Effects of Pyrazine Derivatives and Substituted Positions on the Photoelectric Properties and Electromemory Performance of D–A–D Series Compounds
Abstract
:1. Introduction
2. Results and Discussion
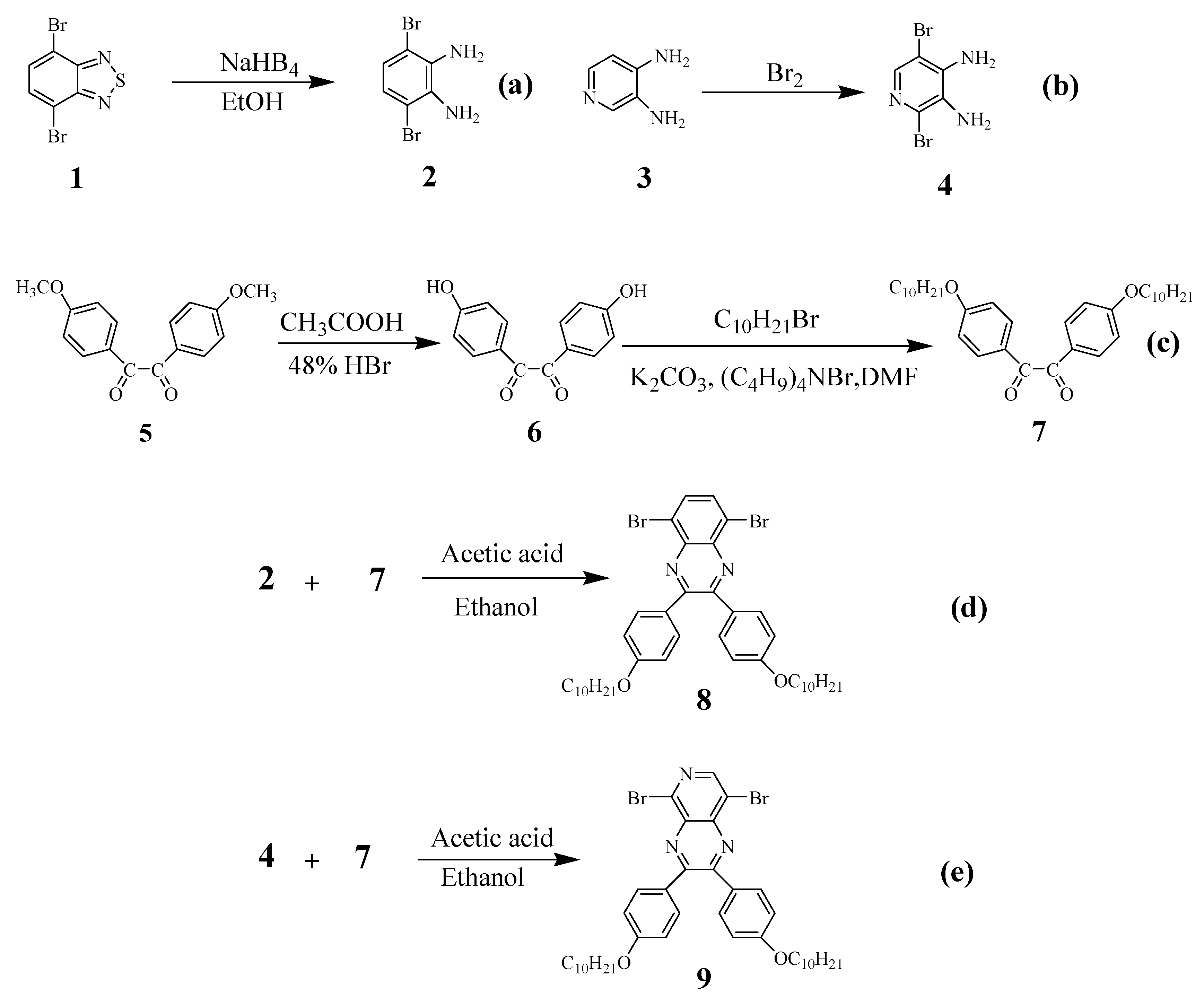
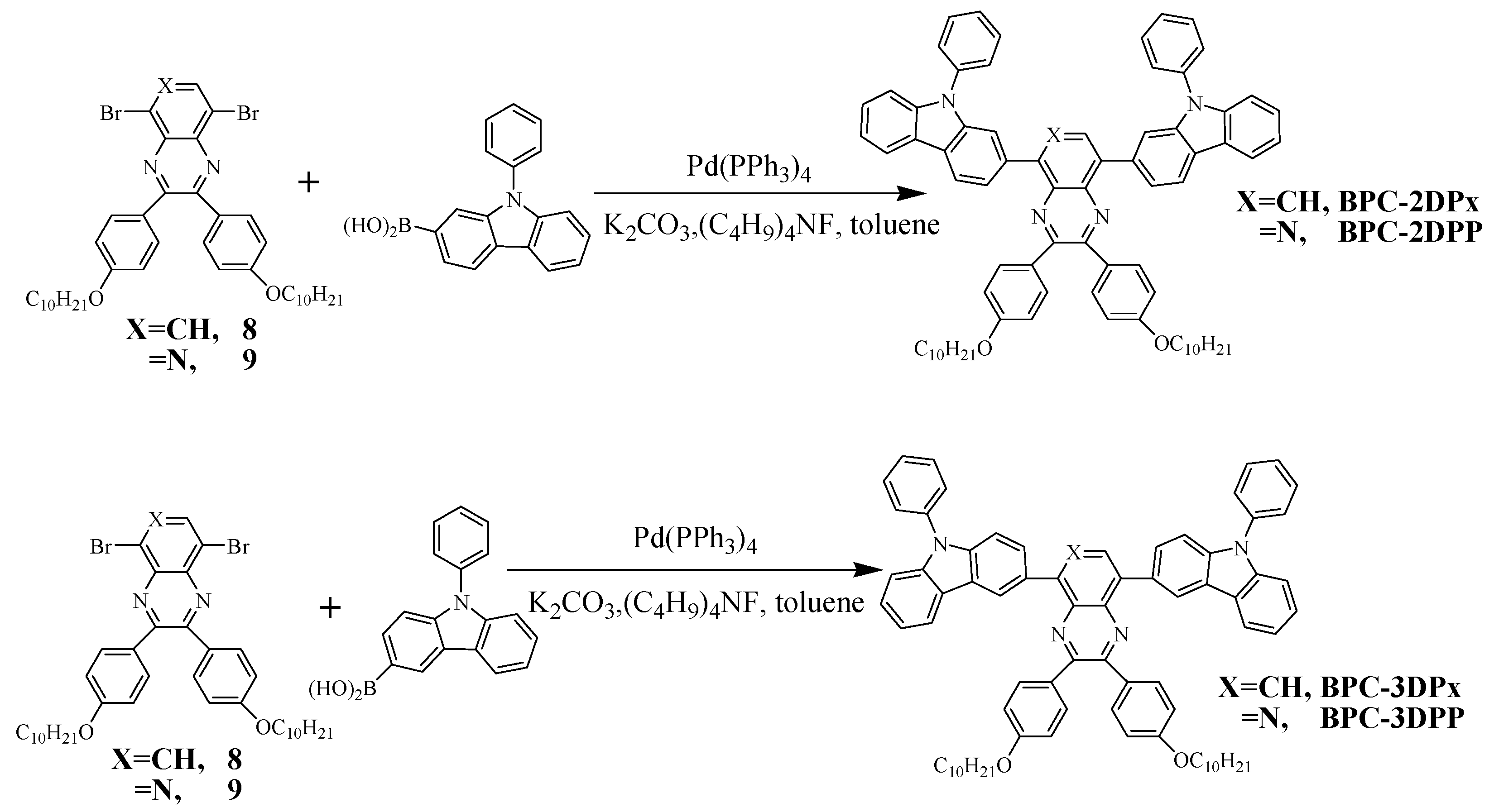
2.1. Synthesis of the Four Fluorescent Compounds
2.2. Optimization of Molecular Energy Levels
2.3. UV-vis Absorption Spectra
2.4. Fluorescent Emission Spectra
2.5. Cyclic Voltammetry
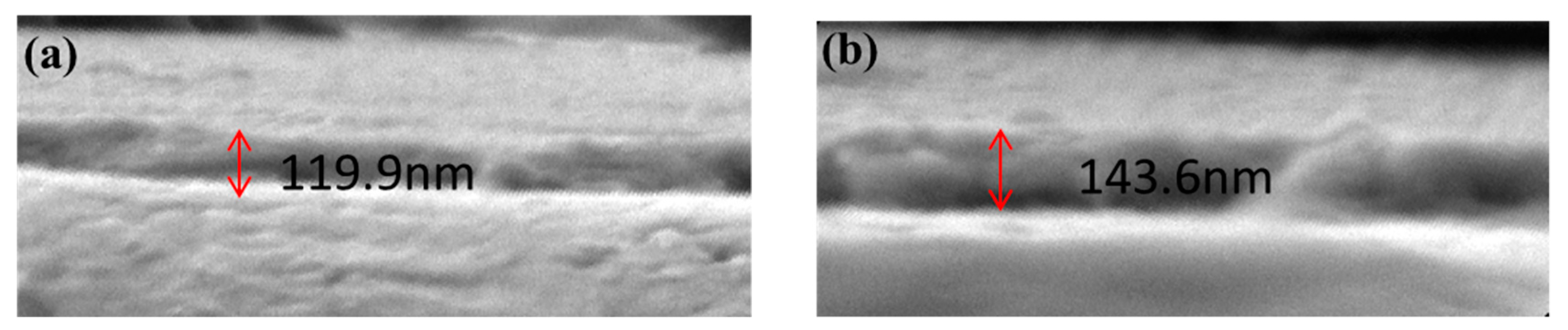
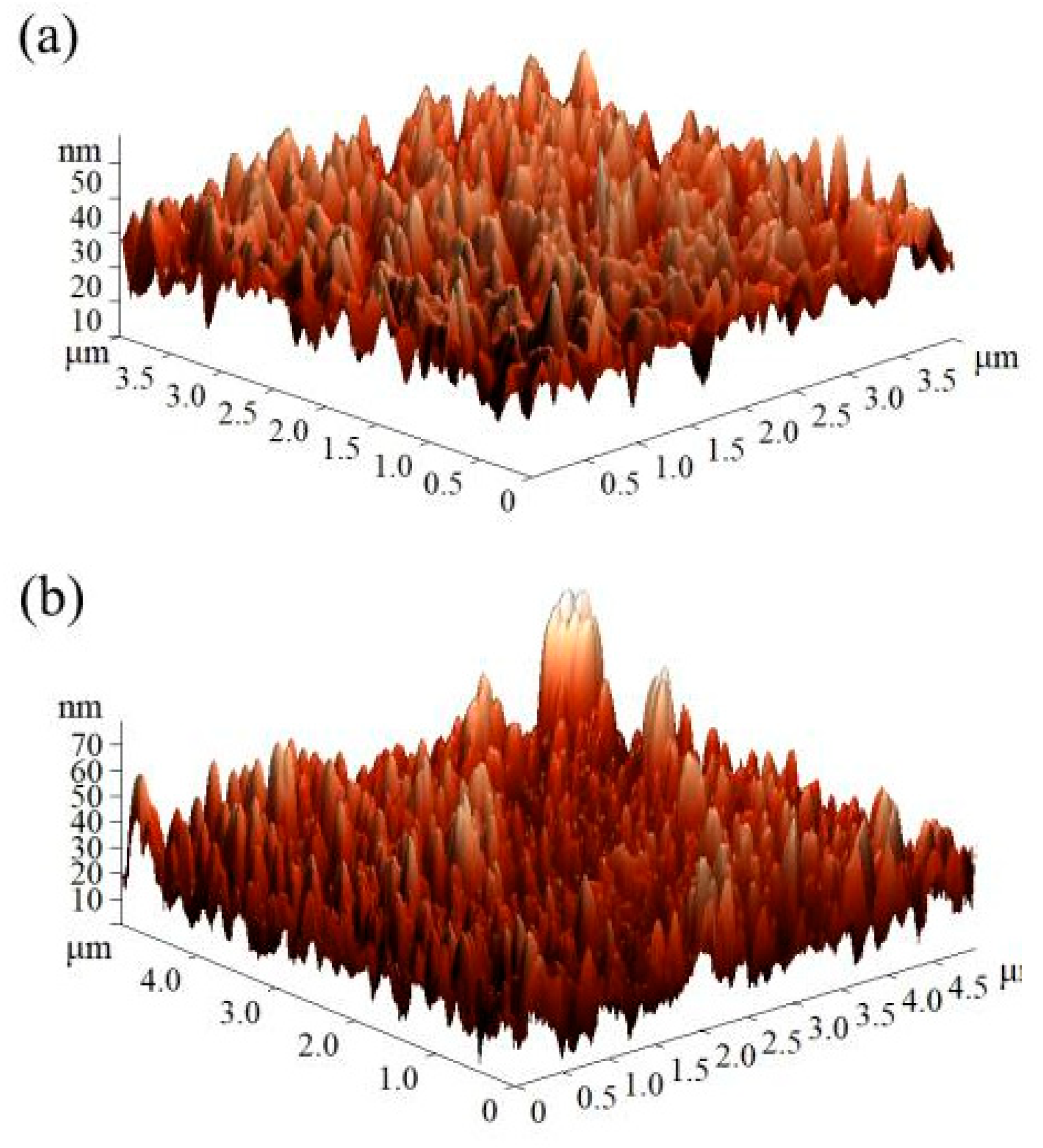
2.6. Thermostability Analysis
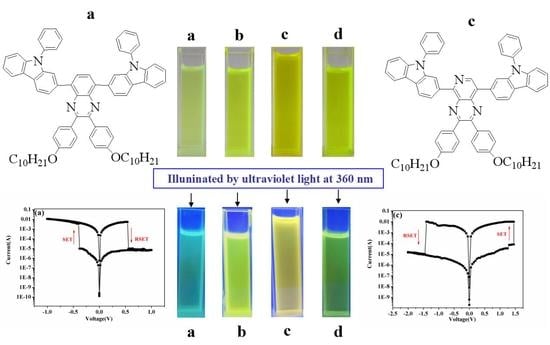
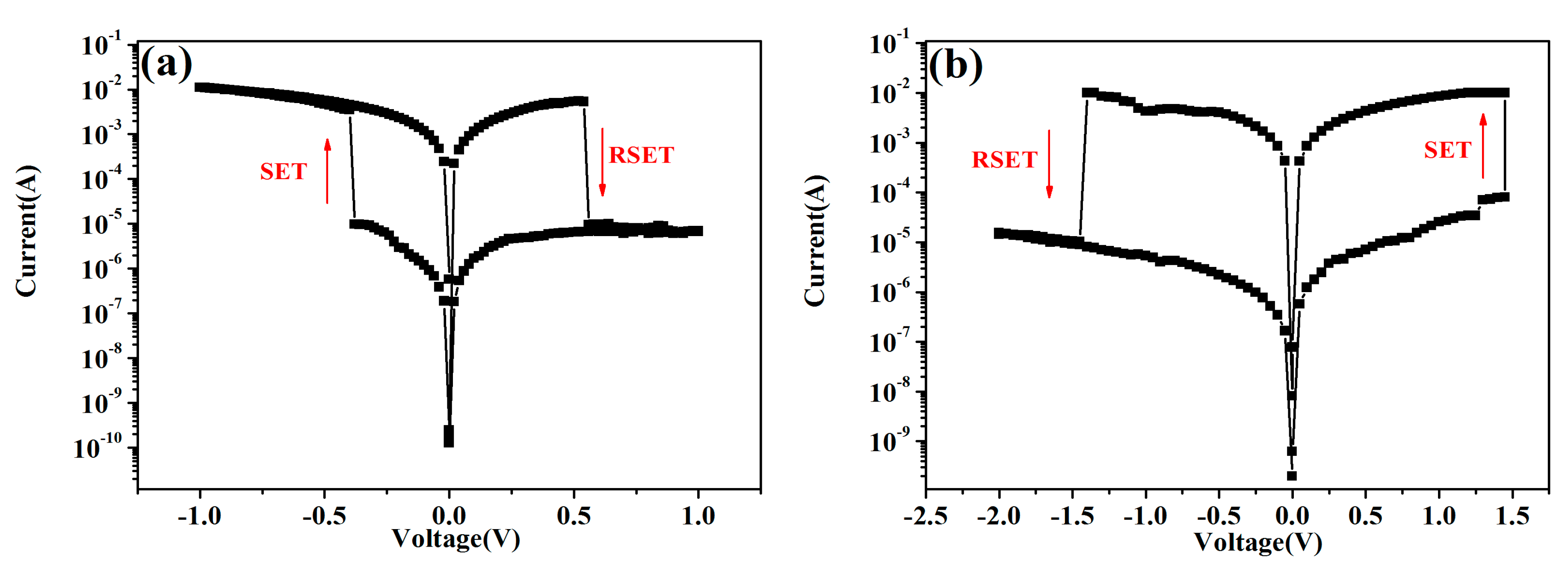
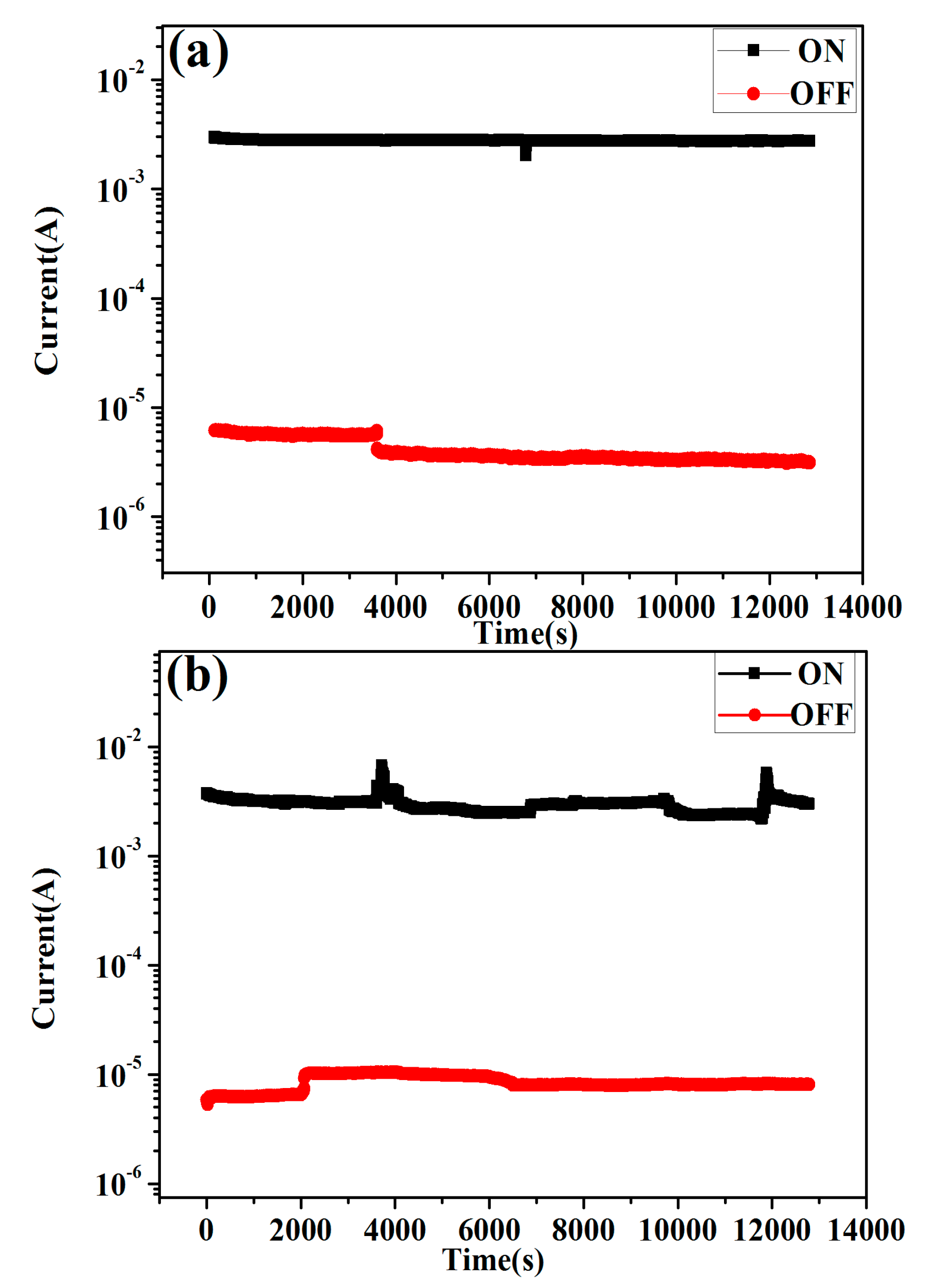
2.7. Performance of the Memory Devices
3. Experimental
3.1. Materials
3.2. Characterization
3.3. Synthesis
3.3.1. Synthesis of the Acceptor Unit
3.3.2. Synthesis of the Four D–A–D Compounds
3.4. Memory Device Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nar, I.; Atsay, A.; AItIndal, A.; Hamuryudan, E. o-carborane, ferrocene, and phthalocyanine triad for high-mobility organic field-effect transistors. Inorg. Chem. 2018, 57, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Rausch, A.F.; Murphy, L.; Williams, J.A.G.; Yersin, H. Improving the performance of Pt(II) complexes for blue light emission by enhancing the molecular rigidity. Inorg. Chem. 2012, 51, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fortney, A.; Washington, K.E.; Bulumulla, C.; Huang, P.; Dissanayake, D.; Biewer, M.C.; Kowalewski, T.; Stefan, M.C. Systematic investigation of benzodithiophene-benzothiadiazole isomers for organic photovoltaics. ACS Appl. Mater. Interfaces 2016, 8, 33025–33033. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, J.C.; Ahn, T.; Lee, S.K. Processable small molecules for application to organic solar cells. J. Nanosci. Nanotechnol. 2016, 16, 8670–8673. [Google Scholar] [CrossRef]
- Sista, P.; Nguyen, H.; Murphy, J.W.; Hao, J.; Dei, D.K.; Palaniappan, K.; Servello, J.; Kularatne, R.S.; Gnade, B.E.; Xue, B.F. Synthesis and electronic properties of semiconducting polymers containing benzodithiophene with alkyl phenylethynyl substituents. Macromolecules 2010, 43, 8063–8070. [Google Scholar] [CrossRef]
- Jonforsen, M.; Jonforsen, T.; Spjuth, L.; Inganas, O.; Andersson, M.R. Synthesis and characterization of poly(quinoxaline vinylene)s and poly(pyridopyrazine vinylene)s with phenyl substituted side-groups. Synth. Met. 2002, 131, 53–59. [Google Scholar] [CrossRef]
- Liu, B.; Giordano, F.; Pei, K.; Decoppet, J.D.; Zhu, W.H.; Zakeeruddin, S.M.; Gratzel, M. Molecular engineering of pyrido[3,4-b]pyrazine-based donor-acceptor-π-acceptor organic sensitizers: Effect of auxiliary acceptor in cobalt- and iodine-based electrolytes. Chem-Eur. J. 2015, 21, 18654–18661. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Wu, Y.Z.; Wu, W.J.; Zhang, Q.; Chen, B.Q.; Tian, H.; Zhu, W.H. Constructing organic D-A-π-A-featured sensitizers with a quinoxaline unit for high-efficiency solar Cells: The effect of an auxiliary acceptor on the absorption and the energy level alignment. Chem.-Eur. J. 2012, 18, 8190–8200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Wan, M.; Zhao, J.S.; Cui, C.S.; Liu, J.F. Effects of alkyl or alkoxy side chains on the electrochromic properties of four ambipolar donor-acceptor typle polymers. RSC Adv. 2014, 4, 52712–52726. [Google Scholar] [CrossRef]
- Lee, P.L.; Hsu, H.L.C.; Lee, J.F.; Chuang, H.Y.; Lin, P.Y. New conjugated copolymers based on benzo[1,2-b; 3,4-b’]dithiophene and derivatives of benzo[g]quinoxaline for bulk heterojunction solar cells. J. Polym. Sci. Part A Pol. Chem. 2011, 49, 662–670. [Google Scholar] [CrossRef]
- Ying, W.J.; Yang, J.B.; Wielopolski, M.; Moehl, T.; Moser, J.E.; Comte, P.; Hua, J.L.; Zakeeruddin, S.M.; Tian, H.; Grazel, M. New pyrido[3,4-b]pyrazine-based sensitizers for efficient and stable dye-sensitized solar cells. Chem. Sci. 2014, 5, 206–214. [Google Scholar] [CrossRef]
- Lu, X.F.; Feng, Q.Y.; Lan, T.; Zhou, G.; Wang, Z.S. Molecular engineering of quinoxaline-based organic sensitizers for highly efficient and stable dye-sensitized solar cells. Chem. Mater. 2012, 24, 3179–3187. [Google Scholar] [CrossRef]
- Lu, X.F.; Fan, S.H.; Wu, J.H.; Jia, X.W.; Wang, Z.S.; Zhou, G. Controlling the charge transfer in D-A-D chromophores based on pyrazine derivatives. J. Org. Chem. 2014, 79, 6480–6489. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Lee, H.J.; Kim, J.H.; Park, S.Y.; Park, S.M.; Dai, L.; Baek, J.B. Novel quinoxaline-based organic sensitizers for dye-sensitized solar cells. Org. Lett. 2011, 13, 3880–3883. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Zhu, W.G.; Tan, H.; Peng, Q. A novel D2-A-D1-A-D2-type donor-acceptor conjugated small molecule based on a benzo[1,2-b:4,5-b’]dithiophene core for solution processed organic photovoltaic cells. Chem. Phys. Lett. 2017, 667, 254–259. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sakai, H.; Yuasa, J.; Araki, Y.; Wada, T.; Sakanoue, T.; Takeobu, T.; Kawai, T.; Hasobe, T. Synthetic control of the excited-State dynamics and circularly polarized luminescence of fluorescent “Push-Pull” tetrathia[9]helicenes. Chem-Eur. J. 2016, 22, 4263–4273. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, G.; Sivakumar, E.K.T.; Ganesamootrhy, R.; Thangamuthu, R.; Sakthivel, P. Review of carbazole based conjugated molecules for highly efficient organic solar cell application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Vollbrecht, J.; Bock, H.; Wiebeler, C.; Schumacher, S.; Kitzerow, H. Polycyclic Aromatic Hydrocarbons Obtained by Lateral Core Extension of Mesogenic Perylenes: Absorption and Optoelectronic Properties. Chem-Eur. J. 2014, 20, 12026–12031. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, J.; Wiebeler, C.; Neuba, A.; Bock, H.; Schumacher, S.; Kitzerow, H. Bay-Extended, Distorted Perylene Esters Showing Visible Luminescence after Ultraviolet Excitation: Photophysical and Electrochemical Analysis. J. Phys. Chem. C 2016, 120, 7839–7848. [Google Scholar] [CrossRef]
- Kong, L.Q.; Wang, M.; Ju, X.P.; Zhao, J.S.; Zhang, Y.; Xie, Y. The availability of neutral cyan, green, blue and purple colors from simple D–A type polymers with commercially available thiophene derivatives as the donor units. Polymers 2017, 9, 656. [Google Scholar] [CrossRef]
- Wolf, J.; Babics, M.; Wang, K.; Saleem, Q.; Liang, R.Z.; Hansen, M.R.; Beaujuge, P.M. Benzo[1,2-b:4,5-b’]dithiophene-pyrido[3,4-b]pyrazine small-molecule donors for bulk heterojunction solar cells. Chem. Mater. 2016, 28, 2058–2066. [Google Scholar] [CrossRef]
- Kitazawa, D.; Watanabe, N.; Yamamoto, S.; Tsukamoto, J. Quinoxaline-based donor polymers for organic solar cells. J. Photopolym. Polym. Sci. Technol. 2010, 23, 293–296. [Google Scholar] [CrossRef]
- Song, X.J.; Wang, M.; Kong, L.Q.; Zhao, J.S. Effects of the acceptor pattern and substitution position on the properties of N-phenyl-carbazolyl based donor-acceptor-donor molecules. RSC Adv. 2017, 7, 18189–18198. [Google Scholar] [CrossRef]
- Sharma, B.; Sarothia, Y.; Singh, R.; Kan, Z.P.; Keivanidis, P.E.; Jacob, J. Synthesis and characterization of light-absorbing cyclopentadithiophene-based donor-acceptor copolymers. Polym. Int. 2016, 65, 57–65. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, H.; Hou, X.Y.; Hu, X.A.; Ng, S.C. A novel low band gap conjugated polymer based on N-substituted dithieno[3,2-b:2’, 3’-d]pyrrole. Synth. Met. 2010, 160, 1438–1441. [Google Scholar] [CrossRef]
- Ooyama, Y.; Inoue, S.; Nagano, T.; Kushimoto, K.; Ohshita, J.; Imae, I.; Komaguchi, K.; Harima, Y. Dye-sensitized solar cells based on donor-acceptor π-conjugated fluorescent dyes with a pyridine ring as an electron-withdrawing anchoring group. Angew. Chem. Int. Ed. 2011, 50, 7429–7433. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.P.; Kong, L.Q.; Zhao, J.S.; Bai, G.Y. Synthesis and electrochemical capacitive performance of thieno[3,4-b]pyrazine-based Donor-Acceptor type copolymers used as supercapacitor electrode material. Electrochim. Acta 2017, 238, 36–48. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Chen, H.C.; Yang, Y.S.; Chang, S.H.; Wu, P.J.; Chu, Y.Y.; Wu, C.G. Comprehensive Study of Pyrido[3,4-b]pyrazine-Based D-pi-a Copolymer for Efficient Polymer Solar Cells. J. Polym. Sci. Part A: Pol. Chem. 2016, 54, 1822–1833. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, B.C.; Tian, W.J.; Yang, B.; Li, M. The comparative investigation on the optical properties and electronic structures of the alkoxy-tuned 1,3,-oxadiazole derivatives. Mater. Chem. Phys. 2010, 119, 243–248. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Fan, H.J.; Li, Y.F.; Zhan, X.W. Thiazole-based organic semiconductors for organic electronics. Adv. Mater. 2012, 24, 3087–3106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Jiang, K.J.; Chen, Q.; Li, G.; Yang, L.M. Pyrazino-[2,3-f][1,10]phenanthroline as a new anchoring group of organic dyes for dye-sensitized solar cells. RSC Adv. 2015, 5, 46206–46209. [Google Scholar] [CrossRef]
- Henriksson, P.; Lindqvist, C.; Abdisa, B.; Wang, E.G.; George, Z.; Kroon, R.; Muller, C.; Yohannes, T.; Inganas, O.; Andersson, M.R. Stability study of quinoxaline and pyrido pyrazine based co-polymers for solar cell applications. Sol. Energy Mater. Sol. Cells 2017, 4, 138–143. [Google Scholar] [CrossRef]
- Lee, B.L.; Yamamoto, T. Syntheses of New Alternating CT-Type Copolymers of Thiophene and Pyrido[3,4-b]pyrazine Units: Their Optical and Electrochemical Properties in Comparison with Similar CT Copolymers of Thiophene with Pyridine and Quinoxaline. Macromolecules 1999, 32, 1375–1382. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D.; Wang, M.; Kong, L.Q.; Zhao, J.S. Donor-acceptor type polymers containing the 2,3-bis(2-pyridyl)-5,8-dibromoquinoxaline acceptor and different thiophene donors: Electrochemical, spectroelectrochemistry and electrochromic properties. New J. Chem. 2016, 40, 2178–2188. [Google Scholar] [CrossRef]
- Wang, T.; Feng, L.L.; Yuan, J.; Jiang, L.H.; Deng, W.; Zhang, Z.G.; Li, Y.F.; Zou, Y.P. Side-chain fluorination on the pyrido[3,4-b]pyrazine unit towards efficient photovoltaic polymers. Sci. China Chem. 2018, 61, 206–214. [Google Scholar] [CrossRef]
- Zhu, X.H.; Xu, L.Y.; Wang, M.; Wang, Z.; Liu, R.M.; Zhao, J.S. Electrosyntheses and characterization of a soluble blue fluorescent copolymer based on pyrene and cabazole. Int. J. Eletrochem. Sci 2011, 6, 1730–1746. [Google Scholar]
- Rodrigues, P.C.; Berlim, L.S.; Azevedo, D.; Saavedra, N.C.; Prasadr, P.N.; Schreine, W.H.; Atvars, T.D.Z.; Akcelrud, L. Electronic structure and optical properties of an alternated fluorene-benzothiadiazole copolymer: Interplay between experimental and theoretical data. J. Phys. Chem. A 2012, 116, 3681–3690. [Google Scholar] [CrossRef] [PubMed]
- Frizon, T.E.A.; Martinez, J.C.V.; Westrup, J.L.; Duarte, R.D.; Zapp, E.; Domiciano, K.G.; Rodembusch, F.S.; Dal-Bó, A.G. 2,1,3-Benzothiadiazole-based fluorophores, Synthesis, electrochemical, thermal and photophysical characterization. Dyes Pigm. 2016, 135, 26–35. [Google Scholar] [CrossRef]
- Zhang, F.F.; Zhou, C.H.; Yan, J.P. New progress of researches in carbazole compounds. Chin. J. Org. Chem. 2010, 30, 783–796. [Google Scholar]
- Basavaraja, J.; Inamdar, S.R.; Kumar, H.M.S. Solvents effect on the absorption and fluorescence spectra of 7-diethylamino-3-thenoylcoumarin: Evaluation and correlation between solvatochromism and solvent polarity parameters. Spectrochim. Acta Part A 2015, 137, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zhuang, H.; Liu, H.F.; Li, H.; Xu, Q.F.; Lu, J.M.; Wang, L.H. Benzothiazole derivatives containing different electron acceptors exhibiting totally different data-storage performances. J. Mater. Chem. C 2014, 2, 5673–5680. [Google Scholar] [CrossRef]
- Xin, Y.; Zhao, X.F.; Jiang, X.K.; Yang, Q.; Huang, J.H.; Wang, R.R.; Zheng, C.; Wang, Y.J.H. Bistable electrical switching and nonvolatile memory effects by doping different amounts of GO in poly(9,9-dioctylfluorene-2,7-diyl). RSC Adv. 2018, 8, 6878–6886. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhou, Q.H.; Li, Y.; Zhang, Q.J.; Li, H.; Xu, Q.F.; Li, N.J.; Lu, J.M.; Wang, L.H. Adjustment of on-state retention ability based on new donor-acceptor imides through structural tailoring for volatile device applications. ACS Appl. Mater. Interface 2014, 6, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Zhang, Q.J.; Zhu, Y.X.; Xu, X.F.; Liu, H.F.; Li, N.J.; Xu, Q.F.; Li, H.; Lu, J.M.; Wang, L.H. Effects of terminal electron acceptor strength on film morphology and ternary memory performance of triphenylamine donor based devices. J. Mater. Chem. C 2013, 1, 3816–3824. [Google Scholar] [CrossRef]
- Lang, Q.D.; Lim, S.L.; Song, Y.; Zhu, C.X.; Chan, D.S.H.; Kang, E.T.; Neoh, K.G. Nonvolatile polymer memory device based on bistable electrical switching in a thin film of poly(N-vinylcarbazole) with covalently bonded C60. Langmuir 2007, 23, 312–319. [Google Scholar] [CrossRef] [PubMed]


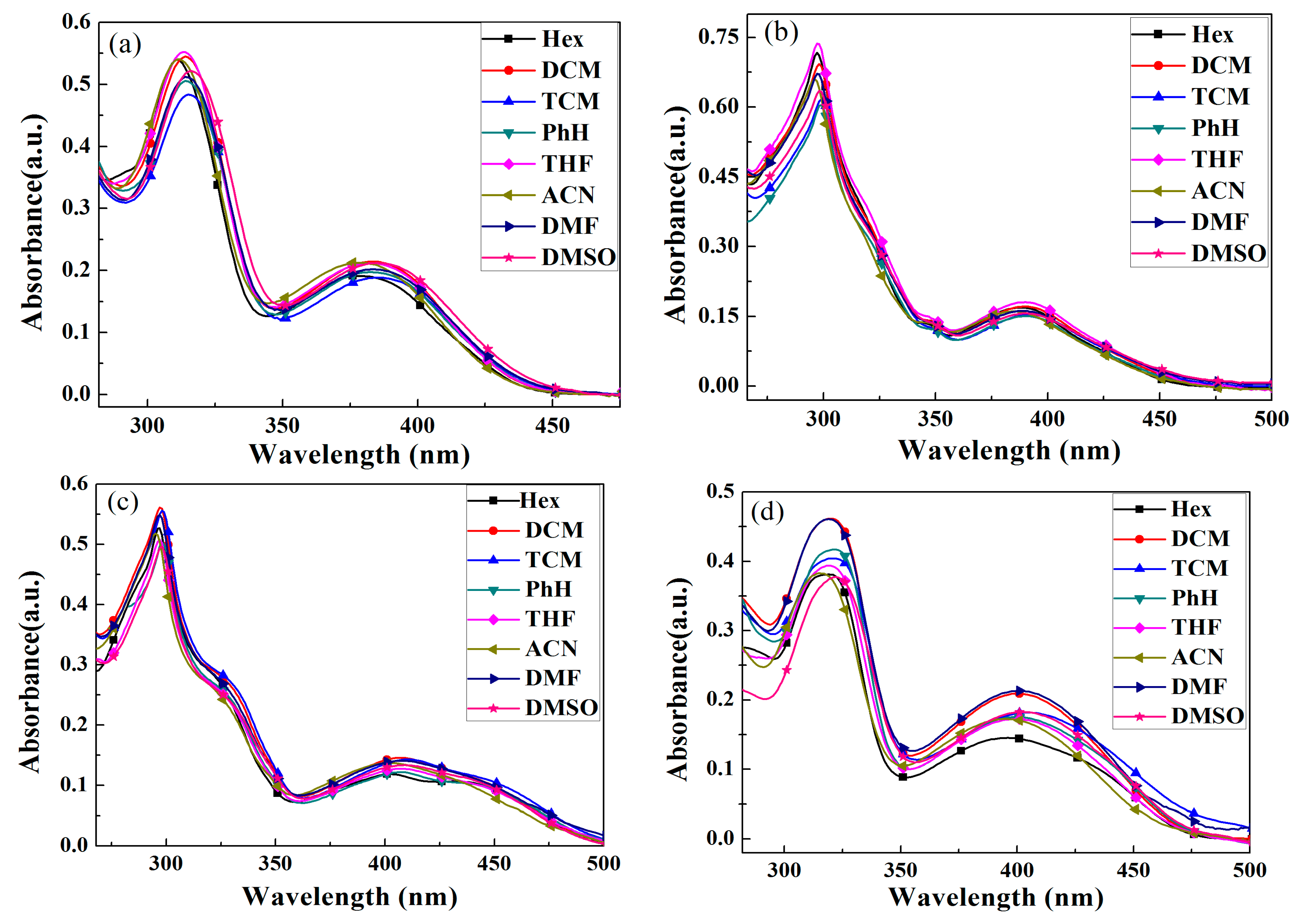
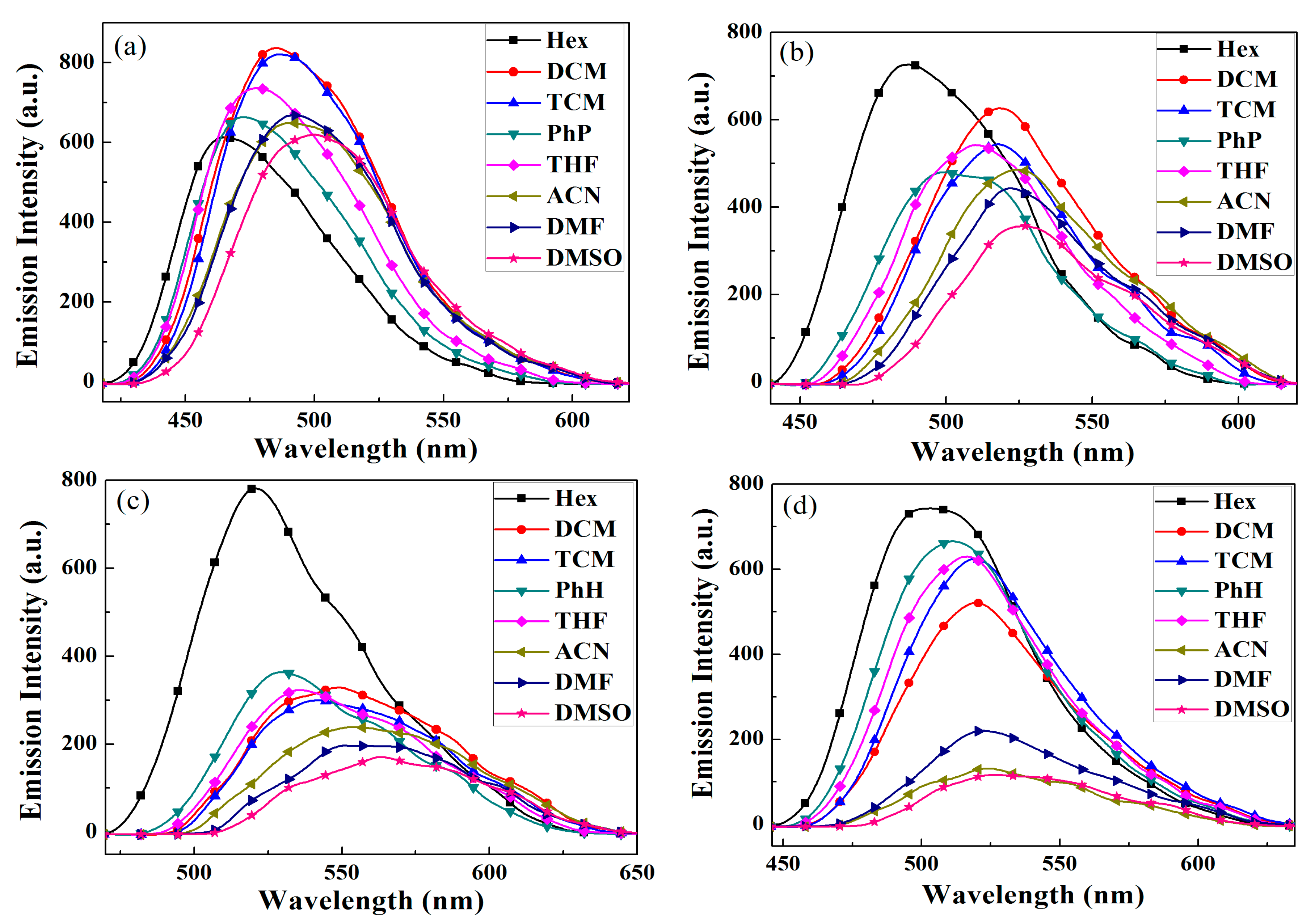



| Solvent | BPC-2DPx | BPC-3DPx | BPC-2DPP | BPC-3DPP |
|---|---|---|---|---|
| Hex | 311, 379 | 297, 388 | 296, 403 | 319, 396 |
| PhH | 314, 383 | 299, 390 | 298, 408 | 321, 399 |
| THF | 313, 383 | 298, 390 | 297, 408 | 319, 399 |
| DCM | 314, 383 | 298, 390 | 297, 408 | 320, 400 |
| TCM | 315, 384 | 299, 391 | 299, 410 | 320, 403 |
| DMF | 314, 383 | 297, 389 | 297, 407 | 319, 401 |
| DMSO | 316, 385 | 298, 391 | 298, 410 | 322, 403 |
| ACN | 312, 378 | 296, 386 | 295, 402 | 316, 395 |
| Solvent | BPC-2DPx | BPC-3DPx | BPC-2DPP | BPC-3DPP |
|---|---|---|---|---|
| Hex | 465 | 487 | 520 | 503 |
| PhH | 473 | 500 | 530 | 511 |
| THF | 478 | 510 | 536 | 517 |
| DCM | 485 | 518 | 549 | 520 |
| TCM | 486 | 518 | 542 | 519 |
| DMF | 492 | 522 | 551 | 522 |
| DMSO | 500 | 529 | 563 | 533 |
| ACN | 490 | 525 | 553 | 524 |
| Dihedral Angles (deg) | λmax (nm)/λonset (nm) | Egb (eV) | HOMO c (eV) | LUMO d (eV) | Ege (eV) | HOMO e (eV) | LUMO e (eV) | |
|---|---|---|---|---|---|---|---|---|
| BPC-2DPx | 41.77, 40.92 | 314, 383/440 | 2.82 | −5.55 | −2.73 | 3.32 | −5.37 | −2.05 |
| BPC-3DPx | 37.28, 36.86 | 298, 390/459 | 2.70 | −5.49 | −2.79 | 3.09 | −5.09 | −2.00 |
| BPC-2DPP | 39.42, 19.42 | 297, 408/501 | 2.48 | −5.54 | −3.06 | 2.94 | −5.35 | −2.41 |
| BPC-3DPP | 36.21, 15.23 | 320, 400/473 | 2.62 | −5.68 | −3.06 | 2.80 | −5.13 | −2.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Kong, L.; Du, H.; Li, X.; Feng, H.; Zhao, J.; Xie, Y. Effects of Pyrazine Derivatives and Substituted Positions on the Photoelectric Properties and Electromemory Performance of D–A–D Series Compounds. Materials 2018, 11, 2063. https://doi.org/10.3390/ma11102063
Song X, Kong L, Du H, Li X, Feng H, Zhao J, Xie Y. Effects of Pyrazine Derivatives and Substituted Positions on the Photoelectric Properties and Electromemory Performance of D–A–D Series Compounds. Materials. 2018; 11(10):2063. https://doi.org/10.3390/ma11102063
Chicago/Turabian StyleSong, Xuejing, Lingqian Kong, Hongmei Du, Xiangyu Li, Hanlin Feng, Jinsheng Zhao, and Yu Xie. 2018. "Effects of Pyrazine Derivatives and Substituted Positions on the Photoelectric Properties and Electromemory Performance of D–A–D Series Compounds" Materials 11, no. 10: 2063. https://doi.org/10.3390/ma11102063