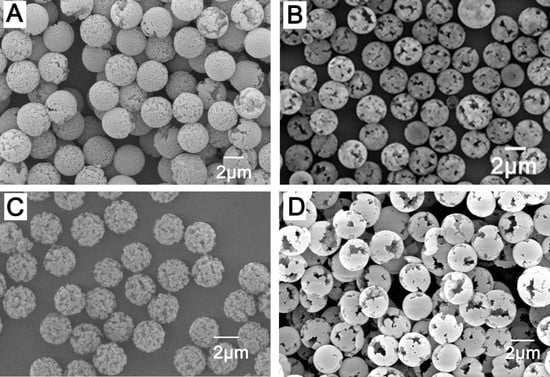
3.1. Surface Morphology of Monodispersed PMMA Seed Microspheres
3.2. Effect of porogen systems on the preparation of monodispersed porous microspheres
3.3. Application of Liquid Chromatography of Monodispersed PMMA Porous Microspheres
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, B.; Tian, C.; Cong, H.; Xu, T. Synthesis of monodisperse poly (styrene-co-divinylbenzene) microspheres with binary porous structures and application in high-performance liquid chromatography. J. Mater. Sci. 2016, 51, 5240–5251. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Zhu, R.; Xiao, L.; Yao, S. Highly sensitive analysis of polycyclic aromatic hydrocarbons in environmental water with porous cellulose/zeolitic imidazolate framework-8 composite microspheres as a novel adsorbent coupled with high-performance liquid chromatography. J. Sep. Sci. 2016, 39, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Naing, N.N.; Li, S.F.Y.; Lee, H.K. Application of porous membrane-protected chitosan microspheres to determine benzene, toluene, ethylbenzene, xylenes and styrene in water. J. Chromatogr. A 2016, 1448, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Y.; Zhang, H.; Zhou, W.; Ma, G. A novel strategy for the preparation of porous microspheres and its application in peptide drug loading. J. Colloid. Interf. Sci. 2016, 478, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.H.; Sone, H.; Omi, S. Preparation of uniform-sized polystyrene-polyacrylamide composite microspheres from a W/O/W emulsion by membrane emulsification technique and subsequent suspension polymerization. Macromolecules 2004, 37, 2954–2964. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Guan, Y.P.; Liu, X.Q.; Liu, H.Z. Preparation and characterization of micron-sized non-porous magnetic polymer microspheres with immobilized metal affinity ligands by modified suspension polymerization. J. Appl. Polym. Sci. 2005, 96, 2174–2180. [Google Scholar] [CrossRef]
- Bamnolker, H.; Margel, S. Dispersion polymerization of styrene in polar solvents: effect of reaction parameters on microsphere surface composition and surface properties, size and size distribution, and molecular weight. J. Polym. Sci. Pol. Chem. 1996, 34, 1857–1871. [Google Scholar] [CrossRef]
- Chao, C.; Zhang, B.; Zhai, R.; Xiang, X.; Liu, J.; Chen, R. Natural nanotube-based biomimetic porous microspheres for significantly enhanced biomolecule immobilization. Acs. Sustain. Chem. Eng. 2013, 2, 396–403. [Google Scholar] [CrossRef]
- Seitz, W.R.; Rooney, M.T.; Miele, E.W.; Wang, H.; Kaval, N.; Zhang, L.; Doherty, S.; Milde, S.; Lenda, J. Derivatized, swellable polymer microspheres for chemical transduction. Anal. Chim. Acta. 1999, 400, 55–64. [Google Scholar] [CrossRef]
- Pich, A.; Bhattacharya, S.; Ghosh, A.; Adler, H.J. Composite magnetic particles: Encapsulation of iron oxide by surfactant-free emulsion polymerization. Polymer 2005, 46, 4596–4603. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Wang, T. Preparation of superparamagnetic polyaniline hybrid hollow microspheres in oil/water emulsion with magnetic nanoparticles as cosurfactant. Chem. Eng. J. 2011, 171, 711–716. [Google Scholar] [CrossRef]
- Shibuya, K.; Nagao, D.; Ishii, H.; Konno, M. Advanced soap-free emulsion polymerization for highly pure, micron-sized, monodisperse polymer particles. Polymer 2014, 55, 535–539. [Google Scholar] [CrossRef]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: a versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv, 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Wang, J.; Cormack, P.A.; Sherrington, D.C.; Khoshdel, E. Monodisperse, molecularly imprinted polymer microspheres prepared by precipitation polymerization for affinity separation applications. Angew. Chem. Int. Edit. 2003, 42, 5336–5338. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, B.; Fu, X.; Zhou, M.; Liu, W.; Bian, G.; Qi, Y.; Yang, X. Synthesis of poly (methacrylic acid)–manganese oxide dihydroxide/silica core–shell and the corresponding hollow microspheres. J. Colloid. Interf. Sci. 2015, 438, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cao, L.; Hanley, T.L.; Caruso, R.A. Facile synthesis of monodisperse mesoporous zirconium titanium oxide microspheres with varying compositions and high surface areas for heavy metal ion sequestration. Adv. Funct. Mater. 2012, 22, 1966–1971. [Google Scholar] [CrossRef]
- Okubo, M.; Ito, A.; Hashiba, A. Production of submicron-sized multihollow polymer particles having high transition temperatures by the stepwise alkali/acid method. Colloid. Polym. Sci., 1996, 274, 428–432. [Google Scholar] [CrossRef]
- Okubo, M.; Mori, H.; Ito, A. Effect of nonionic emulsifier on the production of multihollow polymer particles by the stepwise acid/alkali method. Colloid. Polym. Sci. 2000, 278, 358–363. [Google Scholar] [CrossRef]
- Yu, B.; Xu, T.; Cong, H.; Peng, Q.; Usman, M. Preparation of Porous Poly(Styrene-Divinylbenzene) Microspheres and Their Modification with Diazoresin for Mix-Mode HPLC Separations. Materials 2017, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.J.; Ogawa, S.; Bradley, M. Tuning the pore size and surface area of monodisperse poly (methyl acrylate) beads via parallel seeded polymerisation. Polymer 2005, 46, 2880–2888. [Google Scholar] [CrossRef]
- Unsal, E.; Tolga Çamli, S.; Teksen, T.; Tuncel, M.; Tuncel, A. Hydroxyl functionalized uniform-porous beads, synthesis and chromatographic use. J. Macromol. Sci. 2005, 42, 607–621. [Google Scholar] [CrossRef]
- Ou, J.L.; Wu, M.H.; Chen, H. Swelling of oligomeric polystyrene seed particles to prepare porous microspheres using n-hexane as porogen. J. Mater. Sci. Lett. 2001, 20, 2221–2223. [Google Scholar] [CrossRef]
- Hosoya, K.; Fréchet, J.M. Influence of the seed polymer on the chromatographic properties of size monodisperse polymeric separation media prepared by a multi-step swelling and polymerization method. J. Polym. Sci. Pol. Chem. 1993, 31, 2129–2141. [Google Scholar] [CrossRef]
- Ma, G.H.; Nagai, M.; Omi, S. Synthesis of uniform microspheres with higher content of 2-hydroxyethyl methacrylate by employing SPG (Shirasu porous glass) emulsification technique followed by swelling process of droplets. J. Appl. Polym. Sci. 1997, 66, 1325–1341. [Google Scholar] [CrossRef]
- Huang, F.C.; Ke, C.H.; Kao, C.Y.; Lee, W.C. Preparation and application of partially porous poly (styrene-divinylbenzene) particles for lipase immobilization. J. Appl. Polym. Sci. 2001, 80, 39–46. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Senkal, F.B.; Celik, G.; Arica, M.Y. Preparation and characterization of sulfonyl-hydrazine attached poly(styrene-divinylbenzene) beads for separation of albumin. Colloids Surf. A 2007, 294, 56–63. [Google Scholar] [CrossRef]
- Yue, G.; Luo, Q.; Zhang, J.; Wu, S.L.; Karger, B.L. Ultratrace LC/MS proteomic analysis using 10-microm-i.d. porous layer open tubular poly(styrene-divinylbenzene) capillary columns. Anal. Chem. 2007, 79, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Ohta, H.; Yoshizako, K.; Kimata, K.; Ikegami, T.; Tanaka, N. Preparation of uniformly sized polymeric separation media potentially suitable for small-scale high-performance liquid chromatography and/or capillary electrochromatography. J. Chromatogra. A 1999, 853, 11–20. [Google Scholar] [CrossRef]
- Fujibayashi, T.; Komatsu, Y.; Konishi, N.; Yamori, H.; Okubo, M. Effect of polymer polarity on the shape of “golf ball-like” particles prepared by seeded dispersion polymerization. Ind. Eng. Chem. Res. 2008, 47, 6445–6449. [Google Scholar] [CrossRef]
- Kobayashi, H.; Miyanaga, E.; Okubo, M. Preparation of multihollow polymer particles by seeded emulsion polymerization using seed particles with incorporated nonionic emulsifier. Langmuir 2007, 23, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yang, L.; Wang, M.; Wang, H.; Ge, X.; Ge, X. The mechanism of the formation of multihollow polymer spheres through sulfonated polystyrene particles. Langmuir 2009, 25, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Micale, F.J.; Vanderhoff, J.W.; EI-Aasser, M.S. Synthesis and characterization of monodisperse porous polymer particles. J. Polym. Sci. Part A: Polym. Chem. 1992, 30, 235–244. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yanagida, M.; Hatada, K. High-Performance Liquid Chromatography Using Stereoregular Poly(methyl methacrylate)s as Stationary Phases. Polym. J. 1989, 21, 795–801. [Google Scholar] [CrossRef]
- Tantos, A.; Han, K.H.; Tompa, P. Intrinsic disorder in cell signaling and gene transcription. Mol. Cell. Endocrinol. 2012, 348, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.G.; Jin, A.J.; Ma, K.; Gutierrez-Cruz, G.; Tsai, W.L.; Wang, K. Titin PEVK segment: charge-driven elasticity of the open and flexible polyampholyte. J. Muscle Res. Cell Motil. 2005, 26, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Natively unfolded proteins: a point where biology waits for physics. Protein Sci. 2002, 11, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Lozanov, V.; Petrov, S.; Mitev, V. Simultaneous analysis of amino acid and biogenic polyamines by high-performance liquid chromatography after pre-column derivatization with N-(9-fluorenylmethoxycarbonyloxy) succinimide. J. Chromatogr. A 2004, 1025, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ugelstad, J.; Mfutakamba, H.R.; Mørk, P.C.; Ellingsen, T.; Berge, A.; Schmid, R.; Nustad, K.C. Preparation and application of monodisperse polymer particles. J. Polym. Sci. Polym. Symp. 1985, 72, 225–240. [Google Scholar] [CrossRef]
- Christensen, B.E.; Myhr, M.H.; Aune, O.; Hagen, S.; Berge, A.; Ugelstad, J. Macroporous, monodisperse particles and their application in aqueous size exclusion chromatography of high molecular weight polysaccharides. Carbohyd. Polym. 1996, 29, 217–223. [Google Scholar] [CrossRef]







| Retention time RSD (%) | ||||
|---|---|---|---|---|
| Amino acid | Run to run (n = 5) | Day to day (n = 7) | Column to Column (n = 5) | Continuous 200 times running |
| Argnine | 0.96 | 1.82 | 2.56 | 2.05 |
| Glycine | 0.89 | 1.78 | 2.31 | 1.93 |
| Glutamic acid | 0.93 | 1.90 | 2.48 | 2.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Xue, T.; Pang, L.; Zhang, X.; Shen, Y.; Cong, H. The Effect of Different Porogens on Porous PMMA Microspheres by Seed Swelling Polymerization and Its Application in High-Performance Liquid Chromatography. Materials 2018, 11, 705. https://doi.org/10.3390/ma11050705
Yu B, Xue T, Pang L, Zhang X, Shen Y, Cong H. The Effect of Different Porogens on Porous PMMA Microspheres by Seed Swelling Polymerization and Its Application in High-Performance Liquid Chromatography. Materials. 2018; 11(5):705. https://doi.org/10.3390/ma11050705
Chicago/Turabian StyleYu, Bing, Tingting Xue, Long Pang, Xiulan Zhang, Youqing Shen, and Hailin Cong. 2018. "The Effect of Different Porogens on Porous PMMA Microspheres by Seed Swelling Polymerization and Its Application in High-Performance Liquid Chromatography" Materials 11, no. 5: 705. https://doi.org/10.3390/ma11050705