Study on Flake Formation Behavior and Its Influence Factors in Cr5 Steel
Abstract
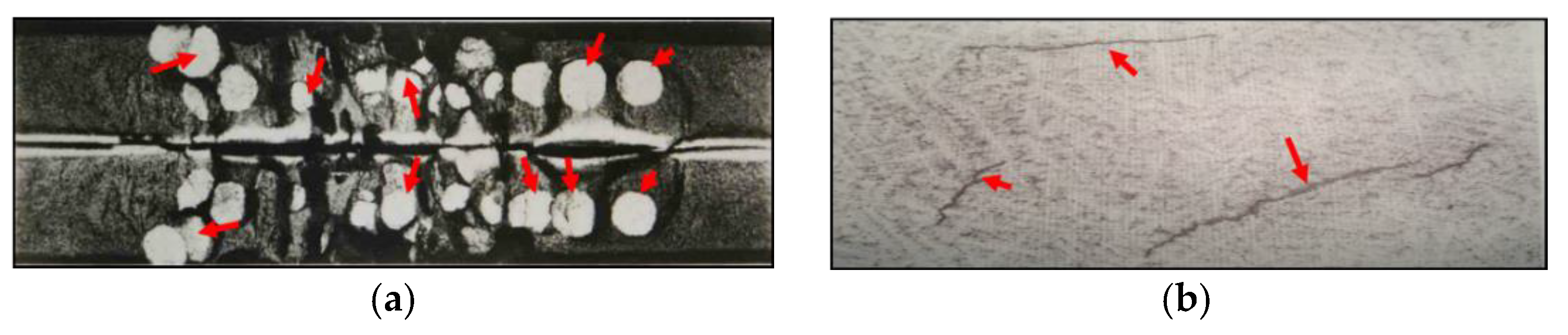
:1. Introduction
2. Quantitative Model of the Three Mechanisms
2.1. Hydrogen Pressure in Trap
2.2. Hydrogen Re-Distribution by Hydrostatic Stress
2.3. Hydrogen Enhanced De-Cohesion (HEDE)
3. Finite Element Analysis Model
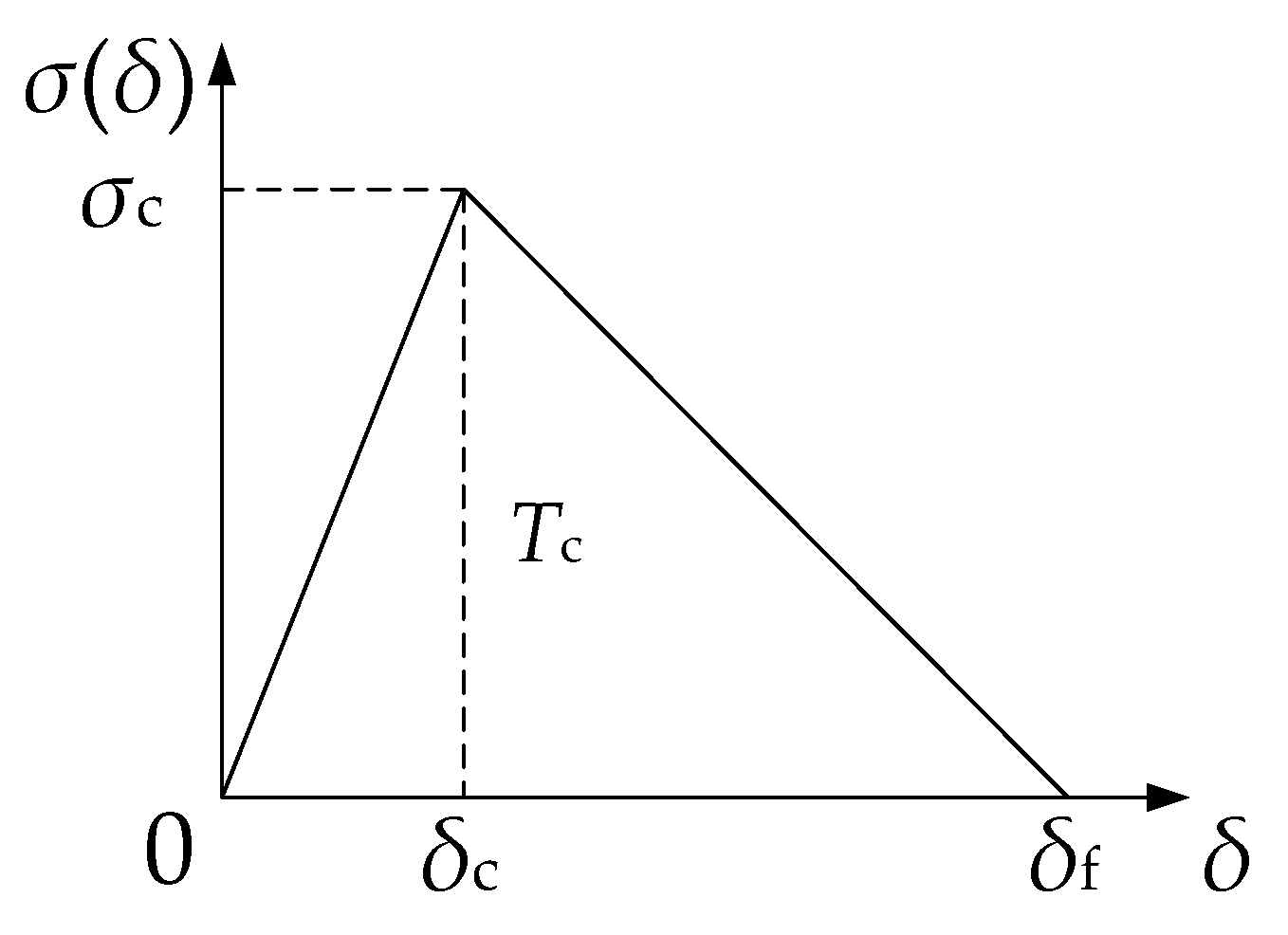
3.1. Traction Separation Law in Cohesive Method
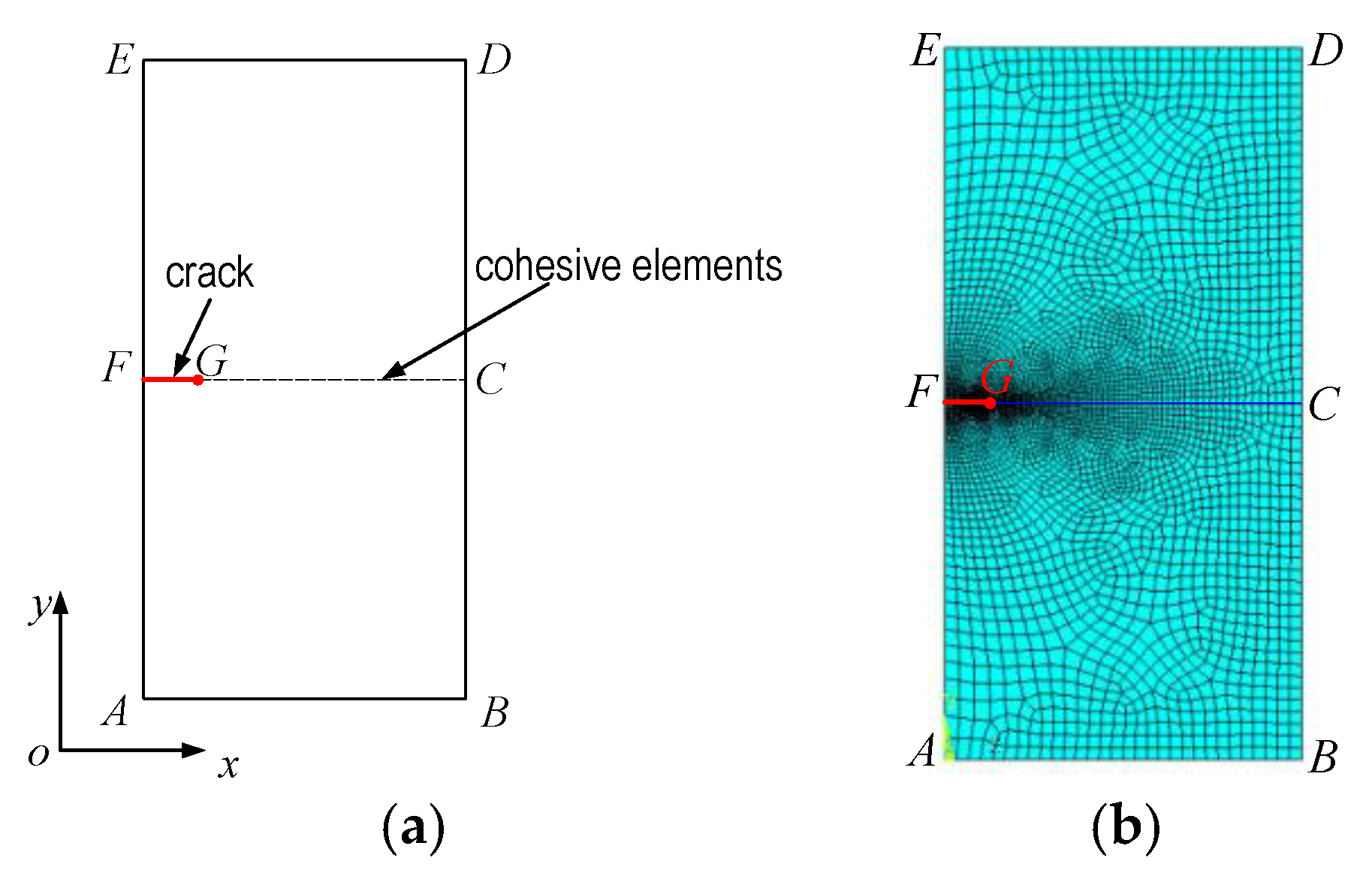
3.2. Finite Element Model
4. Results
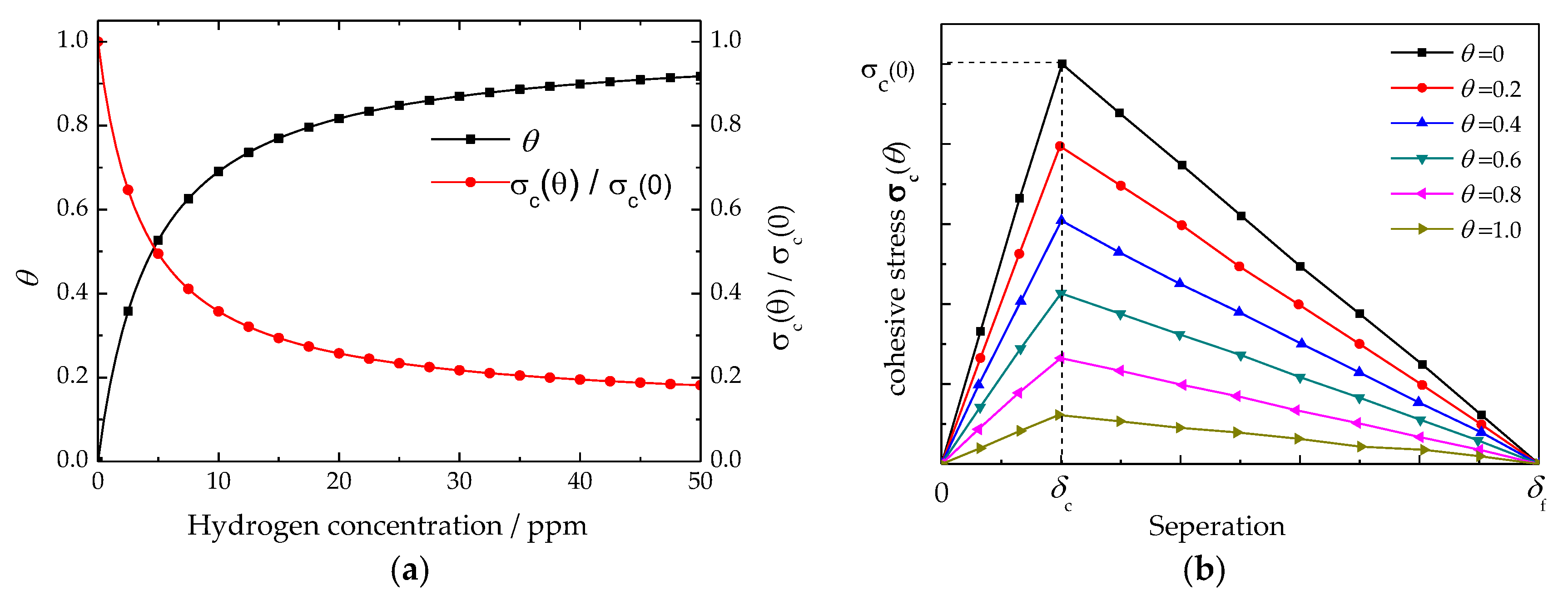
4.1. Influence of Hydrogen on Cohesive Model
4.2. Trap Hydrogen Pressure vs. Hydrogen Content
4.3. Stress Induced Hydrogen Re-Distribution around Crack Tip
4.4. Steel Cohesive Strength and Tensile Stress around Crack Tip
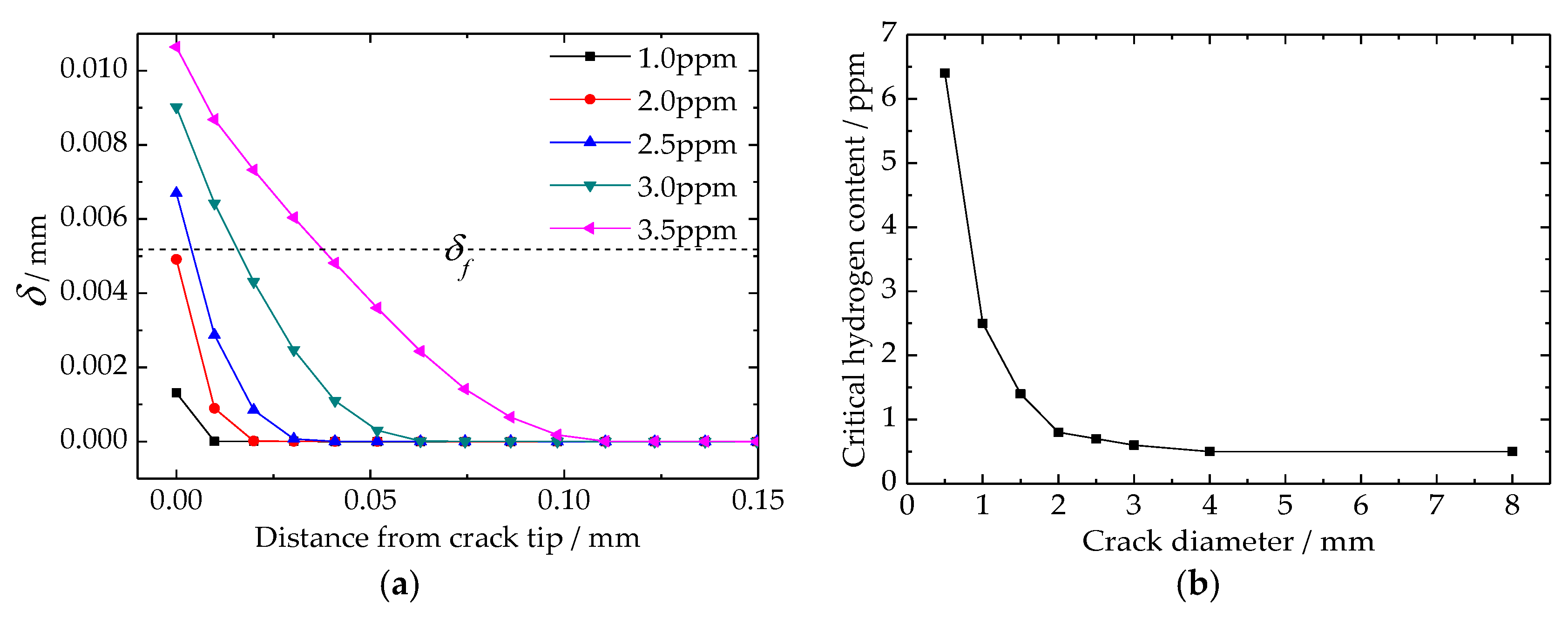
4.5. Critical Hydrogen Content of Flake Formation
5. Discussion
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Voronenko, B.I. Hydrogen and flakes in steel. Met. Sci. Heat Treat. 1997, 39, 462–470. [Google Scholar] [CrossRef]
- Zapffe, C.A.; Sims, C.E. Hydrogen embrittlement, internal stress and defects in steel. Trans. Am. Inst. Min. Met. Petrol. Eng. 1941, 145, 225–261. [Google Scholar]
- Juodkazis, K.; Juodkazytė, J.; Grigucevičienė, A.; Juodkazisb, S. Hydrogen species within the metals: Role of molecular hydrogen ion H2+. Appl. Surf Sci. 2011, 258, 743–747. [Google Scholar] [CrossRef]
- Juodkazytė, J.; Šebeka, B.; Juodkazis, S. Reversible hydrogen evolution and oxidation on Pt electrode mediated by molecular ion. Appl. Surf. Sci. 2014, 290, 13–17. [Google Scholar] [CrossRef]
- Phragmen, G. On the relation between the hydrogen proportion in iron, the temperature and the hydrogen equilibrium pressure. Jemkontorets Ann. 1944, 128, 537–553. [Google Scholar]
- Kazinczy, F.D. A theory of hydrogen embrittlement. J. Iron Steel Inst. 1954, 177, 85–92. [Google Scholar]
- Kazinczy, F.D. On the pressure of hydrogen in cavities of steel. Acta Met. 1959, 7, 525–527. [Google Scholar] [CrossRef]
- Allen-Booth, D.M.; Hewitt, J. A mathematical model describing the effects of micro voids upon the diffusion of hydrogen in iron and steel. Acta Met. 1974, 22, 171–175. [Google Scholar] [CrossRef]
- Lange, G.; Hofman, W. Relation between hydrogen uptake and porosity in iron. Arch. Eisenhu. 1966, 37, 391–397. [Google Scholar]
- Fan, J.K.; Du, F.S.; Huang, H.G. Hydrogen Pressure and Concentration Calculation Models for Cavities in Steel. ICIC Express Lett. 2013, 7, 2741–2746. [Google Scholar]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen embrittlement understood. Met. Mater. Trans. A 2015, 46, 2323–2341. [Google Scholar] [CrossRef]
- Robertson, I.M.; Birnbaum, H.K.; Sofronis, P. Hydrogen effects on plasticity. Dislocat. Solids 2009, 15, 249–293. [Google Scholar]
- Robertson, I.M.; Birnbaum, H.K. An HVEM study of hydrogen effects on the deformation and fracture of nickel. Acta Met. 1986, 34, 353–366. [Google Scholar] [CrossRef]
- Tabata, T.; Birnbaum, H.K. Direct observation of hydrogen enhanced crack propagation in iron. Scr. Metall. 1984, 18, 231–236. [Google Scholar] [CrossRef]
- Petch, N.J. The lowering of fracture-stress due to surface adsorption. Philos. Mag. 1956, 1, 331–337. [Google Scholar] [CrossRef]
- Tromans, D. On surface energy and the hydrogen embrittlement of iron and steels. Acta Met. Mater. 1994, 42, 2043–2049. [Google Scholar] [CrossRef]
- Troiano, A.R. The role of hydrogen and other interstitials in the mechanical behavior of metals. Trans. ASM 1960, 52, 54–80. [Google Scholar] [CrossRef]
- Oriani, R.A. A mechanistic theory of hydrogen embrittlement of steels. Ber. Bunsenges. Phys. Chem. 1972, 76, 848–857. [Google Scholar]
- John, C.S; Gerberich, W.W. The effect of loading mode on hydrogen embrittlement. Met. Trans. 1973, 4, 589–594. [Google Scholar] [CrossRef]
- Oriani, R.A. Whitney Award Lecture—1987: Hydrogen—The versatile embrittler. Corrosion 1987, 43, 390–397. [Google Scholar] [CrossRef]
- Scheider, I.; Pfuff, M.; Dietzel, W. Simulation of hydrogen assisted stress corrosion cracking using the cohesive method. Eng. Fract. Mech. 2003, 75, 4283–4291. [Google Scholar] [CrossRef]
- Olden, V.; Thaulow, C.; Johnsen, R.; Østbyb, E.; Olden, V. Cohesive zone modeling of hydrogen-induced stress cracking in 25% Cr duplex stainless steel. Scr. Mater. 2007, 57, 615–618. [Google Scholar] [CrossRef]
- Olden, V.; Thaulow, C.; Johnsen, R.; Østbyb, E.; Berstadb, T. Application of hydrogen influenced cohesive laws in the prediction of hydrogen induced stress cracking in 25% Cr duplex stainless steel. Eng. Fract. Mech. 2008, 15, 2333–2351. [Google Scholar] [CrossRef]
- Olden, V.; Thaulow, C.; Johnsen, R. Modelling of hydrogen diffusion and hydrogen induced cracking in supermartensitic and duplex stainless steels. Mater. Des. 2008, 29, 1934–1948. [Google Scholar] [CrossRef]
- Olden, V.; Alvaro, A.; Akselsen, O.M. Hydrogen diffusion and hydrogen influenced critical stress intensity in an API X70 pipeline steel welded joint—Experiments and FE simulations. Int. J. Hydrog. Energy 2012, 37, 11474–11486. [Google Scholar] [CrossRef]
- Olden, V.; Alvaro, A.; Akselsen, O.M. 3D cohesive modelling of hydrogen embrittlement in the heat affected zone of an X70 pipeline steel. Int. J. Hydrog. Energy 2013, 38, 7539–7549. [Google Scholar] [CrossRef]
- Olden, V.; Alvaro, A.; Akselsen, O.M. 3D cohesive modelling of hydrogen embrittlement in the heat affected zone of an X70 pipeline steel-Part II. Int. J. Hydrog. Energy 2014, 39, 3528–3541. [Google Scholar] [CrossRef]
- Raykar, N.R.; Maiti, S.K.; Singh Raman, R.K. Modelling of Mode-I stable crack growth under hydrogen assisted stress corrosion cracking. Eng. Fract. Mech. 2011, 78, 3135–3165. [Google Scholar] [CrossRef]
- Brocks, W.; Falkenberg, R.; Scheider, I. Coupling aspects in the simulation of hydrogen induced stress corrosion cracking. Procedia IUTAM 2012, 3, 11–24. [Google Scholar] [CrossRef]
- Yonezu, A.; Hara, T.; Kondo, T.; Hirakata, H.; Minoshima, K. Evaluation of threshold stress intensity factor of hydrogen embrittlement cracking by indentation testing. Mater. Sci. Eng. A 2012, 531, 147–154. [Google Scholar] [CrossRef]
- Charles, Y.; Gasperini, M.; Disashi, J.; Jouinot, P. Numerical modeling of the Disk Pressure Test up to failure under gaseous hydrogen. J. Mater. Process. Technol. 2012, 12, 1761–1770. [Google Scholar] [CrossRef]
- Ben Ali, N.; Estevez, R.; Tanguy, D. Heterogeneity of gain boundaries in 5xxx and 7xxx aluminum alloys and its influence on intergranular toughness. Eng. Fract. Mech. 2013, 97, 1–11. [Google Scholar] [CrossRef]
- Moriconi, C.; Henaff, G.; Halm, D. Cohesive zone modeling of fatigue crack propagation assisted by gaseous hydrogen in metals. Int. J. Fatigue 2014, 68, 56–66. [Google Scholar] [CrossRef]
- Hondros, E.D.; Seah, M.P. The theory of Grain Boundary Segregation in Terms of Surface Adsorption Analogues. Met. Trans. A 1977, 8, 1363–1371. [Google Scholar] [CrossRef]
- Serebrinsky, S.; Carter, E.A.; Ortiz, M. A quantum-mechanically informed continuum model of hydrogen embrittlement. J. Mech. Phys. Solids 2004, 52, 2403–2430. [Google Scholar] [CrossRef]
- Jiang, J.J.; Lin, Z.Y.; Feng, M.L.; Meng, X.-Q.; Cai, X.-J.; Liu, F. Experimental study on temperature influence to fracture toughness of Cr5 cold roll steel. Forg. Stamp. Technol. 2012, 37, 135–139. [Google Scholar]





| Element | Fe | Cr | Mn | Mo | Ni | Si | V | C | P |
|---|---|---|---|---|---|---|---|---|---|
| wt % | 92.94 | 4.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.15 | 0.5 | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Chen, H.; Zhao, W.; Yan, L. Study on Flake Formation Behavior and Its Influence Factors in Cr5 Steel. Materials 2018, 11, 690. https://doi.org/10.3390/ma11050690
Fan J, Chen H, Zhao W, Yan L. Study on Flake Formation Behavior and Its Influence Factors in Cr5 Steel. Materials. 2018; 11(5):690. https://doi.org/10.3390/ma11050690
Chicago/Turabian StyleFan, Junkai, Huitao Chen, Wu Zhao, and Liang Yan. 2018. "Study on Flake Formation Behavior and Its Influence Factors in Cr5 Steel" Materials 11, no. 5: 690. https://doi.org/10.3390/ma11050690
APA StyleFan, J., Chen, H., Zhao, W., & Yan, L. (2018). Study on Flake Formation Behavior and Its Influence Factors in Cr5 Steel. Materials, 11(5), 690. https://doi.org/10.3390/ma11050690





