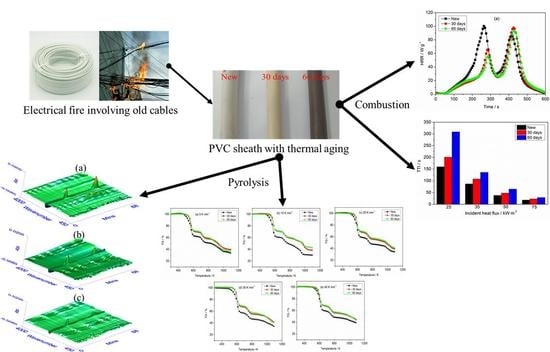
Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter
Abstract
:1. Introduction
2. Experimental
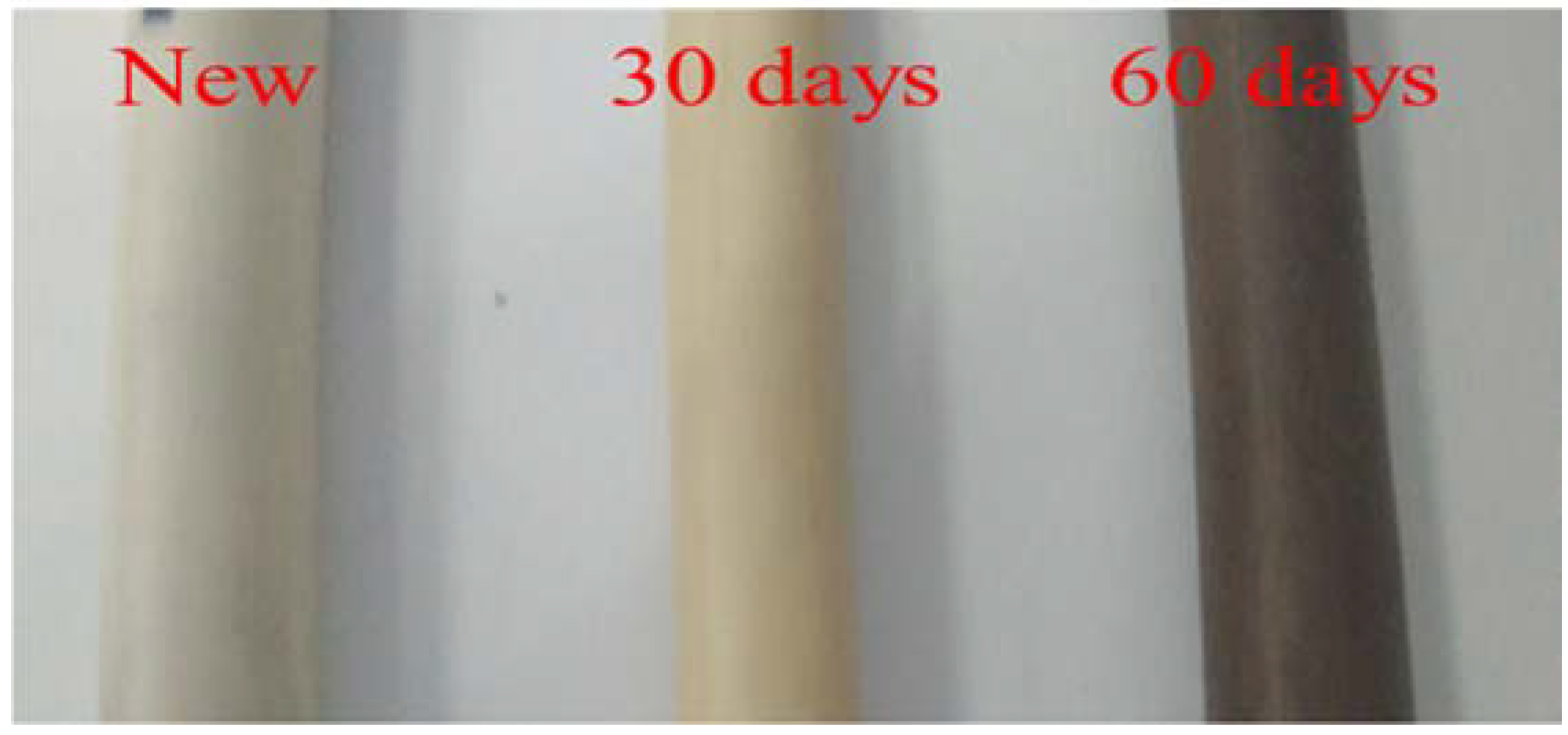
2.1. Sample Preparation
2.2. Thermal Aging
2.3. TG-FTIR Measurements
2.4. Calorimeter Measurements
3. Results and Discussion
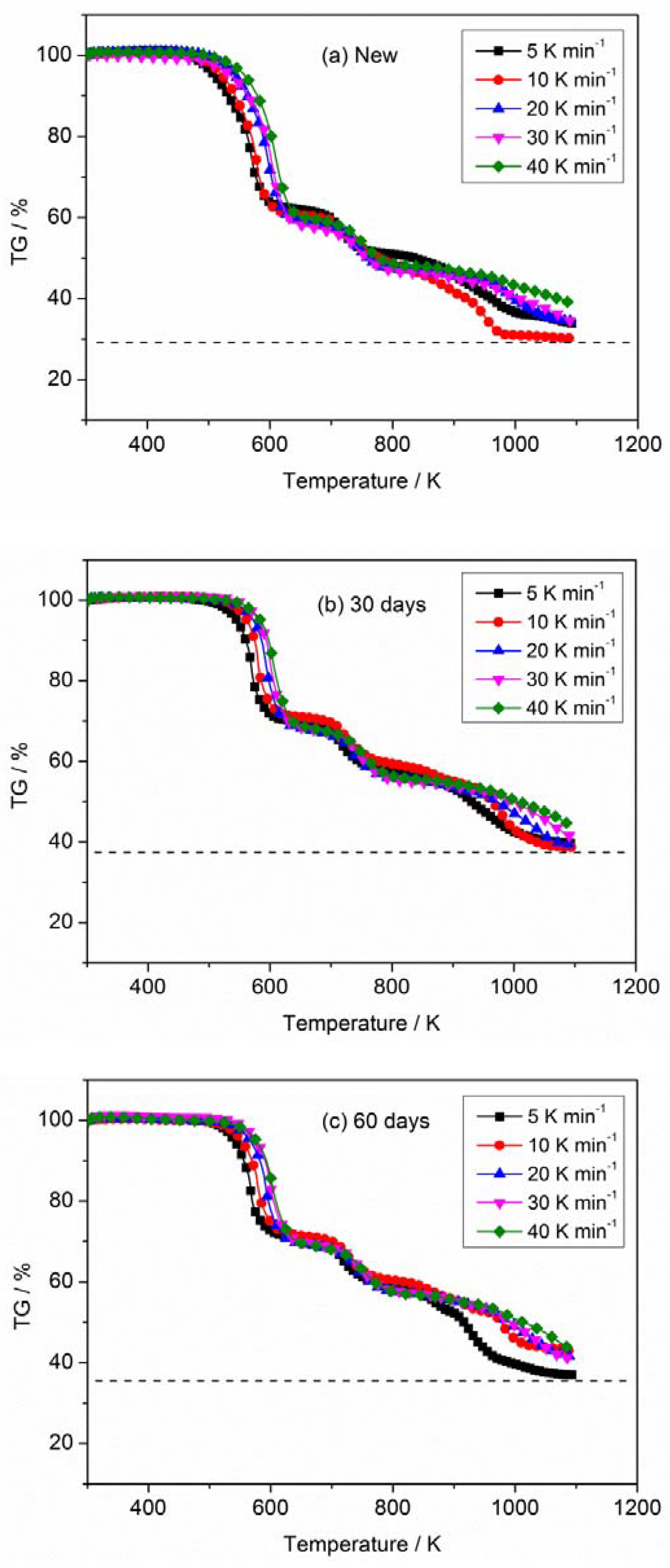
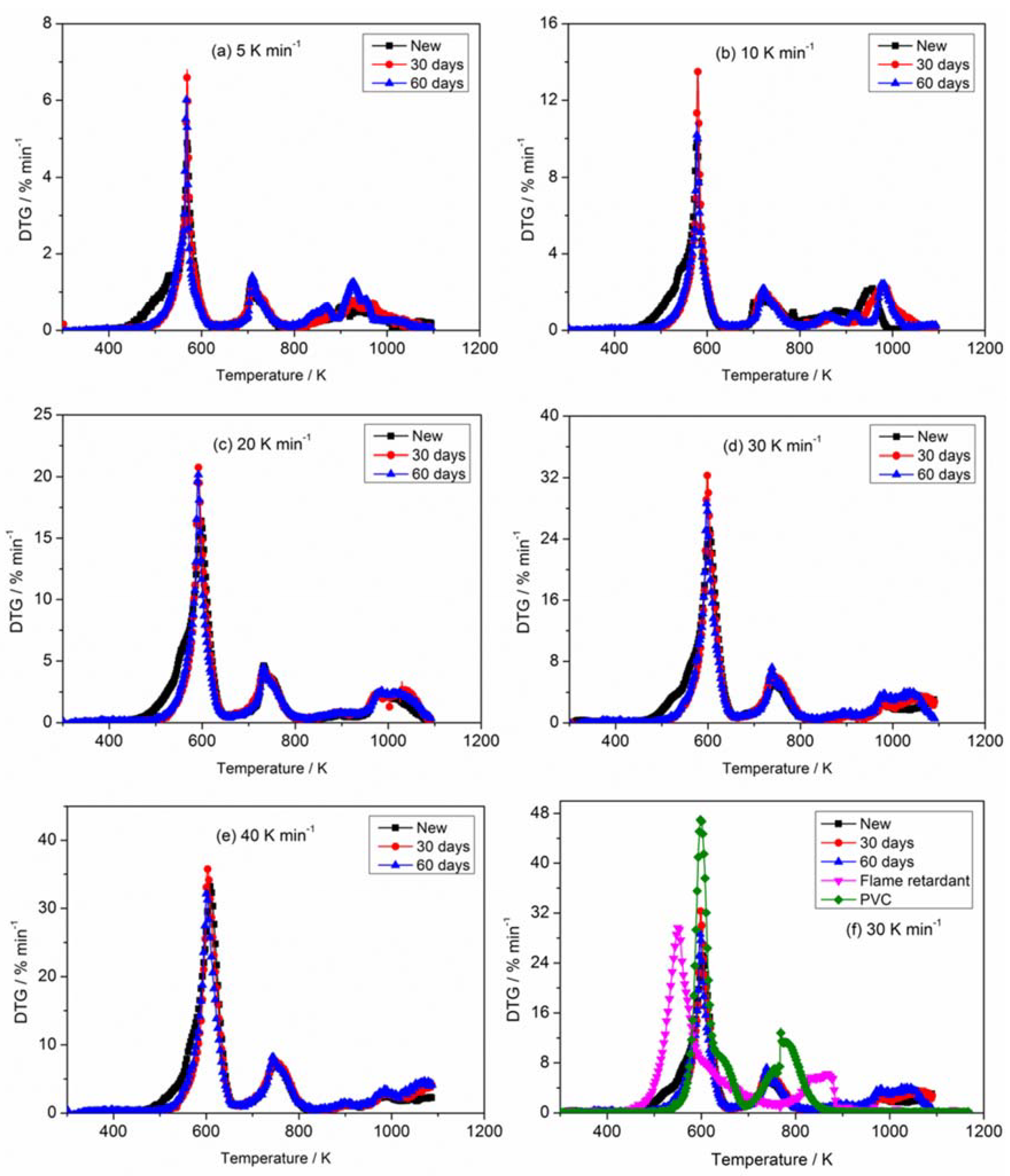
3.1. Thermogravimetric Analysis
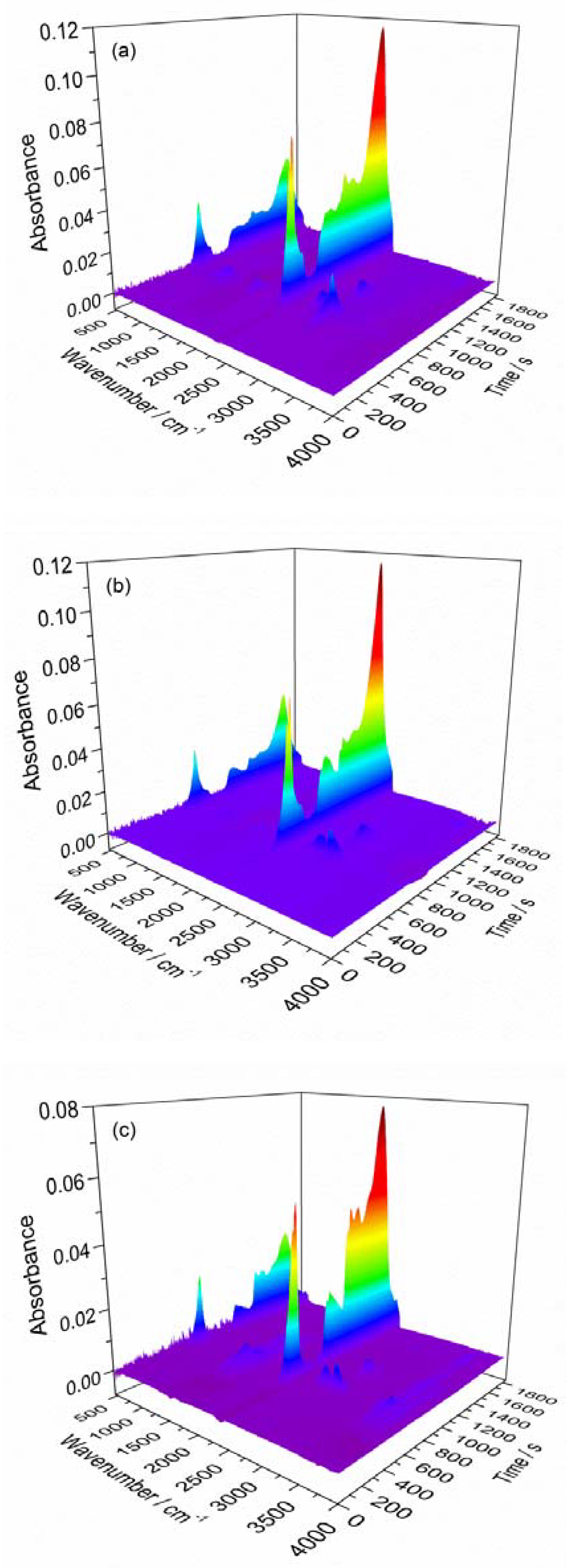
3.2. FTIR Analysis
3.3. MCC Analysis
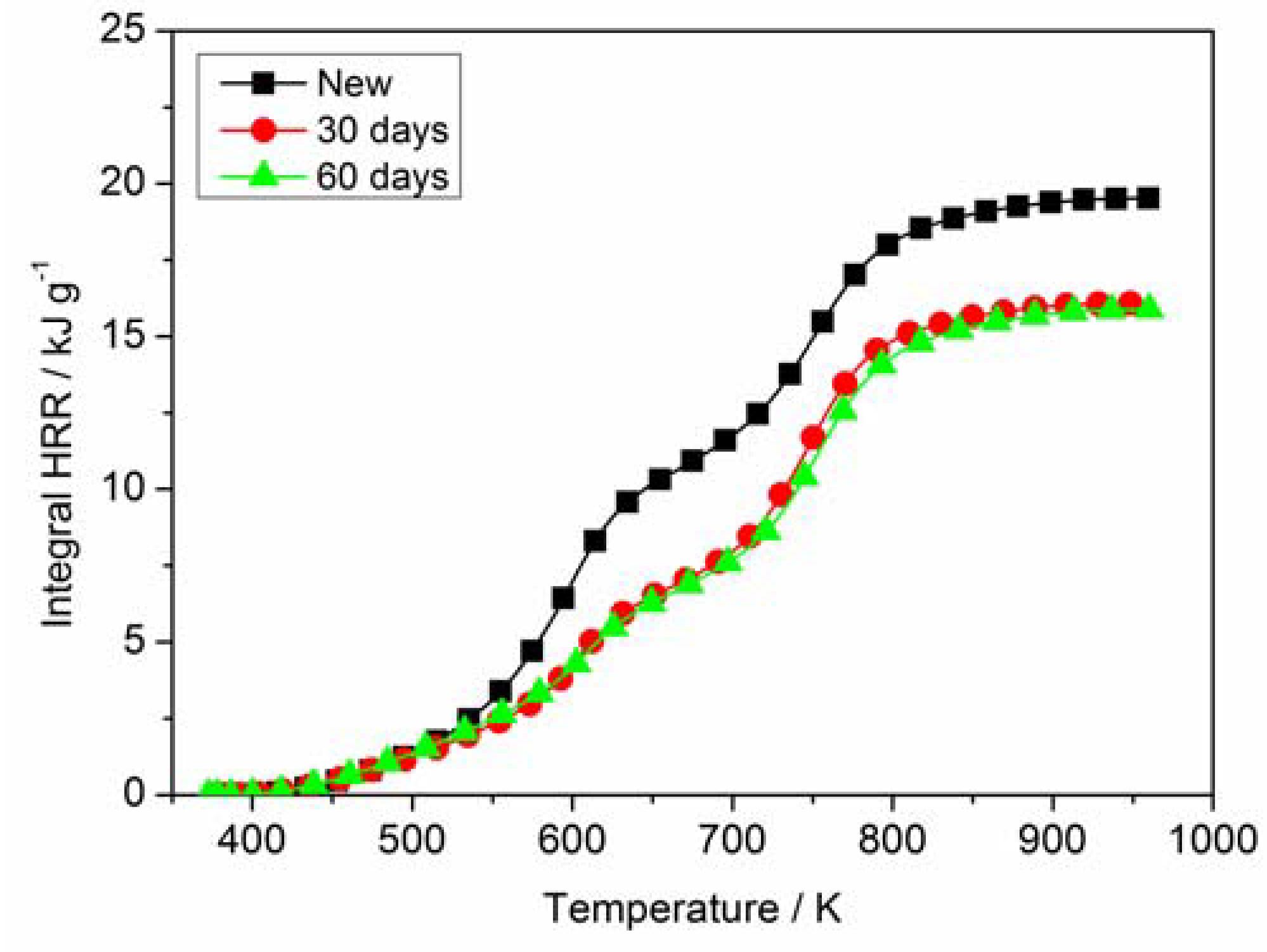
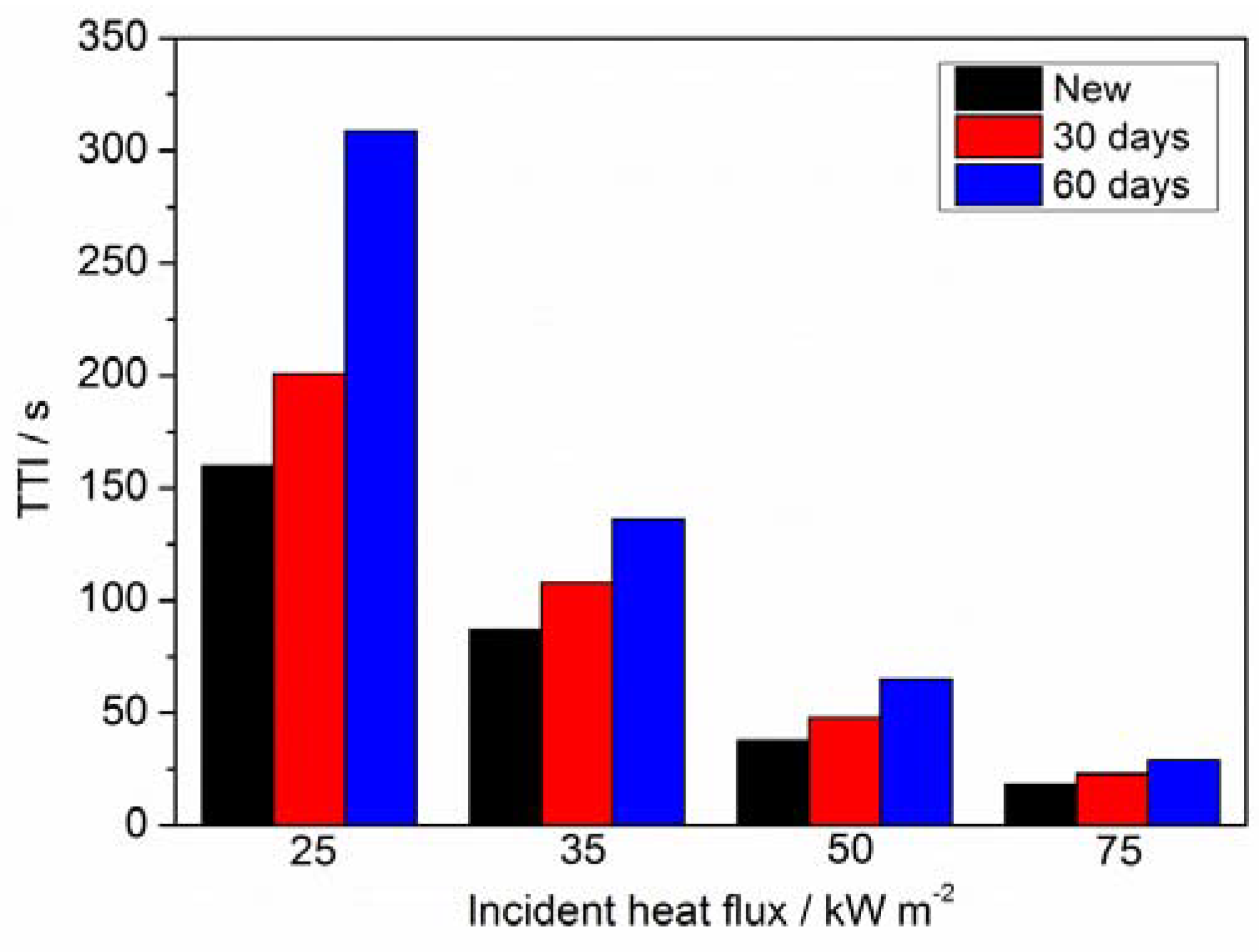
3.4. Cone Calorimetry Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matala, A.; Hostikka, S. Pyrolysis modelling of PVC cable materials. Fire Saf. Sci. 2011, 10, 917–930. [Google Scholar] [CrossRef]
- Emanuelsson, V.; Simonson, M.; Gevert, T. The effect of accelerated ageing of building wires. Fire Mater. 2007, 31, 311–326. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C.; Antonie, S.R.; Chiriac, M.; Precup, M.; Yang, J.; Roy, C. Study of the natural ageing of PVC insulation for electrical cables. Polym. Degrad. Stab. 2000, 67, 209–221. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, H.; Tong, L. Experimental study on the fire protection properties of PVC sheath for old and new cables. J. Hazard. Mater. 2010, 179, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, I.; Yarahmadi, N.; Gevert, T. Effects of accelerated and natural ageing on plasticized polyvinyl chloride (PVC). Polym. Degrad. Stab. 1999, 66, 415–421. [Google Scholar] [CrossRef]
- Meinier, R.; Sonnier, R.; Zavaleta, P.; Suard, S.; Ferry, L. Fire behavior of halogen-free flame retardant electrical cables with the cone calorimeter. J. Hazard. Mater. 2018, 342, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-H.; Lin, Y.-C.; Kuo, C.-P. The study of thermal pyrolysis mechanisms for chloro organic compounds in electric cable and medical wastes. J. Anal. Appl. Pyrolysis 2006, 75, 245–251. [Google Scholar] [CrossRef]
- Henrist, C.; Rulmont, A.; Cloots, R.; Gilbert, B.; Bernard, A.; Beyer, G. Toward the understanding of the thermal degradation of commercially available fire-resistant cable. Mater. Lett. 2000, 46, 160–168. [Google Scholar] [CrossRef]
- Cheng, J.Q.; Jia, W.N.; Shu, Z.J.; Yang, S.S.; Chen, N. Experimental research on rules of releasing of the halogen gas of PVC cable under hydrogenation condition. Fire Sci. Technol. 2007, 26, 383. [Google Scholar]
- Wang, C.; Liu, H.; Zhang, J.; Yang, S.; Zhang, Z.; Zhao, W. Thermal Degradation of Flame-retarded high-voltage Cable Sheath and Insulation via TG-FTIR. J. Anal. Appl. Pyrolysis 2018, 134, 167–175. [Google Scholar] [CrossRef]
- Huggett, C.; Levin, B.C. Toxicity of the pyrolysis and combustion products of poly(vinyl chlorides): A literature assessment. Fire Mater. 1987, 11, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Arlman, E. Thermal and oxidative decomposition of polyvinyl chloride. J. Polym. Sci. 1954, 12, 547–558. [Google Scholar] [CrossRef]
- Hjertberg, T.; Sörvik, E.M. On the influence of HCl on the thermal degradation of poly(vinyl chloride). J. Appl. Polym. Sci. 1978, 22, 2415–2426. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Ferry, L. Effects of ageing on the fire behaviour of flame-retarded polymers: A review. Polym. Int. 2015, 64, 313–328. [Google Scholar] [CrossRef]
- Beneš, M.; Milanov, N.; Matuschek, G.; Kettrup, A.; Plaček, V.; Balek, V. Thermal degradation of PVC cable insulation studied by simultaneous TG-FTIR and TG-EGA methods. J. Therm. Anal. Calorim. 2004, 78, 621–630. [Google Scholar] [CrossRef]
- Gao, Y. Study on pyrolysis characteristics of insulative PVC material used for fire retardant cable. Eng. Plast. Appl. 2007, 35, 44–47. [Google Scholar]
- Courty, L.; Garo, J. External heating of electrical cables and auto-ignition investigation. J. Hazard. Mater. 2017, 321, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pello, A.; Hasegawa, H.; Staggs, K.; Lipska-Quinn, A.; Alvares, N. A study of the fire performance of electrical cables. Fire Saf. Sci. 1991, 3, 237–247. [Google Scholar] [CrossRef]
- Andersson, P.; Rosell, L.; Simonson, M.; Emanuelsson, V. Small and Large Scale Fire Experiments with Electric Cables under Well-Ventilated and Vitiated Conditions. Fire Technol. 2004, 40, 247–262. [Google Scholar] [CrossRef]
- McGrattan, K.B.; Lock, A.J.; Marsh, N.D.; Nyden, M.R. Cable Heat Release, Ignition, and Spread in Tray Installations during Fire (CHRISTIFIRE): Phase 1-Horizontal Trays. Available online: https://www.nist.gov/publications/cable-heat-release-ignition-and-spread-tray-installations-during-fire-christifire-0 (accessed on 11 July 2012).
- McGrattan, K.B.; Bareham, S.D. Cable Heat Release, Ignition, and Spread in Tray Installations during Fire (CHRISTIFIRE) Phase 2: Vertical Shafts and Corridors; NUREG/CR-7010; United States Nuclear Regulatory Commission (U.S.NCR): Washington, DC, USA, 2013; Volume 2. [Google Scholar]
- Grayson, S.; Van Hees, P.; Green, A.M.; Breulet, H.; Vercellotti, U. Assessing the fire performance of electric cables (FIPEC). Fire Mater. 2001, 25, 49–60. [Google Scholar] [CrossRef]
- Quennehen, P.; Royaud, I.; Seytre, G.; Gain, O.; Rain, R.; Espilit, T.; François, S. Determination of the aging mechanism of single core cables with PVC insulation. Polym. Degrad. Stab. 2015, 119, 96–104. [Google Scholar] [CrossRef]
- Ekelund, M.; Edin, H.; Gedde, U.W. Long-term performance of poly(vinyl chloride) cables. Part 1: Mechanical and electrical performances. Polym. Degrad. Stab. 2007, 92, 617–629. [Google Scholar] [CrossRef]
- Ekelund, M.; Azhdar, B.; Hedenqvist, M.S.; Gedde, U.W. Long-term performance of poly (vinyl chloride) cables, Part 2: Migration of plasticizer. Polym. Degrad. Stab. 2008, 93, 1704–1710. [Google Scholar] [CrossRef]
- Gumargalieva, K.; Ivanov, V.; Zaikov, G.; Moiseev, J.V.; Pokholok, T. Problems of ageing and stabilization of poly (vinyl chloride). Polym. Degrad. Stab. 1996, 52, 73–79. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J. An Experimental Study on the Fire Characteristics of New and Aged Building Wires Using a Cone Calorimeter. Available online: https://link.springer.com/article/10.1007/s10973-018-7626-8 (accessed on 14 August 2018).
- Pimentel Real, L.E.; Ferraria, A.M.; do Rego, A.M.B. The influence of weathering conditions on the properties of poly(vinyl chloride) for outdoor applications. An analytical study using surface analysis techniques. Polym. Test. 2007, 26, 77–87. [Google Scholar] [CrossRef]
- ASTM D7309-13. Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Zhu, H.; Jiang, X.; Yan, J.; Chi, Y.; Cen, K. TG-FTIR analysis of PVC thermal degradation and HCl removal. J. Anal. Appl. Pyrolysis 2008, 82, 1–9. [Google Scholar] [CrossRef]
- Conesa, J.A.; Marcilla, A.; Font, R.; Caballero, J.A. Thermogravimetric studies on the thermal decomposition of polyethylene. J. Anal. Appl. Pyrolysis 1996, 36, 1–15. [Google Scholar] [CrossRef]
- Park, J.W.; Oh, S.C.; Lee, H.P.; Kim, H.T.; Yoo, K.O. A kinetic analysis of thermal degradation of polymers using a dynamic method. Polym. Degrad. Stab. 2000, 67, 535–540. [Google Scholar] [CrossRef]
- Yang, J.; Miranda, R.; Roy, C. Using the DTG curve fitting method to determine the apparent kinetic parameters of thermal decomposition of polymers. Polym. Degrad. Stab. 2001, 73, 455–461. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Clough, R.L. Aging effects on fire-retardant additives in polymers. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 767–780. [Google Scholar] [CrossRef]



| Sample | C (%) | H (%) | O (%) | N (%) | Cl (%) | Pb | Sb | Ca |
|---|---|---|---|---|---|---|---|---|
| New | 33.17 | 4.16 | 19.66 | 0.07 | 40.67 | 0.32 | 1.7 | 0.25 |
| 30 days | 30.61 | 3.63 | 20.43 | 0.08 | 37.38 | 3.91 | 1.62 | 2.34 |
| 60 days | 28.21 | 3.24 | 22.24 | 0.06 | 34.61 | 5.39 | 1.56 | 4.69 |
| Sample | Heating Rate (K min−1) | Tonset (K) | DTGpeak (% min−1) | Residuemass (%) |
|---|---|---|---|---|
| New | 5 | 510.41 | 4.88 | 33.72 |
| 10 | 522.36 | 9.91 | 30.17 | |
| 20 | 543.28 | 16.46 | 34.47 | |
| 30 | 552.96 | 25.18 | 34.47 | |
| 40 | 559.12 | 33.22 | 39.07 | |
| 30 days | 5 | 545.84 | 6.69 | 39.63 |
| 10 | 561.77 | 13.58 | 38.70 | |
| 20 | 572.44 | 20.70 | 39.42 | |
| 30 | 580.89 | 32.68 | 41.50 | |
| 40 | 584.08 | 35.97 | 44.36 | |
| 60 days | 5 | 540.75 | 6.29 | 36.91 |
| 10 | 552.54 | 10.78 | 42.87 | |
| 20 | 566.09 | 20.44 | 41.50 | |
| 30 | 576.14 | 28.77 | 41.11 | |
| 40 | 580.24 | 32.75 | 43.42 |
| Sample | Gas | Absorbance | |||
|---|---|---|---|---|---|
| 8.64 min | 10.83 min | 16.80 min | 30.14 min | ||
| New | HCl | 7.26 × 10−3 | 1.00 × 10−2 | 4.33 × 10−3 | 1.40 × 10−3 |
| CO2 | 1.07 × 10−2 | 6.49 × 10−2 | 3.59 × 10−2 | 1.81 × 10−2 | |
| H2O | 2.95 × 10−3 | 5.50 × 10−3 | 6.57 × 10−4 | 5.47 × 10−4 | |
| 30 days | HCl | 7.17 × 10−4 | 1.70 × 10−3 | 3.59 × 10−3 | 2.96 × 10−4 |
| CO2 | 1.29 × 10−2 | 5.24 × 10−2 | 2.79 × 10−2 | 1.27 × 10−2 | |
| H2O | 1.31 × 10−5 | 1.25 × 10−3 | 7.66 × 10−5 | 1.54 × 10−5 | |
| 60 days | HCl | 3.99 × 10−4 | 1.79 × 10−3 | 5.20 × 10−3 | 1.18 × 10−3 |
| CO2 | 1.42 × 10−2 | 5.23 × 10−2 | 2.26 × 10−2 | 7.08 × 10−3 | |
| H2O | 4.71 × 10−3 | 6.35 × 10−3 | 3.74 × 10−3 | 9.39 × 10−4 | |
| Sample | HRC (J g−1 K−1) | PHRR (W g−1) | TPHRR (°C) | THR (kJ g−1) |
|---|---|---|---|---|
| New | 100 | 100.1 | 324.2 | 19.5 |
| 30 days | 104 | 98.0 | 471.4 | 16.2 |
| 60 days | 97 | 93.1 | 476.8 | 15.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 2018, 11, 1997. https://doi.org/10.3390/ma11101997
Wang Z, Wei R, Wang X, He J, Wang J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials. 2018; 11(10):1997. https://doi.org/10.3390/ma11101997
Chicago/Turabian StyleWang, Zhi, Ruichao Wei, Xuehui Wang, Junjiang He, and Jian Wang. 2018. "Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter" Materials 11, no. 10: 1997. https://doi.org/10.3390/ma11101997