Efficient Extraction of a Docosahexaenoic Acid (DHA)-Rich Lipid Fraction from Thraustochytrium sp. Using Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Strain
2.2. Harvesting and Freeze-Drying
2.3. Analytical Determination of Total Lipid Content
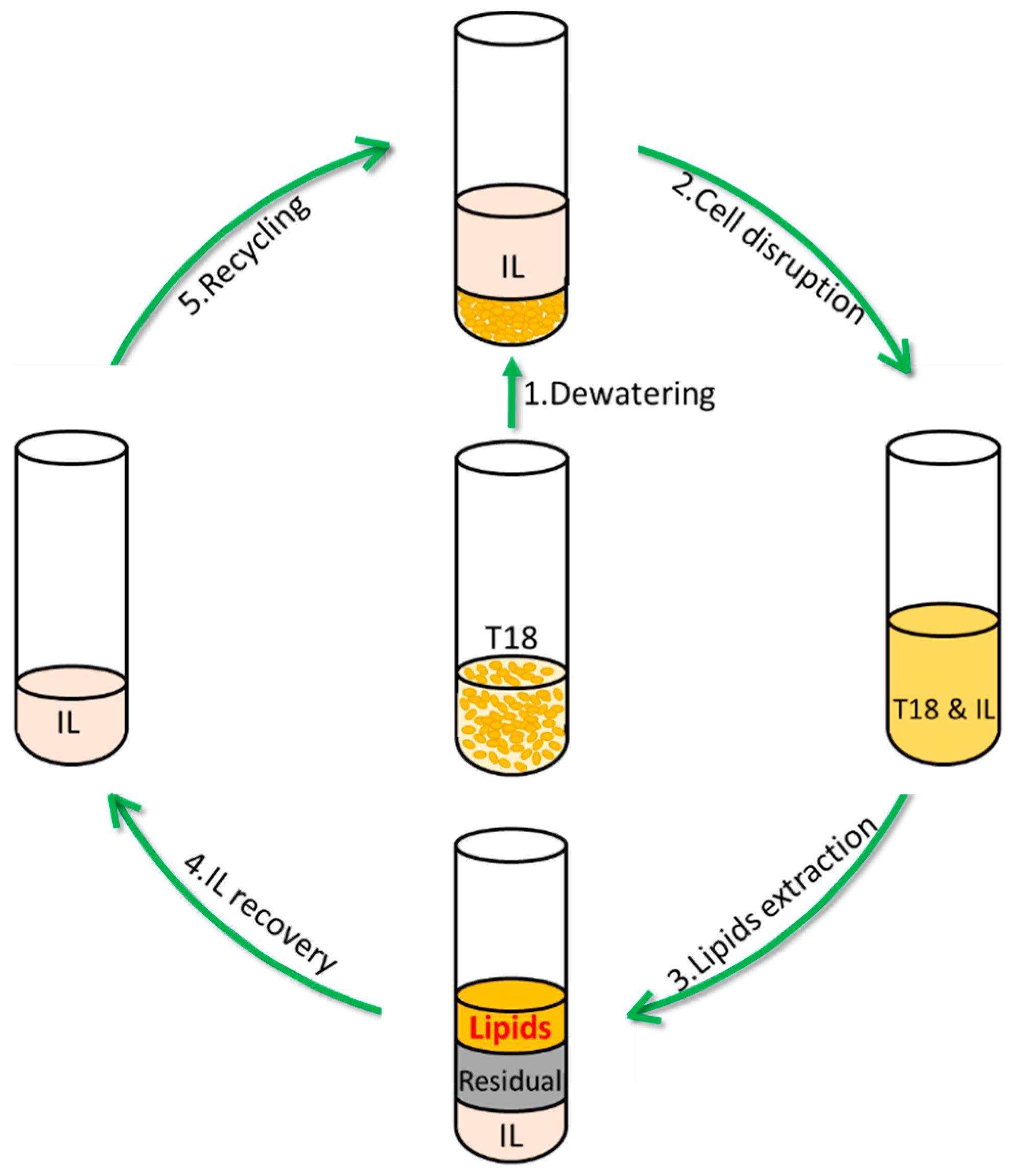
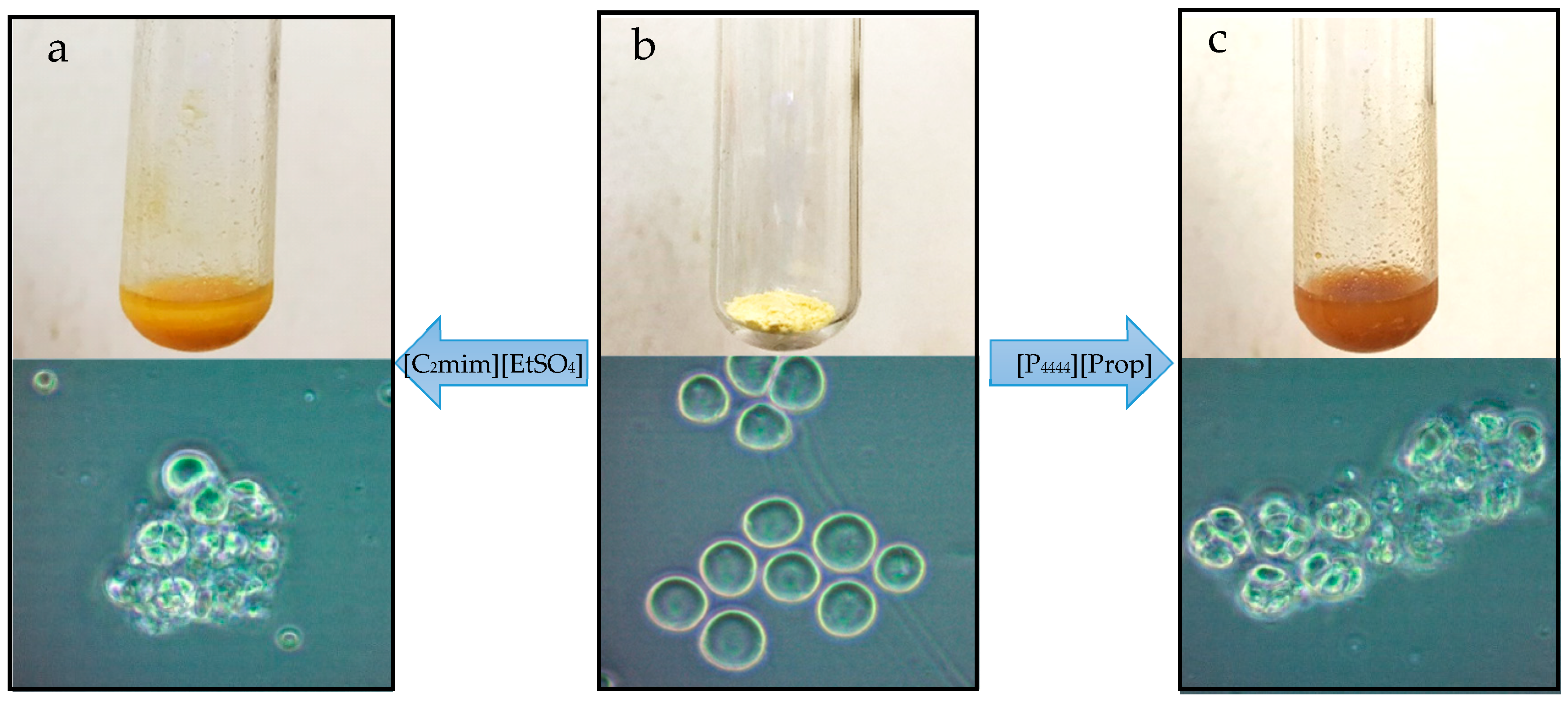
2.4. IL Pretreatment
2.5. IL Recycling
2.6. Lipid Composition
3. Results and Discussion
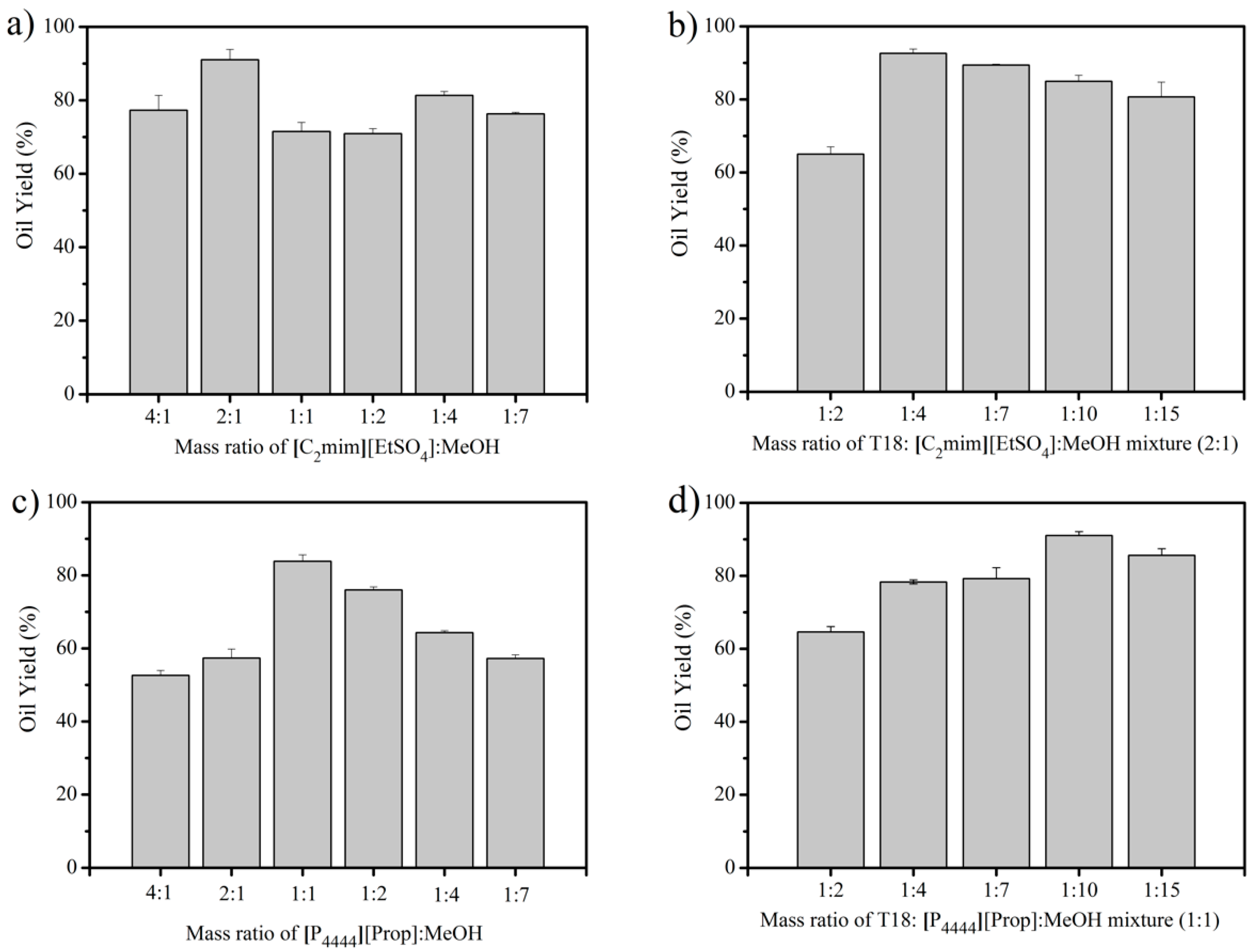
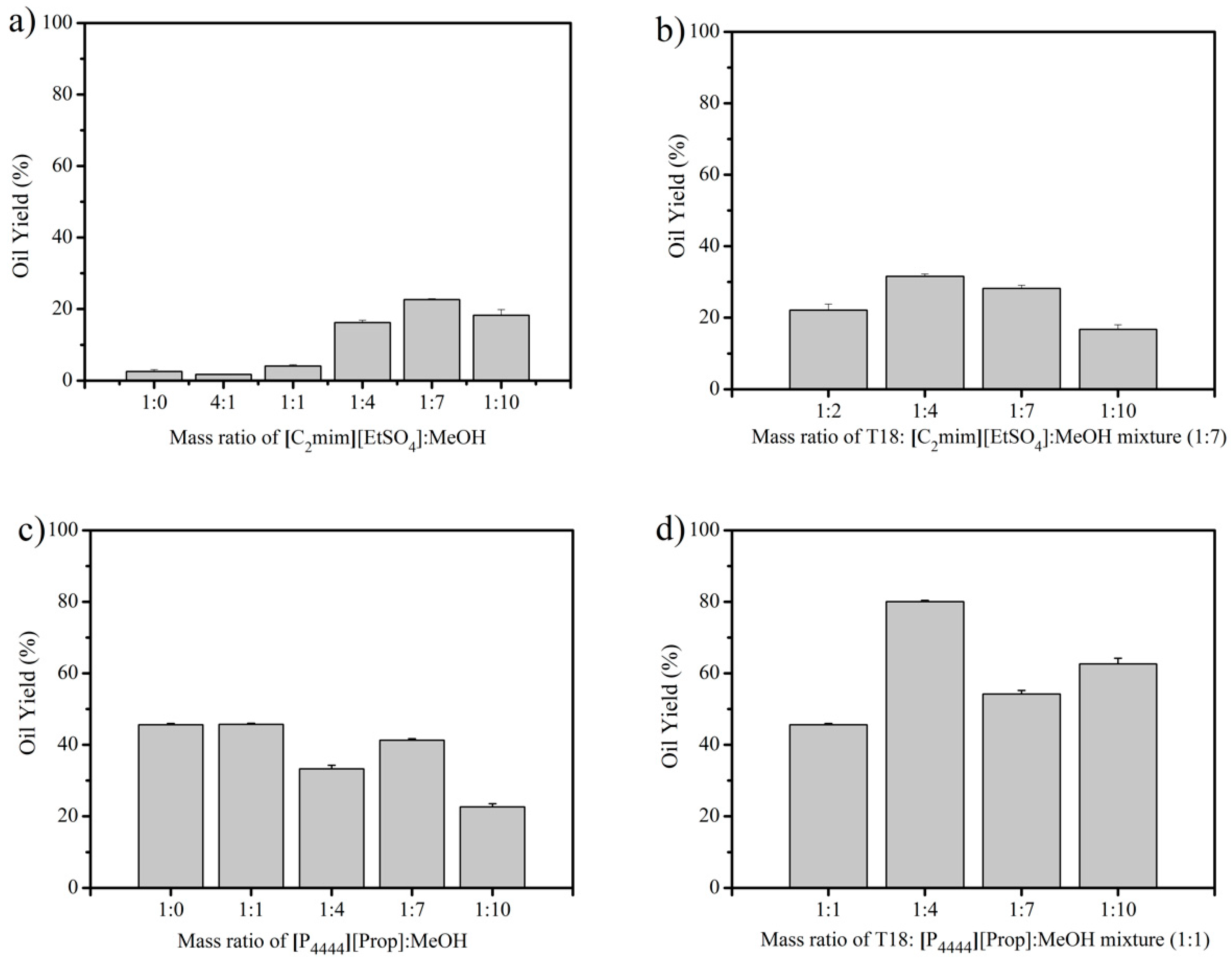
3.1. IL Extraction of Oils from Dried T18.
3.2. IL Extraction of Oils from Wet T18
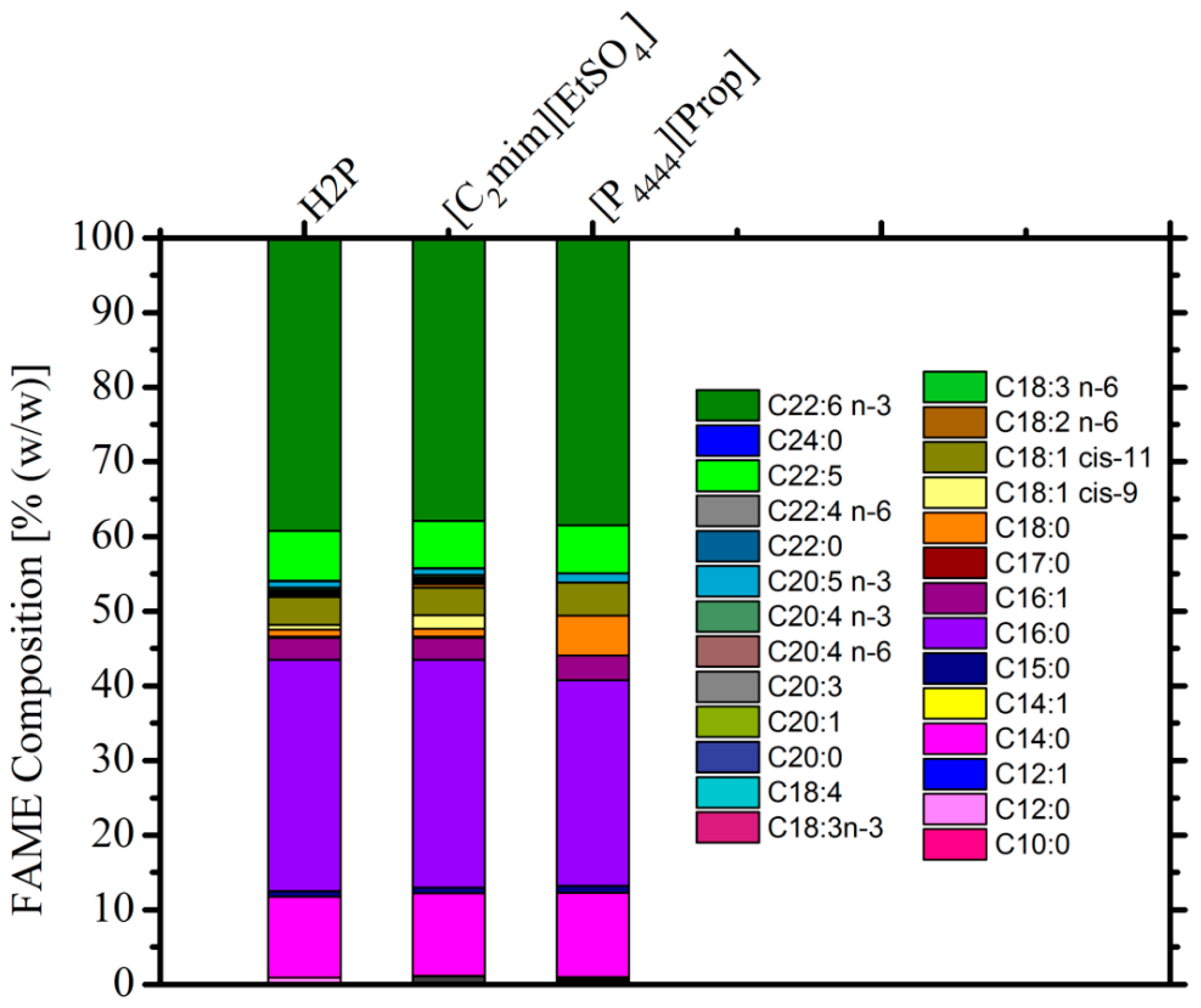
3.3. Composition of Extracted Lipids
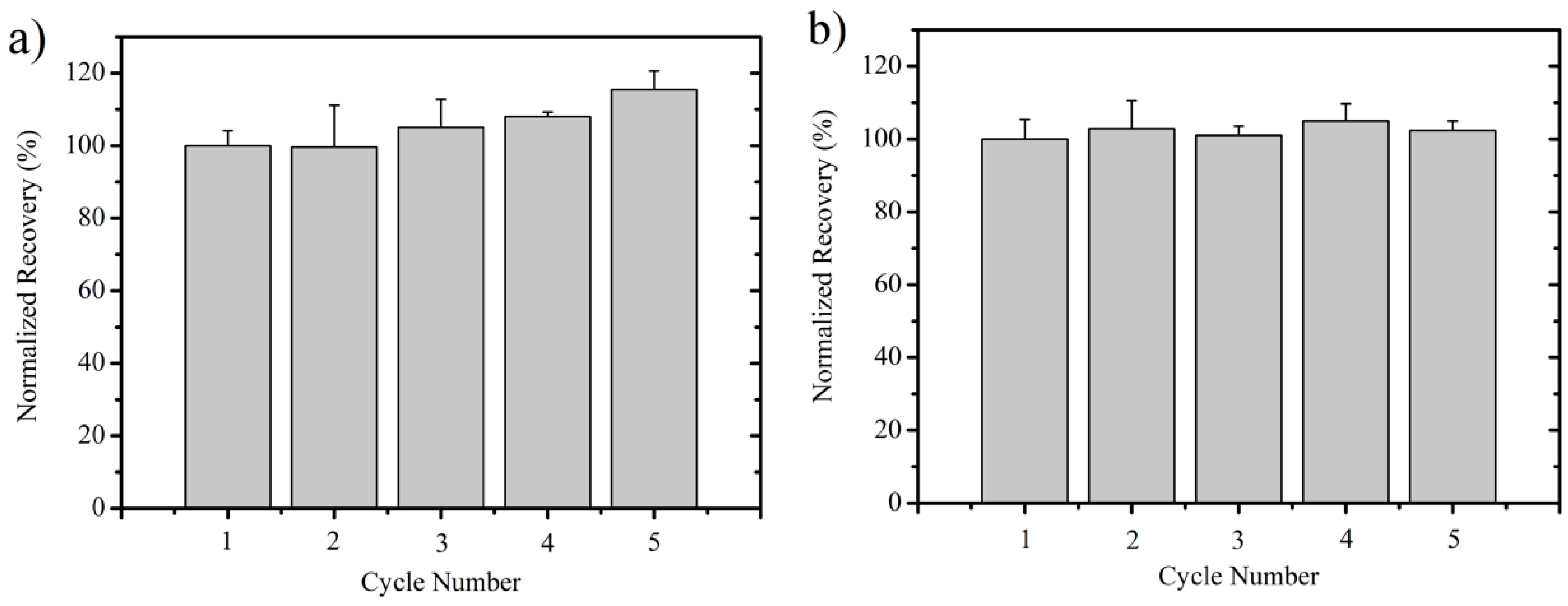
3.4. Ionic Liquid Recycling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, S.A.; Jung, J.Y.; Kim, K.; Kwon, J.H.; Lee, J.S.; Kim, S.W.; Park, J.Y.; Yang, J.W. Effects of molten-salt/ionic-liquid mixture on extraction of docosahexaenoic acid (DHA)-rich lipids from Aurantiochytrium sp. KRS101. Bioprocess Biosyst. Eng. 2014, 37, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Sijtsma, L.; De Swaaf, M.E. Biotechnological production and applications of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. Appl. Microbiol. Biotechnol. 2004, 64, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Qin, C.; Wang, H.; Li, S.; Tian, S. Study on supercritical extraction of lipids and enrichment of DHA from oil-rich microalgae. J. Supercrit. Fluids 2011, 57, 44–49. [Google Scholar] [CrossRef]
- Tapiero, H.; Nguyen Ba, G.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Kang, J.; Leaf, A. Antiarrhythmic effects of polyunsaturated fatty acids. Recent studies. Circulation 1996, 94, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.W.; Murphy, E.W.; McCarty, H.B.; Snyder, B.D.; Schrank, C.S.; McCann, P.J.; Crimmins, B.S. Variation in the essential fatty acids EPA and DHA in fillets of fish from the Great Lakes region. J. Great Lakes Res. 2017, 43, 150–160. [Google Scholar] [CrossRef]
- Routray, W.; Dave, D.; Ramakrishnan, V.V.; Murphy, W.; Salmon, C. Production of High Quality Fish Oil by Enzymatic Protein Hydrolysis from Cultured Atlantic Salmon By-Products: Investigation on Effect of Various Extraction Parameters Using Central Composite Rotatable Design. Waste Biomass Valoriz. 2018, 9, 2003–2014. [Google Scholar] [CrossRef]
- Kuo, C.; Liao, H.; Wang, Y.; Wang, H.D.; Shieh, C.; Tseng, C. Highly efficient extraction of EPA/DHA-enriched oil from cobia liver using homogenization plus sonication. Eur. J. Lipid Sci. Technol. 2017, 119, 1600466. [Google Scholar] [CrossRef]
- De Oliveira, D.A.S.B.; Licodiedoff, S.; Furigo, A., Jr.; Ninow, J.L.; Bork, J.A. Enzymatic extraction of oil from yellowfin tuna (Thunnus albacares) by-products: A comparison with other extraction methods. Int. J. Food Sci. Technol. 2017, 52, 699–705. [Google Scholar] [CrossRef]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; Lopes da Silva, T. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Lee Chang, K.J.; Nichols, P.D.; Blackburn, S.I. Hydroformylation of vegetable oils and the potential use of hydroformylated fatty acids. Lipid Technol. 2013, 25, 199–203. [Google Scholar] [CrossRef]
- Armenta, R.E.; Valentine, M.C. Single-cell oils as a source of omega-3 fatty acids: An overview of recent advances. J. Am. Oil Chem. Soc. 2013, 90, 167–182. [Google Scholar] [CrossRef]
- Lowrey, J.; Armenta, R.E.; Brooks, M.S. Recycling of lipid-extracted hydrolysate as nitrogen supplementation for production of thraustochytrid biomass. J. Ind. Microbiol. Biotechnol. 2016, 43, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Taoka, Y.; Nagano, N.; Okita, Y.; Izumida, H.; Sugimoto, S.; Hayashi, M. Extracellular Enzymes Produced by Marine Eukaryotes, Thraustochytrids. Biosci. Biotechnol. Biochem. 2009, 73, 180–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiorni, L.; Jain, R.; Raghukumar, S.; Aggarwal, R.K. Thraustochytrium gaertnerium sp. nov.: A new thraustochytrid stramenopilan protist from mangroves of Goa, India. Protist 2005, 156, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, L.; Pusceddu, A.; Danovaro, R. Enzymatic activities of epiphytic and benthic thraustochytrids involved in organic matter degradation. Aquat. Microb. Ecol. 2005, 41, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.D.; Armenta, R.E.; Berryman, K.T.; Norman, A.W. Use of raw glycerol to produce oil rich in polyunsaturated fatty acids by a thraustochytrid. Enzyme Microb. Technol. 2011, 48, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Orr, V.; Plechkova, N.V.; Seddon, K.R.; Rehmann, L. Disruption and Wet Extraction of the Microalgae Chlorella vulgaris Using Room-temperature Ionic Liquids. ACS Sustain. Chem. Eng. 2016, 4, 591–600. [Google Scholar] [CrossRef]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Rizzo, A.M.; Pari, L. Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl. Energy 2017, 185, 963–972. [Google Scholar] [CrossRef]
- Patil, P.D.; Dandamudi, K.P.R.; Wang, J.; Deng, Q.; Deng, S. Extraction of bio-oils from algae with supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2018, 135, 60–68. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar]
- Jin, F.; Enomoto, H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: Chemistry of acid/base-catalysed and oxidation reactions. Energy Environ. Sci. 2011, 4, 382–397. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Darley, W.M.; Porter, D.; Fuller, M.S. Cell Wall Composition and Synthesis via Golgi-Directed Schizochytrium aggregatum, with a Note on Thraustochytrium sp. Arch. Mikrobiol. 1973, 106, 89–106. [Google Scholar] [CrossRef]
- Hustedt, I.C.N.; Cleve, D.C. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar]
- Kim, Y.H.; Choi, Y.K.; Park, J.; Lee, S.; Yang, Y.H.; Kim, H.J.; Park, T.J.; Hwan Kim, Y.; Lee, S.H. Ionic liquid-mediated extraction of lipids from algal biomass. Bioresour. Technol. 2012, 109, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.; Chhotaray, P.K.; Agrawal, A.; Gardas, R.L.; Tamilarasan, K.; Rajesh, M. Protic ionic liquid-assisted cell disruption and lipid extraction from fresh water Chlorella and Chlorococcum microalgae. Algal Res. 2017, 25, 228–236. [Google Scholar] [CrossRef]
- Ward, V.C.A.; Munch, G.; Cicek, N.; Rehmann, L. Direct Conversion of the Oleaginous Yeast Rhodosporidium diobovatum to Biodiesel Using the Ionic Liquid [C2mim][EtSO4]. ACS Sustain. Chem. Eng. 2017, 5, 5562–5570. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, J.; Welton, T.; Armstrong, D.W. Characterizing Ionic Liquids On the Basis of Multiple Solvation Interactions. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Oh, Y.K.; Park, S.C.; Lee, J.W.; Park, J.Y. Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris. Renew. Energy 2013, 54, 156–160. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, S.; Kim, M.H.; Choi, Y.K.; Yang, Y.H.; Kim, H.J.; Kim, H.; Kim, H.S.; Song, K.G.; Lee, S.H. Ultrasound-assisted extraction of lipids from Chlorella vulgaris using [Bmim][MeSO4]. Biomass Bioenergy 2013, 56, 99–103. [Google Scholar] [CrossRef]
- Teixeira, R.E. Energy-efficient extraction of fuel and chemical feedstocks from algae. Green Chem. 2012, 14, 419–427. [Google Scholar] [CrossRef]
- Adamová, G.; Gardas, R.L.; Nieuwenhuyzen, M.; Puga, A.V.; Rebelo, L.P.N.; Robertson, A.J.; Seddon, K.R. Alkyltributylphosphonium chloride ionic liquids: Synthesis, physicochemical properties and crystal structure. Dalton Trans. 2012, 41, 8316–8332. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
| Algal Species | Operating Conditions | Extraction Solvent/Method | Yield | Reference |
|---|---|---|---|---|
| Chlorella sp. | ILs: cells, 10:1 (w/w). Incubated for 24 h at room temperature with constant low speed stirring. | Oil yield (mg/g algae) | [28] | |
| Bligh and Dyer | 38.13 | |||
| Butyrolactam formate | 48.0 ± 0.4 | |||
| Butyrolactam acetate | 39.1 ± 5.0 | |||
| Butyrolactam hexanoate | 46.8 ± 5.8 | |||
| Caprolactam formate | 36.3 ± 6.8 | |||
| Caprolactam acetate | 38.3 ± 4.5 | |||
| Caprolactam hexanoate | 42.9 ± 2.0 PAF 15.4 ± 2.6 | |||
| Propylammonium formate | 15.4 ± 2.6 | |||
| Propylammonium acetate | 12.8 ± 4.9 | |||
| 3-Hydroxypropylammonium formate | 8.1 ± 2.1 | |||
| 3-Hydroxypropylammonium acetate | 10.1 ± 1.2 | |||
| Chlorococcum sp. | ILs: cells, 10:1 (w/w). Incubated for 24 h at room temperature with constant low speed stirring. | Bligh and Dyer | 11.55 mg/g | [28] |
| Butyrolactam formate | 36.4 ± 1.4 | |||
| Butyrolactam acetate | 44.4 ± 1.8 | |||
| Butyrolactam hexanoate | 51.1 ± 1.9 | |||
| Caprolactam formate | 45.7 ± 2.5 | |||
| Caprolactam acetate | 49.1 ± 2.3 | |||
| Caprolactam hexanoate | 46.7 ± 2.0 | |||
| Propylammonium formate | 18.9 ± 6.3 | |||
| Propylammonium acetate | 16.7 ± 3.2 | |||
| 3-Hydroxypropylammonium formate | 13.4 ± 4.7 | |||
| 3-Hydroxypropylammonium acetate | 5.9 ± 2.7 | |||
| Aurantiochytrium sp. | FeCl3 H2O:[Emim]OAc, 5:1 (w/w). 90 °C for 60 min (5% Aurantiochytrium sp. loading, w/w) | DHA content (mg/g lipid) | [29] | |
| Bligh and Dyer | 235.2 | |||
| Hexane | 259.4 | |||
| Hexane:Methanol = 7:3 | 275.7 | |||
| 1-ethyl-3-methyl imidazolium acetate | 301.3 | |||
| Chlorella vulgaris | C. vulgaris (500 mg) was mixed with a mixture of 2.5 mL IL and 2.5 mL methanol under magnetic stirring at 65 °C for 18 h. | Lipid contents (%) | [27] | |
| Bligh and Dyer | 11.1 | |||
| [Bmim][CF3SO3] | 19.0 | |||
| [Bmim][MeSO4] | 17.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ward, V.; Dennis, D.; Plechkova, N.V.; Armenta, R.; Rehmann, L. Efficient Extraction of a Docosahexaenoic Acid (DHA)-Rich Lipid Fraction from Thraustochytrium sp. Using Ionic Liquids. Materials 2018, 11, 1986. https://doi.org/10.3390/ma11101986
Zhang Y, Ward V, Dennis D, Plechkova NV, Armenta R, Rehmann L. Efficient Extraction of a Docosahexaenoic Acid (DHA)-Rich Lipid Fraction from Thraustochytrium sp. Using Ionic Liquids. Materials. 2018; 11(10):1986. https://doi.org/10.3390/ma11101986
Chicago/Turabian StyleZhang, Yujie, Valerie Ward, Dorothy Dennis, Natalia V. Plechkova, Roberto Armenta, and Lars Rehmann. 2018. "Efficient Extraction of a Docosahexaenoic Acid (DHA)-Rich Lipid Fraction from Thraustochytrium sp. Using Ionic Liquids" Materials 11, no. 10: 1986. https://doi.org/10.3390/ma11101986





