Synthesis of α-Fe2O3 and Fe-Mn Oxide Foams with Highly Tunable Magnetic Properties by the Replication Method from Polyurethane Templates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
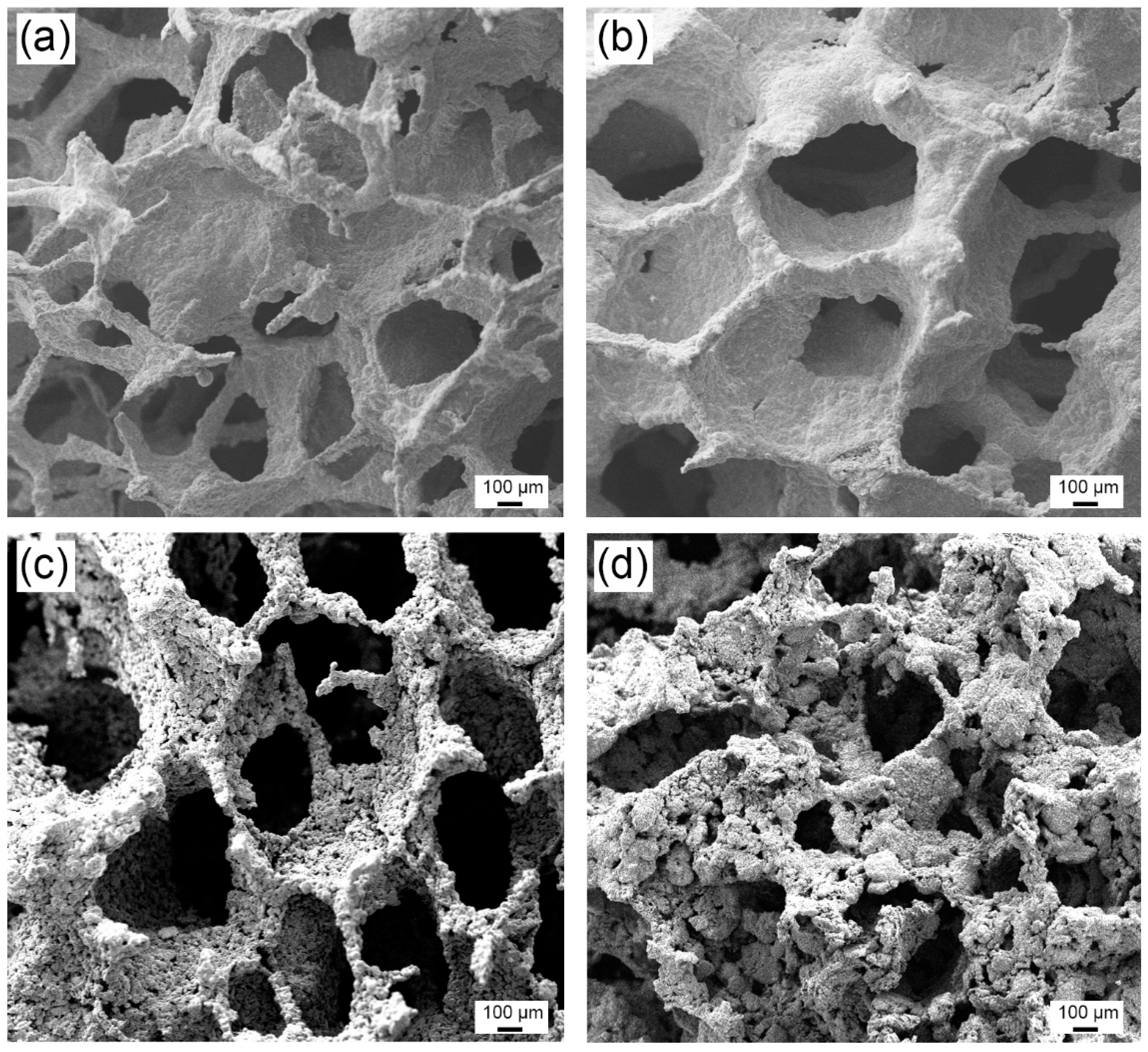
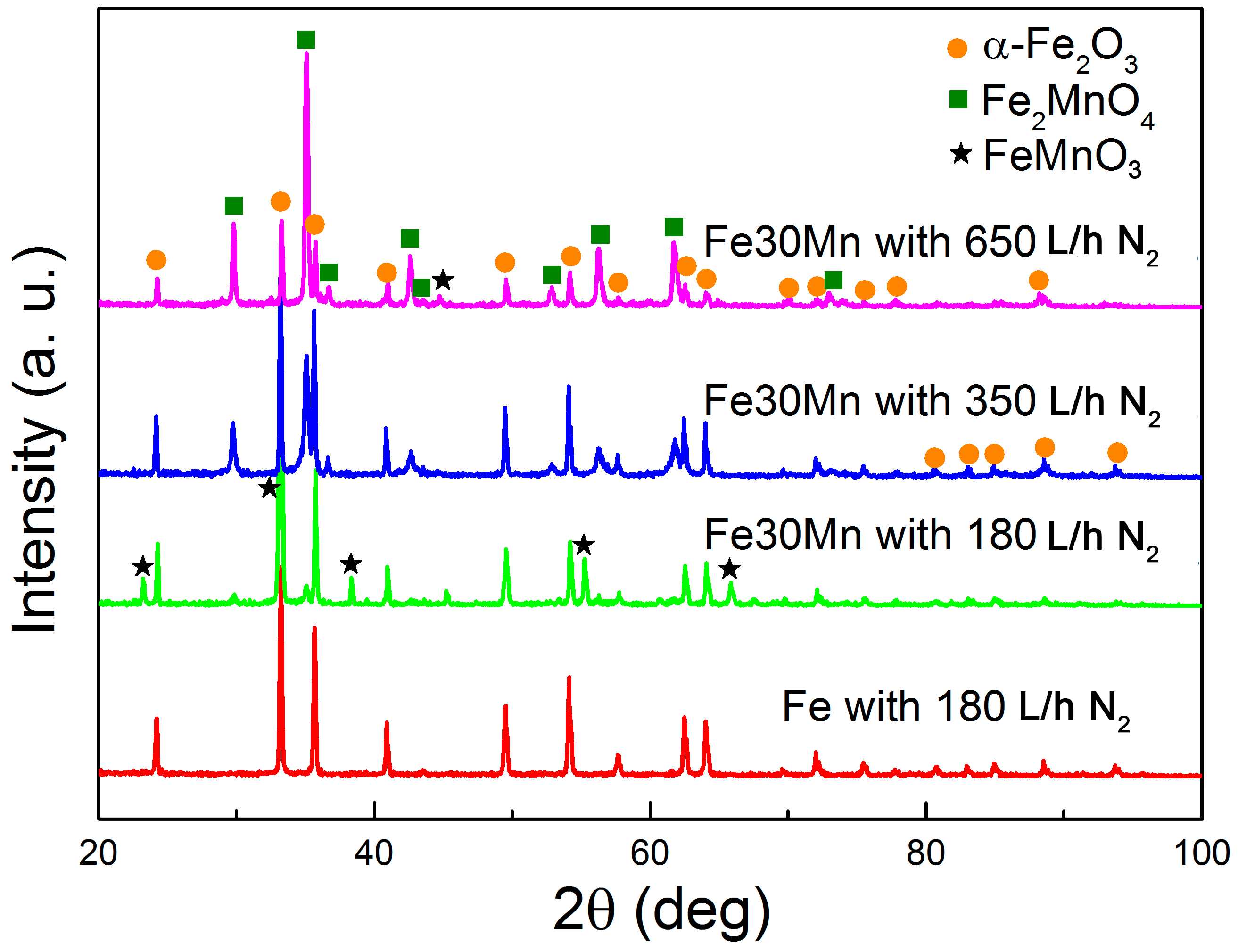
3.1. Microstructure and Compositional Analyses
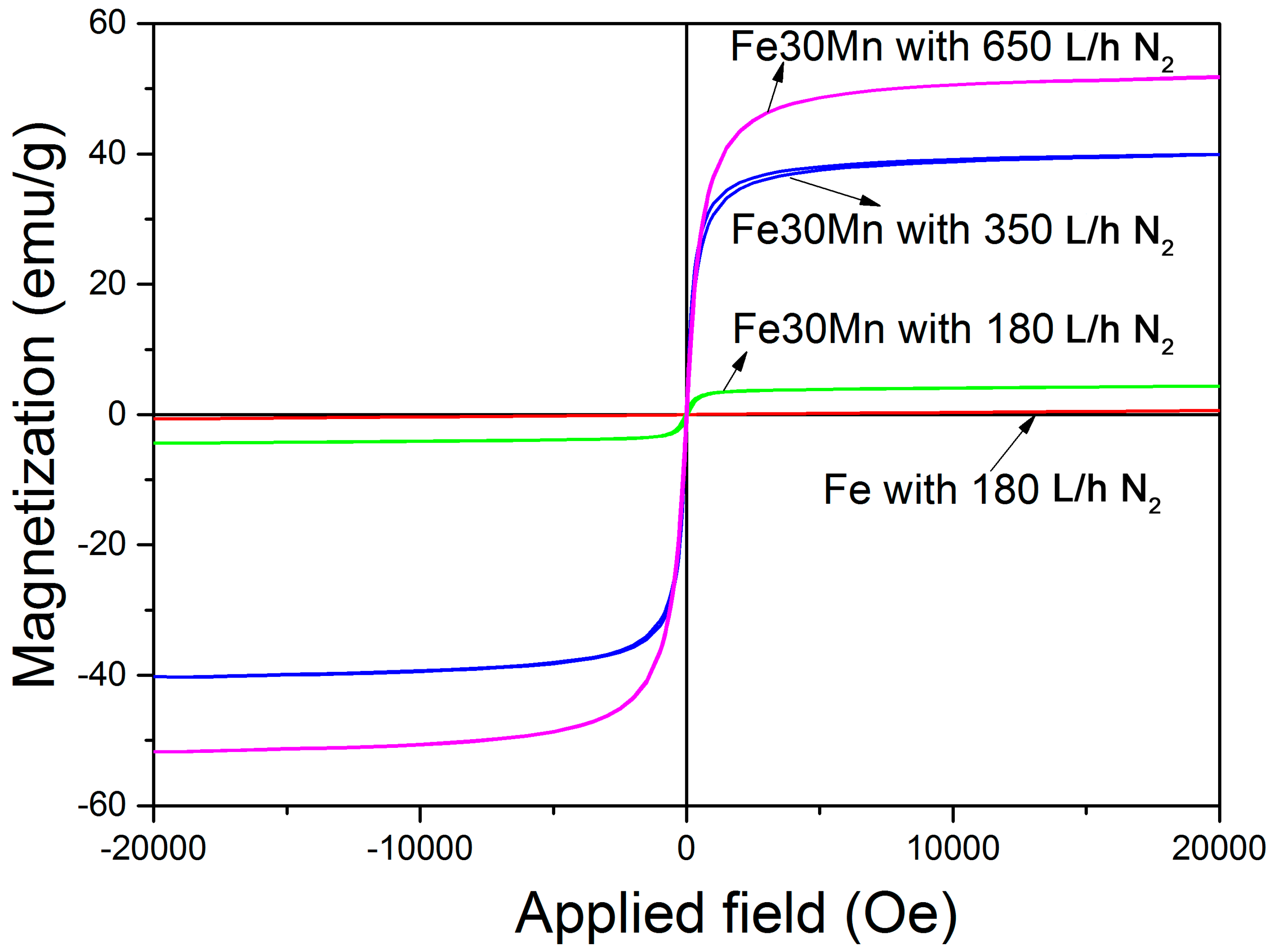
3.2. Magnetic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seymour, R.B.; Kauffman, G.B. Polyurethanes: A Class of Modern Versatile Materials. J. Chem. Educ. 1992, 69, 909–910. [Google Scholar] [CrossRef]
- Karl, S.; Somers, A.V. Method of Making Porous Ceramic Articles. U.S. Patent 3,090,094 A, 21 May 1963. [Google Scholar]
- Park, K.Y.; Lee, S.E.; Kim, C.G.; Han, J.H. Fabrication and electromagnetic characteristics of electromagnetic wave absorbing sandwich structures. Compos. Sci. Technol. 2006, 66, 576–584. [Google Scholar] [CrossRef]
- Chen, Z.P.; Xu, C.; Ma, C.Q.; Ren, W.C.; Cheng, H.M. Lightweight and flexible graphene foam composites for high-performance electromagnetic interference shielding. Adv. Mater. 2013, 25, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Chen, Z.P.; Thomas, A.V.; Ren, W.C.; Cheng, H.M.; Koratkar, N. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network. Sci. Rep. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Buciuman, F.C.; Czarnetzki, B.K. Preparation and characterization of ceramic foam supported nanocrystalline zeolite catalysts. Catal. Today 2001, 69, 337–342. [Google Scholar] [CrossRef]
- Chen, N.; Pan, Q. Versatile fabrication of ultralight magnetic foams and application for oil-water separation. ACS Nano 2013, 7, 6875–6883. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jung, Y.S.; Cavanagh, A.S.; Kim, G.H.; George, S.M.; Dillon, A.C.; Kim, J.K.; Lee, J. Fe3O4 nanoparticles confined in mesocellular carbon foam for high performance anode materials for lithium-ion batteries. Adv. Funct. Mater. 2011, 21, 2430–2438. [Google Scholar] [CrossRef]
- Williams, E.J.A.E.; Evans, J.R.G. Expanded ceramic foam. J. Mater. Sci. 1996, 31, 559–563. [Google Scholar] [CrossRef]
- Wang, S.R.; Geng, H.R.; Hui, L.H.; Wang, Y.Z. Reticulated porous multiphase ceramics with improved compressive strength and fracture toughness. J. Mater. Eng. Perform. 2007, 16, 113–118. [Google Scholar] [CrossRef]
- Bhaduri, S.B. Science and technology of ceramic foams. Adv. Perform. Mater. 1994, 1, 205–220. [Google Scholar] [CrossRef]
- Arrance, F.C. Battery Electrode and Battery, and Process for Preparing Said Electrode. U.S. Patent 3,287,166 A, 22 November 1996. [Google Scholar]
- Antsiferov, V.N.; Makarov, A.M.; Khramtsov, V.D. High-porosity permeable cellular metals and alloys in catalytic processes of gas cleaning. Adv. Eng. Mater. 2005, 7, 77–91. [Google Scholar] [CrossRef]
- Roesler, J.F. Application of polyurethane foam filters for respirable dust separation. J. Air. Pollut. Control Assoc. 1996, 16, 30–34. [Google Scholar] [CrossRef]
- Haack, D.P.; Butcher, K.R.; Kim, T.; Lu, T.J. Novel Lightweight Metal Foam Heat Exchangers; Porvair Fuel Cell Technology, Inc.: Hendersonville, NC, USA, 2001. [Google Scholar]
- Quadbeck, P.; Stephani, G.; Kümmel, K.; Adler, J.; Standke, G. Synthesis and properties of open-celled metal foams. Mater. Sci. Forum 2007, 534–536, 1005–1008. [Google Scholar] [CrossRef]
- Quadbeck, P.; Kümmel, K.; Hauser, R.; Standke, G.; Adler, J.; Stephani, G. Open cell metal foams-application-oriented structure and material selection. In Proceedings of the International Conference on Cellular Materials, Dresden, Germany, 27–29 October 2010; pp. 279–288. [Google Scholar]
- Quadbeck, P.; Kümmel, K.; Hauser, R.; Standke, G.; Adler, J.; Stephani, G.; Kieback, B. Structural and material design of open-cell powder metallurgical foams. Adv. Eng. Mater. 2011, 13, 1024–1030. [Google Scholar] [CrossRef]
- Zhang, H.; Pinjala, D.; Joshi, Y.K.; Wong, T.N.; Toh, K.C.; Iyer, M.K. Fluid flow and heat transfer in liquid cooled foam heat sinks for electronic packages. IEEE Trans. Comp. Packag. Technol. 2005, 28, 272–280. [Google Scholar] [CrossRef]
- Li, J.P.; Li, S.H.; Groot, K.D.; Layrolle, P. Improvement of porous titanium with thicker struts. Key Eng. Mater. 2003, 240–242, 547–550. [Google Scholar] [CrossRef]
- Manonukul, A.; Tange, M.; Srikudvien, P. Rheological properties of commercially pure titanium slurry for metallic foam production using replica impregnation method. Powder Technol. 2014, 266, 129–134. [Google Scholar] [CrossRef]
- Biswas, P.; Ramavath, P.; Nair, C.M.; Suresh, M.B.; Ravi, N.; Johnson, R. Quasi-static compression behavior of nickel oxide, nickel oxide: Zirconia, nickel:zirconia and nickel foams. Ceram. Int. 2016, 42, 10572–10578. [Google Scholar] [CrossRef]
- Zuo, X.; Barbiellini, B.; Vittoria, C. Calculation of exchange constants in manganese ferrite (MnFe2O4). J. Magn. Magn. Mater. 2004, 272, 306–311. [Google Scholar] [CrossRef]
- Padella, F.; Alvani, C.; Barbera, A.L.; Ennas, G.; Liberatore, R.; Varsano, F. Mechanosynthesis and process characterization of nanostructured manganese ferrite. Mater. Chem. Phys. 2005, 90, 172–177. [Google Scholar] [CrossRef]
- Durán, F.G.; Barbero, B.P.; Cadús, L.E.; Rojas, C.; Centeno, M.A.; Odriozola, J.A. Manganese and iron oxides as combustion catalysts of volatile organic compounds. Appl. Catal. B 2009, 92, 194–201. [Google Scholar] [CrossRef]
- Quiroga, M.M.B.; Barbero, B.P.; Cadus, L.E. Synthesis of a catalyst of Mn–Fe–O by mechano-chemical reaction. Appl. Catal. A 2014, 474, 26–33. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Hamdeh, H.H.; Ho, J.C.; O’shea, M.J.; Walker, J.C. Mössbauer studies of manganese ferrite fine particles processed by ball-milling. J. Magn. Magn. Mater. 2000, 220, 139–146. [Google Scholar] [CrossRef]
- Amighian, J.; Mozaffari, M.; Nasr, B. Preparation of nano-sized manganese ferrite (MnFe2O4) via coprecipitation method. Phys. Status Solidi C 2006, 3, 3188–3192. [Google Scholar] [CrossRef]
- Baldi, M.; Escribano, V.S.; Amores, J.M.G.; Milella, F.; Busca, G. Characterization of manganese and iron oxides as combustion catalysts for propane and propene. Appl. Catal. B 1998, 17, L175–L182. [Google Scholar] [CrossRef]
- Kwon, W.H.; Kang, J.Y.; Lee, J.G.; Lee, S.W.; Chae, K.P. Synthesis and magnetic properties of Zn, Co and Ni substituted manganese ferrite powders by sol-gel method. J. Magn. 2010, 15, 159–164. [Google Scholar] [CrossRef]
- Rafique, M.Y.; Qing, P.L.; Javeb, Q.U.A.; Iqbal, M.Z.; Mei, Q.H.; Farooq, M.H.; Gang, G.Z.; Tanveer, M. Growth of monodisperse nanospheres of MnFe2O4 with enhanced magnetic and optical properties. Chin. Phys. B 2013, 22. [Google Scholar] [CrossRef]
- Rayaprol, S.; Kaushik, S.D.; Babu, P.D.; Siruguri, V. Structure and magnetism of FeMnO3. AIP Conf. Proc. 2013, 1512, 1132–1133. [Google Scholar]
| Components | Fe or Fe 30Mn | Binder | Distilled Water |
|---|---|---|---|
| Mass percent (%) | 50 | 17 | 33 |
| Sample | Mn (at %) | Fe (at %) | O (at %) |
|---|---|---|---|
| Fe-O (180 L/h) | 0 | 39 | 61 |
| Fe-Mn-O (180 L/h) | 12 | 28 | 60 |
| Fe-Mn-O (350 L/h) | 16 | 26 | 58 |
| Fe-Mn-O (650 L/h) | 18 | 24 | 56 |
| Alloy | Phase | Cell Parameters (Å) | % | |
|---|---|---|---|---|
| Fe-O (180 L/h) | hematite | Fe2O3 | a = 5.036; c = 13.748 | 100 |
| Fe-Mn-O (180 L/h) | hematite | Fe2O3 | a = 5.038; c = 13.741 | 60 |
| bixbyite | FeMnO3 | a = 9.417 | 31.5 | |
| jacobsite | Fe2MnO4 | a = 8.489 | 8.5 | |
| Fe-Mn-O (350 L/h) | hematite | Fe2O3 | a = 5.036; c = 13.741 | 51 |
| jacobsite | Fe2MnO4 | a = 8.483 | 49 | |
| Fe-Mn-O (650 L/h) | Hematite | Fe2O3 | a = 5.036; c = 13.745 | 26 |
| jacobsite | Fe2MnO4 | a = 8.5028 | 74 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Fornell, J.; Zhang, H.; Solsona, P.; Barό, M.D.; Suriñach, S.; Pellicer, E.; Sort, J. Synthesis of α-Fe2O3 and Fe-Mn Oxide Foams with Highly Tunable Magnetic Properties by the Replication Method from Polyurethane Templates. Materials 2018, 11, 280. https://doi.org/10.3390/ma11020280
Feng Y, Fornell J, Zhang H, Solsona P, Barό MD, Suriñach S, Pellicer E, Sort J. Synthesis of α-Fe2O3 and Fe-Mn Oxide Foams with Highly Tunable Magnetic Properties by the Replication Method from Polyurethane Templates. Materials. 2018; 11(2):280. https://doi.org/10.3390/ma11020280
Chicago/Turabian StyleFeng, Yuping, Jordina Fornell, Huiyan Zhang, Pau Solsona, Maria Dolors Barό, Santiago Suriñach, Eva Pellicer, and Jordi Sort. 2018. "Synthesis of α-Fe2O3 and Fe-Mn Oxide Foams with Highly Tunable Magnetic Properties by the Replication Method from Polyurethane Templates" Materials 11, no. 2: 280. https://doi.org/10.3390/ma11020280







