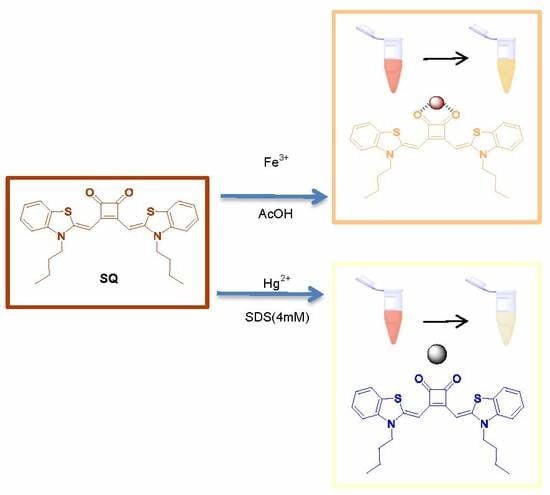
Dual Sensing Performance of 1,2-Squaraine for the Colorimetric Detection of Fe3+ and Hg2+ Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. General Procedures for UV-Vis Experiments
2.3. Job’s Plot Measurements
2.4. Competition Tests
3. Results
3.1. Spectral Properties of SQ
3.2. Colorimetric Sensing for Fe3+ and Hg2+
3.3. Binding Constant (Ka) and Limit of Detection (LOD) for Fe3+ and Hg2+
3.4. Complexation Mechanism of SQ–Fe3+ and SQ–Hg2+
3.5. Preliminary Analytical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Gao, X.H.; Shi, W.; Ma, H.M. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent progress on the development of chemosensors for gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ye, B.; Xia, G.; Zhao, X.; Wang, H. A colorimetric and fluorescent chemosensor for the highly sensitive detection of CO2 gas: Experiment and DFT calculation. Sens. Actuators B Chem. 2016, 233, 76–82. [Google Scholar] [CrossRef]
- Qin, W.; Dou, W.; Leen, V.; Dehan, W.; Van der Auwerar, M.; Boens, N. A ratiometric fluorescent BODIPY-based probe for transition and heavy metal ions. RSC Adv. 2016, 6, 7806–7816. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Qin, J.; Wang, G.; Wang, B.; Fu, A.; Yang, Z. Development of a simple pyrazine-derived “turn on” Al3+ fluorescent sensor with high selectivity and sensitivity. Inorg. Chim. Acta 2015, 430, 91–95. [Google Scholar] [CrossRef]
- Xu, X.X.; Zheng, X.L.; Fan, X.X.; Su, Y.T.; Zhan, X.Q.; Zheng, H. Semicarbazide-based naphthalimide as a highly selective and sensitive colorimetric and “turn-on” fluorescent chemodosimeter for Cu2+. Sens. Actuators B 2016, 227, 191–197. [Google Scholar]
- Sinha, S.; Mukherjee, T.; Mathew, J.; Mukhopadhyay, S.K.; Ghosh, S. Triazole-based Zn2+-specific molecular marker for fluorescence bioimaging. Anal. Chim. Acta 2014, 822, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, C.; Chen, H.; Mack, J.; Guo, Z.; Shen, Z. A red fluorescent turn-on probe for hydrogen sulfide and its application in living cells. Chem. Commun. 2013, 49, 7510–7512. [Google Scholar] [CrossRef] [PubMed]
- Uglov, A.; Bessmertnykh-Lemeune, A.; Guilard, R.; Averin, A.; Beletskaya, I. Optical methods for the detection of heavy metal ions. Russ. Chem. Rev. 2014, 83, 196–224. [Google Scholar] [CrossRef] [Green Version]
- Li, H.D.; Li, L.L.; Yin, B.Z. Highly selective fluorescent chemosensor for Fe3+ detection based on diaza-18-crown-6 ether appended with dual coumarins. Chem. Commun. 2014, 42, 1–4. [Google Scholar] [CrossRef]
- Bhorge, Y.R.; Tsai, H.T.; Huang, K.F.; Pape, A.J.; Janaki, S.N.; Chen, Y.P. A new pyrene-based schiff-base: A selective colorimetric and fluorescent chemosensor for detection of Cu(II) and Fe(III). Spectrochim. Acta Part. A 2014, 130, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Aisen, A.; Wessling-Resnick, M.; Leibold, E.A. Iron metabolism. Curr. Opin. Chem. Biol. 1999, 3, 200–206. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Richardson, D.R. Future of toxicology iron chelators and differing modes of action and toxicity: The changing face of iron chelation therapy. Chem. Res. Toxicol. 2007, 20, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C. Iron deficiency and erythropoiesis: New diagnostic approaches. Clin. Chem. 2003, 49, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Natale, F.D.; Lancia, A.; Molino, A.; Natale, M.D.; Karatza, D.; Musmarra, D. Capture of mercury ions by natural and industrial materials. J. Hazard. Mater. 2006, 132, 220–225. [Google Scholar] [PubMed]
- Fitzgerald, W.F.; Lamborg, C.H.; Hammerschmidt, C.R. Marine biogeochemical cycling of mercury. Chem. Rev. 2007, 107, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury–current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar]
- Carvalho, C.M.L.; Chew, E.H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [PubMed]
- Lunvongsa, S.; Oshima, M.; Motomizu, S. Determination of total and dissolved amount of iron in water samples using catalytic spectrophotometric flow injection analysis. Talanta 2006, 68, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.C.; Segundo, M.A.; Lima, J.C.; Rangel, A.S. Spectrophotometric determination of iron and boron in soil extracts using a multi-syringe flow injection system. Talanta 2005, 66, 703–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timerbaev, A.R.; Dabek-Zlotorzynska, E.; Marc van den Hoop, A.G.T. Inorganic environmental analysis by capillary electrophoresis. Analyst 1999, 124, 811–826. [Google Scholar] [CrossRef]
- Vanloot, P.; Coulomb, B.; Brach-Papa, C.; Sergent, M.; Boudenne, J.L. Multivariate optimization of solid-phase extraction applied to iron determination in finished waters. Chemosphere 2007, 69, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Shamspur, T.; Sheikhshoaie, I.; mashhadizadeh, M.H. Flame atomic absorption spectroscopy (FAAS) determination of iron(III) after preconcentration on to modified analcime zeolite with 5-((4-nitrophenylazo)-N-(2′,4′-dimethoxyphenyl)) salicylaldimine by column method. J. Anal. At. Spectrom. 2005, 20, 476–478. [Google Scholar] [CrossRef]
- Lang, L.; Horvat, M.; Bloom, N.S. An improved speciation method for mercury by GC/CVAFS after aqueous phase ethylation and room temperature precollection. Talanta 1994, 41, 371–379. [Google Scholar] [CrossRef]
- Fabbri, D.; Lombardo, M.; Trombinit, C.; Vassura, I. A new procedure for the speciation of mercury in water based on the transformation of mercury(II) and methylmercury(II) into stable acetylides followed by HPLC analysis. Appl. Organomet. Chem. 1995, 9, 713–718. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Hu, W.; Zhang, L.; Huang, Q.; Ma, T. Highly selective fluorescence enhancement chemosensor for Hg2+ based on rhodamine and its application in living cells and aqueous media. Sens. Actuator B Chem. 2013, 183, 290–296. [Google Scholar] [CrossRef]
- Dong, Z.; Tian, X.; Chen, Y.; Hou, J.; Guo, Y.; Sun, J.; Ma, J. A highly selective fluorescent chemosensor for Hg2+ based on rhodamine B and its application as a molecular logic gate. Dyes. Pigm. 2013, 97, 324–329. [Google Scholar] [CrossRef]
- Ghosh, K.; Tarafdar, D. A new quinoline-based chemosensor in ratiometric sensing of Hg2+ ions. Supramol. Chem. 2012, 25, 127–132. [Google Scholar] [CrossRef]
- Qazi, M.A.; Ocak, U.; Ocak, M.; Memon, S. An excellent copper selective chemosensor based on calix[4]arene framework. Anal. Chim. Acta 2013, 761, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Sargsyan, G.; Olive, A.M.; Balaz, M. Highly Sensitive and Selective Spectroscopic Detection of Mercury(II) in Water by Using Pyridyl porphyrin-DNA Conjugates. Chem. Eur. J. 2013, 19, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, F.; Zou, Y.; Chen, L.; Yang, N.; Zhou, X. Recognition of Cu2+ and Hg2+ in physiological conditions by a new rhodamine based dual channel fluorescent probe. Sens. Actuators B Chem. 2014, 192, 512–521. [Google Scholar] [CrossRef]
- Kim, H.; Rao, B.A.; Jeong, J.W.; Mallick, S.; Kang, S.M.; Choi, J.S.; Lee, C.S.; Son, Y.A. A highly selective dual-channel Cu2+ and Al3+ chemodosimeter in aqueous systems: Sensing in living cells and microfluidic flows. Sens. Actuators B Chem. 2015, 210, 173–182. [Google Scholar] [CrossRef]
- Shahid, M.; Razi, S.S.; Srivastava, P.; Ali, R.; Maiti, B.; Misra, A. A useful scaffold based on acenaphthene exhibiting Cu2+ induced excimer fluorescence and sensing cyanide via Cu2+ isplacement approach. Tetrahedron 2012, 68, 9076–9080. [Google Scholar] [CrossRef]
- Sun, J.; Ye, B.; Xia, G.; Wang, H. A multi-responsive squaraine-based“turn on” fluorescent chemosensor for highly sensitive detection of Al3+, Zn2+ and Cd2+ in aqueous media and its biological application. Sens. Actuators B Chem. 2017, 249, 386–394. [Google Scholar] [CrossRef]
- Jeong, J.; Rao, B.A.; Son, Y.A. Dual sensing performance of a rhodamine-derived scaffold for the determination of Cu2+ and Ce4+ in aqueous media. Sens. Actuators B Chem. 2015, 220, 1254–1265. [Google Scholar] [CrossRef]
- Jo, T.G.; Bok, K.H.; Han, J.; Lim, M.H.; Kim, C. Colorimetric detection of Fe3+ and Fe2+ and sequential fluorescent detection of Al3+ and pyrophosphate by an imidazole-based chemosensor in a near-perfect aqueous solution. Dyes Pigm. 2017, 139, 136–147. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xue, S.; Liang, Q.; Li, Z.; Xu, S. Highly selective and sensitive colorimetric and fluorescent chemosensor of Fe3+ and Cu2+ based on 2,3,3-trimethylnaphto[1,2-d] squaraine. RSC. Adv. 2016, 6, 6540–6550. [Google Scholar] [CrossRef]
- Wei, T.B.; Zhang, P.; Shi, B.B.; Chen, P.; Lin, Q.; Liu, J.; Zhang, Y.M. A highly selective chemosensor for colorimetric detection of Fe3+ and fluorescence turn-on response of Zn2+. Dyes. Pigm. 2013, 97, 297–302. [Google Scholar] [CrossRef]
- Inoue, T.; Pandy, S.S.; Fujikawa, N.; Yamaguchi, Y.; Hayase, S. Synthesis and characterization of squaric acid based NIR dyes for their application towards dye-sensitized solar cells. J. Photochem. Photobiol. A. 2010, 213, 23–29. [Google Scholar] [CrossRef]
- Maeda, T.; Nakao, H.; Kito, H.; Ichinose, H.; Yagi, S.; Nakazumi, H. Far-red absorbing squarylium dyes with terminally connected electron-accepting units for organic dye-sensitized solar cells. Dyes Pigm. 2011, 90, 275–283. [Google Scholar] [CrossRef]
- Zhu, H.J.; Lin, Y.H.; Wang, G.M.; Chen, Y.Q.; Lin, X.H.; Fu, N.Y. A coordination driven deaggregation approach toward Hg2+-specific chemosensors based on thioether linked squaraine-aniline dyads. Sens. Actuators B 2014, 198, 201–209. [Google Scholar] [CrossRef]
- Li, B.H.; Li, W.W.; Xu, Y.Q.; Li, J.; Tu, J.; Sun, S. A simple approach for the discrimination of surfactants based on the control of squaraine aggregation. Chem. Commun. 2015, 51, 14652–14655. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Z.; Malkovskiy, A.; Sun, S.; Pang, Y. Aggregation control of squaraines and their use as near-infrared fluorescent sensors for protein. J. Phys. Chem. 2010, 114, 8574–8580. [Google Scholar] [CrossRef] [PubMed]
- McEwen, J.J.; Wallace, K.J. Squaraine dyes in molecular recognition and self-assembly. Chem. Commun. 2009, 42, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, B.; Li, L.; Xiao, J.; Ouyang, S.; Sun, Y.; Pang, A. A colorimetric and near-infrared fluorescent probe with high sensitivity and selectivity for acid phosphatase and inhibitor screening. Chem. Commun. 2014, 50, 8677–8680. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cho, B.; Chan, L.Y.; Kwan, W.L.; Lee, C.L. Development of asymmetrical near infrared squaraines with large stokes shift. RSC. Adv. 2015, 5, 106868–106876. [Google Scholar] [CrossRef]
- Ronchi, E.; Ruffo, R.; Rizzato, S.; Albinati, A.; Beverina, L.; Pagani, G.A. Regioselective synthesis of 1,2- vs. 1,3-Squaraines. Org. Lett. 2011, 12, 3166–3169. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A. Chemistry of squaraine-derived materials: Near-IR Dyes, low band gap systems, and cation sensors. Acc. Chem. Res. 2005, 38, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ros-Lis, J.; Martínez-Máñez, R.; Rurack, K.; Sancenón, F.; Soto, J.; Spieles, M. Highly selective chromogenic signaling of Hg2+ in aqueous media at nanomolar levels employing a squaraine-based reporter. Inorg. Chem. 2004, 43, 5183–5185. [Google Scholar] [CrossRef] [PubMed]
- Basheera, M.; Alex, S.; Thomas, K.; Suresh, C.; Dasa, S. A squaraine-based chemosensor for Hg2+ and Pb2+. Tetrahedron 2006, 62, 605–610. [Google Scholar] [CrossRef]
- Chen, C.; Wang, R.; Guo, L.; Fu, N.; Dong, H.; Yuan, Y. A squaraine-based colorimetric and “turn on” fluorescent sensor for selective detection of Hg2+ in an aqueous medium. Org. Lett. 2011, 13, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Law, K.Y.; Perlstein, J.; Whitten, D.G. Amphiphilic squaraine dye aggregates: Evidence for a cyclic chiral structure as a general supramolecular structure for aggregates of dyes and aromatic molecules. J. Am. Chem. Soc. 1995, 117, 7257–7258. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, B.; Yao, S.; Bondar, M.V.; Belfield, K.D. Controlled Aggregation and Enhanced Two-Photon Absorption of a Water-Soluble Squaraine Dye with a Poly(acrylic acid) Template. Langmuir 2013, 29, 11005–11012. [Google Scholar] [CrossRef] [PubMed]
- You, G.R.; Park, G.J.; Lee, S.A.; Ryu, K.Y.; Kim, C. Chelate-type Schiff base acting as a colorimetric sensor for iron in aqueous solution. Sens. Actuators B Chem. 2015, 215, 188–195. [Google Scholar] [CrossRef]
- Choi, Y.W.; Park, G.J.; Na, Y.J.; Jo, H.Y.; Lee, S.A.; You, G.R. A single Schiff base molecule for recognizing multiple metal ions: A fluorescence sensor for Zn(II) and Al(III) and colorimetric sensor for Fe(II) and Fe(III). Sens. Actuators B Chem. 2014, 194, 343–352. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Xie, X.; Ma, S.; Liu, Y.; Xu, Z.; Gao, Y.; Ye, Y. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B Chem. 2016, 224, 661–667. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y.; Yin, J.; Wei, G.; Du, Y. Design and application of Fe3+ probe for “naked-eye” colorimetric detection in fully aqueous system. Sens. Actuators B Chem. 2011, 160, 1316–1321. [Google Scholar] [CrossRef]
- Lee, S.; Rao, B.A.; Son, Y.A. A highly selective fluorescent chemosensor for Hg2+ based on a squaraine–bis (rhodamine-B) derivative: Part II. Sens. Actuators B Chem. 2015, 210, 3519–3532. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rao, B.A.; Hwang, J.Y.; Son, Y.A. A novel sensing capabilities and structural modification from thiourea to urea derivative by Hg(ClO4)2: Selective dual chemodosimeter for Hg2+ and F− ions. Sens. Actuators B Chem. 2015, 220, 1070–1085. [Google Scholar] [CrossRef]
- Kaur, P.; Sareen, D. The synthesis and development of a dual-analyte colorimetric sensor: Simultaneous estimation of Hg2+ and Fe3+. Dyes. Pigm. 2011, 88, 296–300. [Google Scholar] [CrossRef]
- Ho, T.L. Hard soft acids bases (HSAB) principle and organic chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Huang, J.H.; Sun, Y.Y.; Chou, P.T.; Fang, J.M. Two-stage sensing property via a conjugated donor-acceptor-donor constitution: Application to the visual detection of mercuric Ion. Org. Chem. 2005, 70, 5827–5832. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]












| Sensor | Target | Response Type | LOD (μM) | Reaction Media | Reversibility | Reference |
|---|---|---|---|---|---|---|
| Dansyl based derivative | Fe3+ | Fluorescence | 0.62 | C2H5OH-H2O (1:1, v/v) | No | [57] |
| Rhodamine derivative | Fe3+ | Fluorescence | 0.74 | CH3CN-H2O(1:1, v/v) | Yes | [58] |
| Sugar-functioned coumarin | Fe3+ | Color | 4.6 | H2O | No | [59] |
| Julolidine derivative | Fe3+ | Color | 6.8 | DMF | No | [57] |
| Squaraine -bis(rhodamine-B) derivative | Hg2+ | Fluorescence | 6.48 | CH3CN | Yes | [60] |
| Coumarin-urea derivative | Hg2+ | Fluorescence | 0.45 | CH3CN | Yes | [61] |
| Hetarylazo | Fe3+ | Color | 2.0 | CH3CN | No | [62] |
| Hg2+ | Color | 2.0 | CH3CN | No | ||
| Naphthalimide-rhodamine | Fe3+ | Color | 0.57 | EtOH/PBS buffer (1:1) | No | [63] |
| Hg2+ | Fluorescence | 2.72 | EtOH/PBS buffer (1:1) | Yes | ||
| Our work | Fe3+ | Color | 0.54 | CH3COOH | Yes | / |
| Hg2+ | Color | 1.69 | SDS (4 mM) | No |
| Sample | AAS (μM) | Added (mM) | Found (mM) | Recovery (%) | |
|---|---|---|---|---|---|
| Industrial waste water I | Fe3+ | 2.01 | 1.12 | 1.14 | 102 |
| Hg2+ | 3.20 | 4.30 | 4.41 | 102 | |
| Industrial waste water II | Fe3+ | 4.03 | 2.23 | 2.19 | 98 |
| Hg2+ | 2.43 | 2.85 | 2.77 | 97 | |
| Industrial waste water III | Fe3+ | 6.05 | 4.41 | 4.38 | 99 |
| Hg2+ | 1.65 | 2.20 | 2.17 | 99 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, N.; Xu, M.-M.; Jiang, C.; Wang, J.; Song, G.; Wang, Y. Dual Sensing Performance of 1,2-Squaraine for the Colorimetric Detection of Fe3+ and Hg2+ Ions. Materials 2018, 11, 1998. https://doi.org/10.3390/ma11101998
Liu X, Li N, Xu M-M, Jiang C, Wang J, Song G, Wang Y. Dual Sensing Performance of 1,2-Squaraine for the Colorimetric Detection of Fe3+ and Hg2+ Ions. Materials. 2018; 11(10):1998. https://doi.org/10.3390/ma11101998
Chicago/Turabian StyleLiu, Xiaoqian, Na Li, Min-Min Xu, Chunhui Jiang, Jianhao Wang, Guoqiang Song, and Yong Wang. 2018. "Dual Sensing Performance of 1,2-Squaraine for the Colorimetric Detection of Fe3+ and Hg2+ Ions" Materials 11, no. 10: 1998. https://doi.org/10.3390/ma11101998