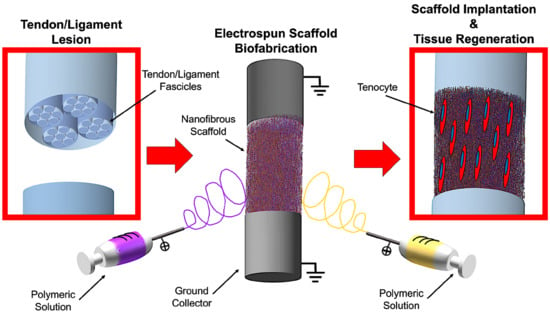
Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments
Abstract
:1. Introduction
2. Methods of the Literature Search and Review
- The following search string was used to retrieve the manuscripts presenting equipment and techniques to produce electrospun scaffolds: “electrospinning AND (review OR technique OR setup OR production process OR equipment OR methods OR scaffold production OR scaffold manufacturing)”.
- The following search string was used to retrieve the manuscripts presenting applications of electrospun scaffolds for regeneration and replacement of tendons and ligaments: “electrospinning AND (review OR regeneration OR repair OR tendon OR ligament OR bone OR muscle OR insertion)”.
3. Tendons and Ligaments: Properties and Replacement
3.1. Morphological and Mechanical Properties of Tendons and Ligaments
3.2. Generic Requirements of Scaffolds for Tendon and Ligament Regeneration
- Biocompatibility: Scaffolds must be biocompatible and made of natural or synthetic materials. This encourages the cells to grow, infiltrate and proliferate on and into the scaffolds, reproducing the physiological collagen. Biocompatibility is also fundamental to prevent and minimize inflammatory phenomena which could compromise the regenerative process [17,23,24,25].
- Biodegradability: Scaffolds need to be progressively degraded by cells and body fluids. Therefore, they must be properly engineered to permit that the degradation rate could allow cells to reproduce the natural collagen without being resorbed too fast. Moreover, the products of the degradation must not produce any inflammatory or toxic effects to cells and their surrounding tissues [17,23,24,25].
- Mechanical Properties: To permit a correct replacement of the site of the lesion, cells have to feel the physiological stiffness of the substrate, and experience physiological levels of strain, and also have to be stimulated to reproduce collagen and proliferate [17,25,26]. For these reasons, the scaffolds need to be designed to provide mechanical properties in the range of the specific tendon or ligament. However, to prevent damages of the surrounding tissues after the suture in the site of the lesion, scaffolds must be less stiff and less strong compared with the host tendon or ligament. Finally, a degree of ductility before the nominal failure load is required to prevent an unexpected and abrupt failure of the scaffolds in case of an overload.
- Morphology: Tendons and ligaments are composed of nanometric and axially aligned collagen fibrils connected in different hierarchical levels. A scaffold designed for tendon and ligament tissue regeneration needs to be produced with the same philosophy. In fact, fiber-like scaffolds permit cells to grow, attach and reproduce the collagen following the direction of alignment of the fibers, contributing to confer tendons’ and ligaments’ morphology and mechanical properties to the regenerated tissue.
- Porosity: Scaffolds also need to be porous to allow the cells’ infiltration [17,24,25,26]. Interconnected networks of porosities are essential for cell nutrition, proliferation, and migration for tissue vascularization and formation of new tissues [27,28]. A porous network structure assists in guiding and promoting new tissue formation [29,30]. Materials with high porosity allow releasing biofactors such as proteins and genes, providing good substrates for nutrient exchange between the cells [31].
4. The Electrospinning Technique: An Introduction
4.1. Electrospinning Operating Principles
- Solution parameters: Polymers and solvents, viscosity, conductivity of solvents and polymers, and concentration of the polymers.
- Electrospinning parameters: Flow rate of the pump, diameter and shape of the needle, applied voltage, distance between the needle and the collector, and shape and movement of the collector.
- Environmental parameters: Temperature and relative humidity.
4.2. Materials for Tendon and Ligament Tissue Regeneration
5. Equipment and Techniques to Produce Electrospun Scaffolds for Tendon and Ligament
5.1. Mats of Nanofibers and Multilayered Scaffolds
5.2. Short and Finite Length Bundles and Yarns
5.3. Continuous Bundles.
5.4. Continuous Yarns
5.5. Tubes and Conduits
5.6. Textiles of Nanofibers
5.7. Multiscale Hierarchical Scaffolds
6. Applications for Tendon and Ligament Regeneration and Replacement
6.1. Preliminary Studies on Electrospun Materials
6.1.1. Flat Electrospun Mats
6.1.2. Multilayered and Co-Electrospun Scaffolds
6.2. Patches and Augmentation Grafts
6.2.1. Patches
6.2.2. Augmentation Grafts
6.3. Multiscale Hierarchical Scaffolds for Massive Replacement
6.3.1. Fascicle-Inspired Bundles and Yarns
6.3.2. Hierarchically Structured Scaffolds
6.4. Bone Insertions
6.5. Muscle Insertions
6.6. Tendon and Ligament Healing and Anti-Adhesion
7. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Pećina, M.; Vukičević, S. Tissue engineering and regenerative orthopaedics (TERO). Int. Orthop. 2014, 38, 1757–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilak, F.; Baaijens, F.P.T. Functional tissue engineering: Ten more years of progress. J. Biomech. 2014, 47, 1931–1932. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.A.; Delgado, L.M.; Azeem, A.; Fuller, K.; Shologu, N.; Keeney, M.; Biggs, M.J.; Pandit, A.; Zeugolis, D.I. Harnessing Hierarchical Nano- and Micro-Fabrication Technologies for Musculoskeletal Tissue Engineering. Adv. Healthc. Mater. 2015, 4, 2488–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastelic, J.; Galeski, A.; Baer, E. The Multicomposite Structure of Tendon. Connect. Tissue Res. 1978, 6, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Listrat, A.; Béchet, D. Hierarchical mechanics of connective tissues: Integrating insights from nano to macroscopic studies. J. Biomed. Nanotechnol. 2014, 10, 2464–2507. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Engineering tendon and ligament tissues: Present developments towards successful clinical products. J. Tissue Eng. Regen. Med. 2013, 4, 673–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, R.A.; McGuinness, G.B. Electrospun nanofibre bundles and yarns for tissue engineering applications: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.A.; Conte, A.A.; Kanski, G.; Turkula, S.; Hu, X.; Kleiner, M.T.; Beachley, V. Mechanical Considerations for Electrospun Nanofibers in Tendon and Ligament Repair. Adv. Healthc. Mater. 2018, 1701277, 1–31. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Laurencin, C.T. Nanofiber technology: Its transformative role in nanomedicine. Nanomedicine 2016, 11, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Ralphs, J.R. Tendon and ligaments—An overview.pdf. Histol. Histopatol. 1997, 12, 1135–1144. [Google Scholar]
- Frank, C.B. Ligament structure, physiology and function. J. Musculoskelet. Neuronal Interact. 2004, 4, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.L.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Regenerative Strategies for the Treatment of Knee Joint Disabilities. In Regenerative Strategies for the Treatment of Knee Joint Disabilities; Oliveira, J.M., Ruis, R.L., Eds.; Springer: New York, NY, USA, 2017; Volume 21, pp. 349–371. ISBN 978-3-319-44783-4. [Google Scholar]
- Murphy, W.; Black, J.; Hastings, G. Handbook of Biomaterial Properties, 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3303-7. [Google Scholar]
- Wang, J.H.C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.; Oryan, A. Tendon and Ligament Tissue Engineering, Healing and Regenerative Medicine. J. Sports Med. Doping Stud. 2013, 3, 1–18. [Google Scholar] [CrossRef]
- Woo, S.L.Y.; Chan, S.S.; Yamaji, T. Biomechanics of knee ligament healing, repair and reconstruction. J. Biomech. 1997, 30, 431–439. [Google Scholar] [CrossRef]
- Woo, S.L.Y.; Abramowitch, S.D.; Kilger, R.; Liang, R. Biomechanics of knee ligaments: Injury, healing, and repair. J. Biomech. 2006, 39, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pioletti, D.; Rakotomanana, L.; Leyvraz, P. Strain rate effect on the mechanical behavior of the anterior cruciate ligament-bone complex. Med. Eng. Phys. 1999, 21, 95–100. [Google Scholar] [CrossRef]
- Yoganandan, N.; Kumaresan, S.; Pintar, F.A. Geometric and Mechanical Properties of Human Cervical. J. Biomech. Eng. 2000, 122, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Mesfar, W.; Moglo, K. Effect of the transverse ligament rupture on the biomechanics of the cervical spine under a compressive loading. Clin. Biomech. 2013, 28, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Moshiri, A.; Oryan, A. Role of tissue engineering in tendon reconstructive surgery and regenerative medicine: Current concepts, approaches and concerns. Hard Tissue 2012, 1, 1–11. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Wang, A.; Zheng, M. Scaffolds for tendon and ligament repair: Review of the efficacy of commercial products. Expert Rev. Med. Devices 2014, 6, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Causa, F.; Netti, P.A.; Ambrosio, L. A multi-functional scaffold for tissue regeneration: The need to engineer a tissue analogue. Biomaterials 2007, 28, 5093–5099. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, E.; Chulia, D.; Pouget, C.; Viana, M. Fabrication of Porous Substrates: A Review of Processes Using Pore Forming Agents in the Biomaterial Field. J. Pharm. Sci. 2008, 97, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walden, G.; Liao, X.; Donell, S.; Raxworthy, M.J.; Riley, G.P.; Saeed, A. A Clinical, Biological, and Biomaterials Perspective into Tendon Injuries and Regeneration. Tissue Eng. Part B Rev. 2017, 23, 44–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, J.F. Electrical Method of Dispersing Fluids. U.S. Patent US745276A, 6 October 1899. [Google Scholar]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Matabola, K.P.; Moutloali, R.M. The influence of electrospinning parameters on the morphology and diameter of poly(vinyledene fluoride) nanofibers-effect of sodium chloride. J. Mater. Sci. 2013, 48, 5475–5482. [Google Scholar] [CrossRef]
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Woo, S.L.; Debski, R.E.; Zeminski, J.; Abramowitch, S.D.; Saw, S.S.C.; Fenwick, J.A. Injury and repair of ligaments and tendons. Annu. Rev. Biomed. Eng. 2000, 2, 83–118. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Paavola, M.; Józsa, L. Aging and degeneration of tendons. In Tendon Injuries; Maffulli, N., Renström, P., Leadbetter, W.B., Eds.; Springer: New York, NY, USA, 2005; pp. 25–31. ISBN 978-1-84628-050-4. [Google Scholar]
- Clayton, R.A.E.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Hannafin, J.A. The Mature Athlete: Aging Tendon and Ligament. Sports Health 2014, 6, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Surrao, D.C.; Hayami, J.W.S.; Waldman, S.D.; Amsden, B.G. Self-crimping, biodegradable, electrospun polymer microfibers. Biomacromolecules 2010, 11, 3624–3629. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Kahn, C.; Frochot, C.; Nouvel, C.; Six, J.L.; De Isla, N.; Luo, L.H.; Cooper-White, J.; Rahouadj, R.; Wang, X. Aligned poly(l-lactic-co-e-caprolactone) electrospun microfibers and knitted structure: A novel composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2010, 94, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, J.; Wang, H.; Li, H.; Di, N.; Song, L.; Li, S.; Li, D.; Xiang, Y.; Liu, W.; et al. Fabrication of Electrospun Poly(l-Lactide-co-ε-Caprolactone)/Collagen Nanoyarn Network as a Novel, Three-Dimensional, Macroporous, Aligned Scaffold for Tendon Tissue Engineering. Tissue Eng. Part C Methods 2013, 19, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, G.; Chen, W.; Ye, X.; Mo, X. A novel electrospun-aligned nanoyarn-reinforced nanofibrous scaffold for tendon tissue engineering. Colloids Surf. B Biointerfaces 2014, 122, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Surrao, D.C.; Waldman, S.D.; Amsden, B.G. Biomimetic poly(lactide) based fibrous scaffolds for ligament tissue engineering. Acta Biomater. 2012, 8, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Lauro, B.B.; Yang, G.; Debski, R.E.; Musahl, V.; Tuan, R.S. Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering. Tissue Eng. Part A 2017, 23, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sensini, A.; Gualandi, C.; Cristofolini, L.; Tozzi, G.; Dicarlo, M.; Teti, G.; Belmonte-Mattioli, M.; Focarete, M.L. Biofabrication of bundles of poly(lactic acid) collagen blends mimicking the fascicles of the human Achille tendon. Biofabrication 2017, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Cristofolini, L.; Focarete, M.L.; Belcari, J.; Zucchelli, A.; Kao, A.; Tozzi, G. High-resolution X-ray tomographic morphological characterisation of electrospun nanofibrous bundles for tendon and ligament regeneration and replacement. J. Microsc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.G.; Handorf, A.M.; Allee, T.J.; Li, W.-J. Braided Nanofibrous Scaffold for Tendon and Ligament Tissue Engineering. Tissue Eng. Part A 2013, 19, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Bao, M.; Li, Q.; Li, B.; Yuan, H.; Zhang, Y. Aligned core–shell structured ultrafine composite fibers of PLLA–collagen for tendon scaffolding. J. Control. Release 2013, 172, e128. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, H.; Liu, H.; Chen, X.; Lu, P.; Zhu, T.; Yang, L.; Yin, Z.; Heng, B.C.; Zhang, Y.; et al. Well-aligned chitosan-based ultrafine fibers committed teno-lineage differentiation of human induced pluripotent stem cells for Achilles tendon regeneration. Biomaterials 2015, 53, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Zhang, E.; Yang, L.; Yuan, H.; Tu, W.; Zhang, H.; Yin, Z.; Shen, W.; Chen, X.; et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 2018, 66, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xie, X.; Pan, G.; Shen, P.; Zhao, J.; Cui, W. Healing improvement after rotator cuff repair using gelatin-grafted poly(l-lactide) electrospun fibrous membranes. J. Surg. Res. 2015, 193, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, R.; Sun, Z.; Su, W.; Pan, G.; Zhao, S.; Zhao, J.; Cui, W. Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing. Acta Biomater. 2017, 61, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Shao, Z.; Dai, L.; Li, L.; Hu, X.; Wang, X.; Zhou, C.; Ao, Y. In Vivo Study of Ligament-Bone Healing after Anterior Cruciate Ligament Reconstruction Using Autologous Tendons withMesenchymal Stem Cells Affinity Peptide Conjugated Electrospun Nanofibrous Scaffold Jingxian. J. Nanomater. 2013, 1–11. [Google Scholar] [CrossRef]
- Ladd, M.R.; Lee, S.J.; Stitzel, J.D.; Atala, A.; Yoo, J.J. Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering. Biomaterials 2011, 32, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, M.; Hu, C.; Wu, F.; Cui, W.; Jin, T.; Fan, C. Tendon healing and anti-adhesion properties of electrospun fibrous membranes containing bFGF loaded nanoparticles. Biomaterials 2013, 34, 4690–4701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, S.; Liu, S.; Chen, S.; Lin, Z.Y.W.; Pan, G.; He, F.; Li, F.; Fan, C.; Cui, W. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials 2015, 61, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.S.; Lewis, M.P.; Loo, S.C.J. Sustained-release of naproxen sodium from electrospun-aligned PLLA–PCL scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 1011–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Zhao, X.; Chen, S.; Pan, G.; Song, J.; He, N.; Li, F.; Cui, W.; Fan, C. Down-regulating ERK1/2 and SMAD2/3 phosphorylation by physical barrier of celecoxib-loaded electrospun fibrous membranes prevents tendon adhesions. Biomaterials 2014, 35, 9920–9929. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, X.; Fan, D.; Yu, S.; Wu, D.; Fan, C.; Cui, W.; Ruan, H. Release of celecoxib from a bi-layer biomimetic tendon sheath to prevent tissue adhesion. Mater. Sci. Eng. C 2016, 61, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef] [PubMed]
- Inui, A.; Kokubu, T.; Mifune, Y.; Sakata, R.; Nishimoto, H.; Nishida, K.; Akisue, T.; Kuroda, R.; Satake, M.; Kaneko, H.; et al. Regeneration of rotator cuff tear using electrospun poly(d,l-Lactide-co-Glycolide) scaffolds in a rabbit model. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Ang, L.T.; Cho-Hong Goh, J.; Toh, S.L. Bioactive nanofibers for fibroblastic differentiation of mesenchymal precursor cells for ligament/tendon tissue engineering applications. Differentiation 2010, 79, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Ouyang, H.; Goh, J.C.-H.; Tay, T.E.; Toh, S.L. Characterization of a Novel Polymeric Scaffold for Potential Application in Tendon/Ligament Tissue Engineering. Tissue Eng. 2006, 12, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.S.; Dimling, A.F.; Plessl, D.S.; Hahn, M.R.; Guelcher, S.A.; Dahlgren, L.A.; Goldstein, A.S. Cellularized Cylindrical Fiber/Hydrogel Composites for Ligament Tissue Engineering. Biomacromolecules 2014, 15, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Full, S.M.; Delman, C.; Gluck, J.M.; Abdmaulen, R.; Shemin, R.J.; Heydarkhan-Hagvall, S. Effect of fiber orientation of collagen-based electrospun meshes on human fibroblasts for ligament tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.S.; Verbridge, S.S.; Dahlgren, L.A.; Kakar, S.; Guelcher, S.A.; Goldstein, A.S. Fiber/collagen composites for ligament tissue engineering: Influence of elastic moduli of sparse aligned fibers on mesenchymal stem cells. J. Biomed. Mater. Res.Part A 2016, 104, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, J.; Dong, S.; Huangfu, X.; Li, B.; Yang, H.; Zhao, J.; Cui, W. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomedicine 2014, 9, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A. Travelling along the Clinical Roadmap: Developing Electrospun Scaffolds for Tendon Repair. Conf. Pap. Sci. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kolluru, P.V.; Lipner, J.; Liu, W.; Xia, Y.; Thomopoulos, S.; Genin, G.M.; Chasiotis, I. Strong and tough mineralized PLGA nanofibers for tendon-to-bone scaffolds. Acta Biomater. 2013, 9, 9442–9450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.; Toh, S.L.; Goh, J.C.H. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qin, T.; Liu, Y.; Li, X.; Li, D.; Jin, Z. Electrospinning of nanofibrous scaffolds with continuous structure and material gradients. Mater. Lett. 2014, 137, 393–397. [Google Scholar] [CrossRef]
- Criscenti, G.; Longoni, A.; Di Luca, A.; De Maria, C.; van Blitterswijk, C.A.; Vozzi, G.; Moroni, L. Triphasic scaffolds for the regeneration of the bone-ligament interface. Biofabrication 2016, 8, 015009. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Vaidya, P.; Gaddam, P.; Whittington, A.R.; Goldstein, A.S. Electrospun meshes possessing region-wise differences in fiber orientation, diameter, chemistry and mechanical properties for engineering bone-ligament-bone tissues. Biotechnol. Bioeng. 2014, 111, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Naghashzargar, E.; Farè, S.; Catto, V.; Bertoldi, S.; Semnani, D.; Karbasi, S.; Tanzi, M.C. Nano/micro hybrid scaffold of PCL or P3HB nanofibers combined with silk fibroin for tendon and ligament tissue engineering. J. Appl. Biomater. Funct. Mater. 2015, 13, e156–e168. [Google Scholar] [CrossRef] [PubMed]
- Gurlek, A.C.; Sevinc, B.; Bayrak, E.; Erisken, C. Synthesis and characterization of polycaprolactone for anterior cruciate ligament regeneration. Mater. Sci. Eng. C 2017, 71, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Olvera, D.; Sathy, B.N.; Carroll, S.F.; Kelly, D.J. Modulating microfibrillar alignment and growth factor stimulation to regulate mesenchymal stem cell differentiation. Acta Biomater. 2017, 64, 148–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepthi, S.; Jeevitha, K.; Nivedhitha Sundaram, M.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid hydrogel coated poly(caprolactone) multiscale bilayer scaffold for ligament regeneration. Chem. Eng. J. 2015, 260, 478–485. [Google Scholar] [CrossRef]
- Pauly, H.M.; Kelly, D.J.; Popat, K.C.; Trujillo, N.A.; Dunne, N.J.; McCarthy, H.O.; Haut Donahue, T.L. Mechanical properties and cellular response of novel electrospun nanofibers for ligament tissue engineering: Effects of orientation and geometry. J. Mech. Behav. Biomed. Mater. 2016, 61, 258–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauly, H.M.; Sathy, B.N.; Olvera, D.; McCarthy, H.O.; Kelly, D.J.; Popat, K.C.; Dunne, N.J.; Haut Donahue, T.L. Hierarchically Structured Electrospun Scaffolds with Chemically Conjugated Growth Factor for Ligament Tissue Engineering. Tissue Eng. Part A 2017, 23, 823–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquette, C.; Sudheesh Kumar, P.T.; Petcu, E.B.; Ivanovski, S. Combining electrospinning and cell sheet technology for the development of a multiscale tissue engineered ligament construct (TELC). J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Chainani, A.; Hippensteel, K.J.; Kishan, A.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Multilayered Electrospun Scaffolds for Tendon Tissue Engineering. Tissue Eng. Part A 2013, 19, 2594–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beason, D.P.; Connizzo, B.K.; Dourte, L.M.; Mauck, R.L.; Soslowsky, L.J.; Steinberg, D.R.; Bernstein, J. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J. Shoulder Elbow Surg. 2012, 21, 245250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, X.; Dong, S.; Yu, J.; Pan, G.; Zhang, Y.; Zhao, J.; Cui, W. A hierarchical, stretchable and stiff fibrous biotemplate engineered using stagger- electrospinning for augmentation of rotator cuff tendon-healing. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 990–1000. [Google Scholar] [CrossRef]
- Manning, C.N.; Schwartz, A.G.; Liu, W.; Xie, J.; Havlioglu, N.; Sakiyama-Elbert, S.E.; Silva, M.J.; Xia, Y.; Gelberman, R.H.; Thomopoulos, S. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater. 2013, 9, 6905–6914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Lin, H.; Rothrauff, B.B.; Yu, S.; Tuan, R.S. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 2016, 35, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosworth, L.A.; Alam, N.; Wong, J.K.; Downes, S. Investigation of 2D and 3D electrospun scaffolds intended for tendon repair. J. Mater. Sci. Mater. Med. 2013, 24, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Rathbone, S.R.; Bradley, R.S.; Cartmell, S.H. Dynamic loading of electrospun yarns guides mesenchymal stem cells towards a tendon lineage. J. Mech. Behav. Biomed. Mater. 2014, 39, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.; Bosworth, L.A.; Wong, R.; O’brien, M.A.; Kriel, H.; Smit, E.; McGrouther, D.A.; Wong, J.K.; Cartmell, S.H. Cell response to sterilized electrospun poly(ε-caprolactone) scaffolds to aid tendon regeneration in vivo. J. Biomed. Mater. Res. Part A 2017, 105, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Chiera, S.; Gershovich, P.; Motta, A.; Reis, R.L.; Gomes, M.E. Enhancing the Biomechanical Performance of Anisotropic Nanofibrous Scaffolds in Tendon Tissue Engineering: Reinforcement with Cellulose Nanocrystals. Adv. Healthc. Mater. 2016, 5, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Levitt, A.S.; Knittel, C.E.; Vallett, R.; Koerner, M.; Dion, G.; Schauer, C.L. Investigation of nanoyarn preparation by modified electrospinning setup. J. Appl. Polym. Sci. 2017, 134, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, O.; Mouthuy, P.A.; Zargar, N.; Lostis, E.; Morrey, M.; Carr, A. A layered electrospun and woven surgical scaffold to enhance endogenous tendon repair. Acta Biomater. 2015, 26, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.L.; Lewis, G.S.; Brown, J.L. Multiscale Poly-(ε-caprolactone) Scaffold Mimicking Non-linearity in Tendon Tissue Mechanics. Regen. Eng. Transl. Med. 2016, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira, M.; Domingues, R.M.A.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. 3D Mimicry of Native-Tissue-Fiber Architecture Guides Tendon-Derived Cells and Adipose Stem Cells into Artificial Tendon Constructs. Small 2017, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Deng, X.; Song, J.; Chen, F. Enhanced biological properties of biomimetic apatite fabricated polycaprolactone/chitosan nanofibrous bio-composite for tendon and ligament regeneration. J. Photochem. Photobiol. B Biol. 2018, 178, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Olsen Horton, C.; Guelcher, S.A.; Goldstein, A.S.; Whittington, A.R. Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament-bone interface. Acta Biomater. 2011, 7, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Guelcher, S.A.; Goldstein, A.S.; Whittington, A.R. Response of bone marrow stromal cells to graded co-electrospun scaffolds and its implications for engineering the ligament-bone interface. Biomaterials 2012, 33, 7727–7735. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ma, B.; Michael, P.L.; Shuler, F.D. Fabrication of Nanofiber Scaffolds With Gradations in Fiber Organization and Their Potential Applications. Macromol. Biosci. 2012, 12, 1336–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhao, J.; Ruan, H.; Tang, T.; Liu, G.; Yu, D.; Cui, W.; Fan, C. Biomimetic Sheath Membrane via Electrospinning for Antiadhesion of Repaired Tendon. Biomacromolecules 2012, 13, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, X.; Chen, S.; Du, C. Osteogenic and tenogenic induction of hBMSCs by an integrated nanofibrous scaffold with chemical and structural mimicry of the bone–ligament connection. J. Mater. Chem. B 2017, 5, 1015–1027. [Google Scholar] [CrossRef]
- Hayami, J.W.S.; Surrao, D.C.; Waldman, S.D.; Amsden, B.G. Design and characterization of a biodegradable composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2010, 92, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Karchin, A.; Wang, Y.-N.; Sanders, J.E. Modulation of gene expression using electrospun scaffolds with templated architecture. J. Biomed. Mater. Res. A 2012, 100, 1605–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Aghdam, M.; Faridi-Majidi, R.; Derakhshan, M.A.; Chegeni, A.; Azami, M. Preparation of collagen/polyurethane/knitted silk as a composite scaffold for tendon tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, R.D.; Dahlgren, L.A.; Goldstein, A.S. Electrospun fibre diameter, not alignment, affects mesenchymal stem cell differentiation into the tendon/ligament lineage. J. Tissue Eng. Regen. Med. 2014, 8, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.P.; Robbins, A.B.; Mohiuddin, S.F.; Jiang, M.; Moreno, M.R.; Cosgriff-Hernandez, E.M. Fabrication of macromolecular gradients in aligned fiber scaffolds using a combination of in-line blending and air-gap electrospinning. Acta Biomater. 2017, 56, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.A.; Huri, P.Y.; Ginn, B.P.; Gilbert-Honick, J.; Somers, S.M.; Temple, J.P.; Mao, H.Q.; Grayson, W.L. Characterization of a novel bioreactor system for 3D cellular mechanobiology studies. Biotechnol. Bioeng. 2016, 113, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.L.; Gong, R.-H.; Porat, I. Nano-coated hybrid yarns using electrospinning. Surf. Coat. Technol. 2010, 204, 3459–3463. [Google Scholar] [CrossRef]
- Dodel, M.; Hemmati Nejad, N.; Bahrami, S.H.; Soleimani, M.; Mohammadi Amirabad, L.; Hanaee-Ahvaz, H.; Atashi, A. Electrical stimulation of somatic human stem cells mediated by composite containing conductive nanofibers for ligament regeneration. Biologicals 2017, 46, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mouthuy, P.A.; Zargar, N.; Hakimi, O.; Lostis, E.; Carr, A. Fabrication of continuous electrospun filaments with potential for use as medical fibres. Biofabrication 2015, 7, 025006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschmann, J.; Meier-Burgisser, G.; Bonavoglia, E.; Neuenschwander, P.; Milleret, V.; Giovanoli, P.; Calcagni, M. Cellular response of healing tissue to DegraPol tube implantation in rabbit Achilles tendon rupture repair: An in vivo histomorphometric study. J. Tissue Eng. Regen. Med. 2013, 7, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Calcagni, M.; Meier Burgisser, G.; Bonavoglia, E.; Neuenschwander, P.; Milleret, V.; Giovanoli, P. Synthesis, characterization and histomorphometric analysis of cellular response to a new elastic DegraPolW polymer for rabbit Achilles tendon rupture repair. J. Tissue Eng. Regen. Med. 2015, 9, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Evrova, O.; Houska, J.; Welti, M.; Bonavoglia, E.; Calcagni, M.; Giovanoli, P.; Vogel, V.; Buschmann, J. Bioactive, Elastic, and Biodegradable Emulsion Electrospun DegraPol Tube Delivering PDGF-BB for Tendon Rupture Repair. Macromol. Biosci. 2016, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Senthil-Kumar, P.; Dubbin, K.; Aznar-Cervantes, S.D.; Datta, N.; Randolph, M.A.; Cenis, J.L.; Rutledge, G.C.; Kochevar, I.E.; Redmond, R.W. A photoactivated nanofiber graft material for augmented Achilles tendon repair. Lasers Surg. Med. 2012, 44, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Liu, W.; Zhang, P.; Jiang, J.; Chen, S. Electrospun silk fibroin mat enhances tendon-bone healing in a rabbit extra-articular model. Biotechnol. Lett. 2016, 38, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.A.; Macossay, J.; Cantu, T.; Zhang, X.; Shamshi Hassan, M.; Esther Salinas, M.; Farhangi, C.S.; Ahmad, H.; Kim, H.; Bowlin, G.L. Imaging, spectroscopy, mechanical, alignment and biocompatibility studies of electrospun medical grade polyurethane (Carbothane™ 3575A) nanofibers and composite nanofibers containing multiwalled carbon nanotubes. J. Mech. Behav. Biomed. Mater. 2015, 41, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xie, J.; Lipner, J.; Yuan, X.; Thomopoulos, S.; Xia, Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009, 9, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Abbasipour, M.; Khajavi, R. Nanofiber bundles and yarns production by electrospinning: A review. Adv. Polym. Technol. 2013, 32, 1–9. [Google Scholar] [CrossRef]
- Ali, U.; Zhou, Y.; Wang, X.; Lin, T. Electrospinning of Continuous Nanofiber Bundles and Twisted Nanofiber Yarns. In Nanofibers-Production, Properties and Functional Applications; InTech: Rijeka, Croatia, 2011; pp. 153–174. [Google Scholar]
- Sahay, R.; Thavasi, V.; Ramakrishna, S. Design modifications in electrospinning setup for advanced applications. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.Y.; Lee, S.C.; Shao, C.L.; Lee, D.R.; Park, S.J.; Kwag, G.B.; Choi, K.J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning Nanofibers as Uniaxially Aligned Arrays and Layer-by-Layer Stacked Films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Liu, L.; Dzenis, Y.A. Analysis of the effects of the residual charge and gap size on electrospun nanofiber alignment in a gap method. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Bazbouz, M.B.; Stylios, G.K. Alignment and Optimization of Nylon 6 Nanofibers by Electrospinning. J. Appl. Polym. Sci. 2008, 107, 3023–3032. [Google Scholar] [CrossRef]
- Kameoka, J.; Czaplewski, D.; Liu, H.; Craighead, H.G. Polymeric nanowire architecture. J. Mater. Chem. 2004, 14, 1503–1505. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakai, H.; Murata, H. A new electrospinning method to control the number and a diameter of uniaxially aligned polymer fibers. Mater. Lett. 2008, 62, 3370–3372. [Google Scholar] [CrossRef]
- Formhals, A. Process and Apparatus for Preparing Artificial Threads. U.S. Patent US1975504A, 2 October 1934. [Google Scholar]
- Formhals, A. Artificial Fiber Construction. U.S. Patent US2109333A, 4 March 1938. [Google Scholar]
- Formhals, A. Method and Apparatus for the Production of Fibers. U.S. Patent US2123992A, 19 July 1938. [Google Scholar]
- Formhals, A. Method and Apparatus for Spinning. U.S. Patent US2349950A, 30 May 1944. [Google Scholar]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ki, C.S.; Park, S.Y.; Kim, H.J.; Jung, H.M.; Woo, K.M.; Lee, J.W.; Park, Y.H. Development of 3-D nanofibrous fibroin scaffold with high porosity by electrospinning: Implications for bone regeneration. Biotechnol. Lett. 2008, 30, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Hattori, S.; Yoshikawa, C.; Yasuda, Y.; Koyama, H.; Takato, T.; Kobayashi, H. Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Mater. Lett. 2009, 63, 754–756. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Xu, H.; Chang, J. Electrospun membranes: Control of the structure and structure related applications in tissue regeneration and drug delivery. J. Mater. Chem. B 2014, 2, 5492–5510. [Google Scholar] [CrossRef]
- Daming, Z.; Jiang, C. Electrospinning of three-dimensional nanofibrous tubes with controllable architectures. Nano Lett. 2008, 8, 3283–3287. [Google Scholar] [CrossRef]
- Nedjari, S.; Hébraud, A.; Eap, S.; Siegwald, S.; Mélart, C.; Benkirane-Jessel, N.; Schlatter, G. Electrostatic template-assisted deposition of microparticles on electrospun nanofibers: Towards microstructured functional biochips for screening applications. RSC Adv. 2015, 5, 83600–83607. [Google Scholar] [CrossRef]
- Kidoaki, S.; Kwon, I.K.; Matsuda, T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 2005, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.M.; Kleinmeyer, J.D.; Hirvonen, J.K.; Tan, N.C.B. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer 2001, 42, 8163–8170. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.E.; Beck Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Theron, A.; Zussman, E.; Yarin, A.L. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology 2001, 12, 384–390. [Google Scholar] [CrossRef]
- Zussman, E.; Rittel, D.; Yarin, A.L. Failure modes of electrospun nanofibers. Appl. Phys. Lett. 2003, 82, 3958–3960. [Google Scholar] [CrossRef]
- Fennessey, S.F.; Farris, R.J. Fabrication of aligned and molecularly oriented electrospun polyacrylonitrile nanofibers and the mechanical behavior of their twisted yarns. Polymer 2004, 45, 4217–4225. [Google Scholar] [CrossRef]
- Liu, C.K.; Sun, R.J.; Lai, K.; Sun, C.Q.; Wang, Y.W. Preparation of short submicron-fiber yarn by an annular collector through electrospinning. Mater. Lett. 2008, 62, 4467–4469. [Google Scholar] [CrossRef]
- Uddin, N.M.; Ko, F.; Xiong, J.; Farouk, B.; Capaldi, F. Process, structure, and properties of electrospun carbon nanotube-reinforced nanocomposite yarns. Res. Lett. Mater. Sci. 2009, 2009. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. Electrospun fibre bundle made of aligned nanofibres over two fixed points. Nanotechnology 2005, 16, 1878–1884. [Google Scholar] [CrossRef]
- Dalton, P.D.; Klee, D.; Möller, M. Electrospinning with dual collection rings. Polymer 2005, 46, 611–614. [Google Scholar] [CrossRef]
- Liu, L.Q.; Eder, M.; Burgert, I.; Tasis, D.; Prato, M.; Wagner, H.D. One-step electrospun nanofiber-based composite ropes. Appl. Phys. Lett. 2007, 90, 1–3. [Google Scholar] [CrossRef]
- Lotus, A.F.; Bender, E.T.; Evans, E.A.; Ramsier, R.D.; Reneker, D.H.; Chase, G.G. Electrical, structural, and chemical properties of semiconducting metal oxide nanofiber yarns. J. Appl. Phys. 2008, 103, 1–6. [Google Scholar] [CrossRef]
- Lotus, A.F.; Bhargava, S.; Bender, E.T.; Evans, E.A.; Ramsier, R.D.; Reneker, D.H.; Chase, G.G. Electrospinning route for the fabrication of p-n junction using nanofiber yarns. J. Appl. Phys. 2009, 106, 1–5. [Google Scholar] [CrossRef]
- Smit, E.; Buttner, U.; Sanderson, R.D. Continuous yarns from electrospun fibers. Polymer 2005, 46, 2419–2423. [Google Scholar] [CrossRef]
- Khil, M.S.; Bhattarai, S.R.; Kim, H.Y.; Kim, S.Z.; Lee, K.H. Novel fabricated matrix via electrospinning for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, J.-C. Process of preparing continuous filament composed of nanofibers 2010.
- Pan, H.; Li, L.; Hu, L.; Cui, X. Continuous aligned polymer fibers produced by a modified electrospinning method. Polymer 2006, 47, 4901–4904. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Zhu, M.; Hsiao, B.S.; Chu, B. Enhanced mechanical performance of self-bundled electrospun fiber yarns via post-treatments. Macromol. Rapid Commun. 2008, 29, 826–831. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Zhu, M.; Yu, H.; Zhou, Z.; Chen, Y.; Hsiao, B.S. Continuous polymer nanofiber yarns prepared by self-bundling electrospinning method. Polymer 2008, 49, 2755–2761. [Google Scholar] [CrossRef]
- Ko, F.; Gogotsi, Y.; Ali, A.; Naguib, N.; Ye, H.; Yang, G.; Li, C.; Willis, P. Electrospinning of continuous carbon nanotube-filled nanofiber yarns. Adv. Mater. 2003, 15, 1161–1165. [Google Scholar] [CrossRef]
- Teo, W.E.; Gopal, R.; Ramaseshan, R.; Fujihara, K.; Ramakrishna, S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer 2007, 48, 3400–3405. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Latifi, M.; Teo, W.-E.; Amani-Tehran, M.; Ramakrishna, S. Producing Continuous Twisted Yarn From Well-Aligned Nanofibers by Water Vortex. Polym. Eng. Sci. 2011, 51, 323–329. [Google Scholar] [CrossRef]
- Dabirian, F.; Hosseini, Y.; Ravandi, S.A.H. Manipulation of the electric field of electrospinning system to produce polyacrylonitrile nanofiber yarn. J. Text. Inst. 2007, 98, 237–241. [Google Scholar] [CrossRef]
- Dabirian, F.; Hosseini, S.A. Novel method for nanofibre yarn production using two differently charged nozzles. Fibres Text. East. Eur. 2009, 17, 45–47. [Google Scholar]
- Afifi, A.M.; Nakano, S.; Yamane, H.; Kimura, Y. Electrospinning of continuous aligning yarns with a ’funnel. Target. Macromol. Mater. Eng. 2010, 295, 660–665. [Google Scholar] [CrossRef]
- Ali, U.; Zhou, Y.; Wang, X.; Lin, T. Direct electrospinning of highly twisted, continuous nanofiber yarns. J. Text. Inst. 2012, 103, 80–88. [Google Scholar] [CrossRef]
- Kanani, A.G.; Bahrami, S.H. Review on Electrospun Nanofibers Scaffold and Biomedical Applications. Trends Biomater. Artif. Organs 2010, 24, 93–115. [Google Scholar]
- Sivolella, S.; Brunello, G.; Ferrarese, N.; Della Puppa, A.; D’Avella, D.; Bressan, E.; Zavan, B. Nanostructured guidance for peripheral nerve injuries: A review with a perspective in the oral and maxillofacial area. Int. J. Mol. Sci. 2014, 15, 3088–3117. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Tonda-Turo, C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 2015, 131, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Bini, T.B.; Gao, S.; Tan, T.C.; Wang, S.; Lim, A.; Hai, L. Ben; Ramakrishna, S. Electrospun poly(l-lactide-co-glycolide) biodegradable polymer nanofibre tubes for peripheral nerve regeneration. Nanotechnology 2004, 15, 1459–1464. [Google Scholar] [CrossRef]
- Stitzel, J.; Liu, J.; Lee, S.J.; Komura, M.; Berry, J.; Soker, S.; Lim, G.; Van Dyke, M.; Czerw, R.; Yoo, J.J.; et al. Controlled fabrication of a biological vascular substitute. Biomaterials 2006, 27, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Ihara, M.; Inoguchi, H.; Kwon, I.K.; Takamizawa, K.; Kidoaki, S. Mechano-active scaffold design of small-diameter artificial graft made of electrospun segmented polyurethane fabrics. J. Biomed. Mater. Res.Part A 2005, 73, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.M.; van Tuijl, S.; Bouten, C.V.C.; Baaijens, F.P.T. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005, 1, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Textile Technologies and Tissue Engineering: A Path Toward Organ Weaving. Adv. Healthc. Mater. 2016, 5, 751–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Han, C.; Hu, X.; Sun, H.; You, C.; Gao, C.; Haiyang, Y. Applications of knitted mesh fabrication techniques to scaffolds for tissue engineering and regenerative medicine. J. Mech. Behav. Biomed. Mater. 2011, 4, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Guilak, F. Composite scaffolds for cartilage tissue engineering. Biorheology 2008, 45, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Guilak, F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng. Part A 2010, 16, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Estes, B.T.; Guilak, F. Multifunctional Hybrid Three-dimensionally Woven Scaffolds for Cartilage Tissue Engineering. Macromol. Biosci. 2010, 10, 1355–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Duan, B.; Liu, P.; Zhang, C.; Qin, X.; Butcher, J.T. Fabrication of Aligned Nano fi ber Polymer Yarn Networks for Anisotropic Soft Tissue Sca ff olds. ACS Appl. Mater. Interfaces 2016, 8, 16950–16960. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Patel, S.; Hashi, C.; Huang, N.F.; Kurpinski, K. Biomimetic Scaffolds 2007.
- Koh, H.S.; Yong, T.; Teo, W.E.; Chan, C.K.; Puhaindran, M.E.; Tan, T.C.; Lim, A.; Lim, B.H.; Ramakrishna, S. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J. Neural Eng. 2010, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, X.; Sun, B.; Wu, T.; Chen, W.; Huang, C.; Ke, Q.; EI-Hamshary, H.A.; Al-Deyab, S.S.; Mo, X. Nerve conduits constructed by electrospun P(LLA-CL) nanofibers and PLLA nanofiber yarns. J. Mater. Chem. B 2015, 3, 8823–8831. [Google Scholar] [CrossRef]
- Bashur, C.A.; Shaffer, R.D.; Dahlgren, L.A.; Guelcher, S.A.; Goldstein, A.S. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng. Part A 2009, 15, 2435–2445. [Google Scholar] [CrossRef] [PubMed]















| Acronym | Extended Name | Application | References |
|---|---|---|---|
| P(LLA-CL) | Poly(l-lactide-co-ε-caprolactone) | Tendon/Ligament | [53] |
| Ligament | [54] | ||
| Tendon | [55,56] | ||
| PDLLA | Poly(d,l-lactic acid) | Ligament | [57] |
| PLDLA | Poly(L-lactide-co-d,l-lactic acid) | Ligament | [57] |
| PLLA | Poly(l-lactic acid) | Tendon/Ligament | [58,59,60,61] |
| Ligament | [57] | ||
| Tendon | [62,63,64,65,66] | ||
| Ligament-to-Bone Interface | [67] | ||
| Tendon-to-Muscle Interface | [68] | ||
| Tendon Anti-Adhesion | [69,70,71] | ||
| PELA | Poly(l-lactic acid)-poly(ethylene glycol) | Tendon Anti-Adhesion | [72,73] |
| PDLLGA | Poly(d,l-lactide-co-glycolic acid) | Ligament | [74] |
| Tendon | [75] | ||
| PLGA | Poly(lactic-co-glycolic acid) | Tendon/Ligament | [76,77] |
| Ligament | [78,79,80] | ||
| Tendon | [81,82] | ||
| Tendon-to-Bone Interface | [83] | ||
| PLLGA | Poly(l-lactic-co-glycolic acid) | Tendon/Ligament | [84] |
| Tendon-to-Bone Interface | [85,86] | ||
| Bone-Ligament-Bone | [87] | ||
| PCL | Poly(ε-caprolactone) | Tendon/Ligament | [58,88] |
| Ligament | [80,89,90,91,92,93,94] | ||
| Tendon | [82,95,96,97,98,99,100,101,102,103,104,105,106,107,108] | ||
| Tendon/Ligament-to-Bone Interface | [109] | ||
| Ligament-to-Bone Interface | [110,111] | ||
| Tendon-to-Bone Interface | [112] | ||
| Tendon-to-Muscle Interface | [68] | ||
| Tendon Anti-Adhesion | [71,113] | ||
| Bone-Ligament-Bone | [87,114] | ||
| PCLDLLA | Poly(ε-caprolactone-co-d,l-lactic acid) | Ligament | [115] |
| PU | Poly(urethane) | Ligament | [79,116,117] |
| Tendon | [118] | ||
| PEUR | Poly(ester urethane) | Ligament | [80] |
| PEUUR | Poly(ester urethane urea) | Tendon/Ligament | [119] |
| Ligament | [74,78] | ||
| Ligament-to-Bone Interface | [111] | ||
| PEUUR2000 | Poly(ester urethane urea) elastomer | Ligament-to-Bone Interface | [110] |
| BPUR10 | Biodegradable Poly(urethane urea) 10 | Tendon-to-Bone Interface | [120] |
| BPUR50 | Biodegradable Poly(urethane urea) 50 | Tendon-to-Bone Interface | [120] |
| PEO | Poly(ethylene oxide) | Tendon/Ligament | [121,122] |
| Tendon | [63,64,96] | ||
| PEGDA | Poly(ethylene glycol diacrylate) | Ligament | [78] |
| PEDOT | Poly(3,4-ethylenedioxythiophene) | Ligament | [123] |
| PDO | Poly(dioxanone) | Tendon | [106,124] |
| PAN | Poly(acrylonitrile) | Tendon | [105] |
| PVDF-TrFe | Poly(vinylidene fluoride-trifluoro ethylene) | Tendon | [105] |
| DP | Biodegradable Poly(ester urethane) block copolymer (DegraPol®) | Tendon Anti-Adhesion | [125,126,127] |
| P3HB | Poly(3-hydroxybutyrate) | Tendon/Ligament | [88] |
| Nylon6.6 | Nylon 6.6 | Tendon/Ligament | [60] |
| SE | Silk | Ligament | [123] |
| Tendon | [128] | ||
| Tendon-to-Bone Interface | [129] | ||
| SF | Silk Fibroin | Tendon | [56] |
| Fibrinogen | Fibrinogen | Tendon/Ligament | [121] |
| Tendon/Ligament | [59,60] | ||
| Coll | Collagen | Tendon | [55,62,118] |
| Tendon-to-Muscle Interface | [68] | ||
| CTS | Chitosan | Tendon/Ligament-to-Bone Interface | [109] |
| Tendon | [63,97,104,108] | ||
| Tendon Anti-Adhesion | [113] | ||
| GT | Gelatin | Tendon | [63] |
| HA | Hyaluronic acid | Tendon Anti-Adhesion | [70,73,113] |
| mGLT | Methacrylated Gelatin | Tendon | [100] |
| Carbothane™ 3575A | Poly(carbonate)-based thermoplastic poly(urethane) | Tendon/Ligament | [130] |
| MWCNTs | Multi Wallen Carbon Nanotubes | Tendon/Ligament | [130] |
| Acronym | Type | Application | References |
|---|---|---|---|
| P(LLA-CL)/Coll | Blend | Tendon | [55] |
| P(LLA-CL)/SF | Blend | Tendon | [56] |
| PLLA/PCL | Blend | Tendon Anti-Adhesion | [71] |
| PLLA/MMC | Core–Shell | Tendon Anti-Adhesion | [70] |
| PLLA/Coll | Blend | Tendon/Ligament | [59,60] |
| Core–Shell | Tendon | [62] | |
| Blend | Tendon-to-Muscle Interface | [68] | |
| PLLA/PEO | Blend | Tendon | [64] |
| PLLA/PEO/CTS/GT | Blend | Tendon | [63] |
| PEO/Fibrinogen | Blend | Tendon/Ligament | [121] |
| PCL/CTS | Blend | Tendon/Ligament-to-Bone Interface | [109] |
| Tendon | [104,108] | ||
| PCL/Coll | Blend | Tendon-to-Muscle Interface | [68] |
| PCL/HA | Blend | Tendon Anti-Adhesion | [113] |
| PCL/PLGA | Blend | Tendon | [82] |
| PLGA/Coll | Blend | Ligament | [79] |
| PLGA/PEGDA | Blend | Ligament | [78] |
| PEUUR/PEGDA | Blend | Ligament | [78] |
| PEUUR/PCL | Blend | Ligament | [80] |
| PU/Coll | Blend | Tendon | [118] |
| PELA/HA | Blend | Tendon Anti-Adhesion | [73] |
| Acronym | Extended Name | Application | References |
|---|---|---|---|
| bFGF | Basic Fibroblast Growth Factor | Tendon/Ligament | [76,84] |
| Tendon Anti-Adhesion | [69] | ||
| Tendon | [81] | ||
| DGNs | Dextran Glassy Nanoparticles | Tendon Anti-Adhesion | [69] |
| Celecoxib | Selective Non-Steroidal Anti-Inflammatory Drug | Tendon Anti-Adhesion | [72,73] |
| MMC | Mitomycin-C | Tendon Anti-Adhesion | [70] |
| TSA | Trichostatin-A | Tendon | [64] |
| HAp | Hydroxyapatite | Tendon/Ligament-to-Bone Interface | [109] |
| Tendon-to-Bone Interface | [85] | ||
| Ligament-to-Bone Interface | [110,111] | ||
| Tendon | [66] | ||
| CNCs | Cellulose Nanocrystals | Tendon | [104,108] |
| CTGF | Connective Tissue Growth Factors | Ligament | [93] |
| PDGF-BB | Platelet Derived Growth Factor-BB | Tendon | [127] |
| TP | Tricalcium Phosphate | Tendon-to-Bone Interface | [83,131] |
| BLM | Biomimetically Prepared Bone-like Mineral | Bone-Ligament-Bone | [114] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. https://doi.org/10.3390/ma11101963
Sensini A, Cristofolini L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials. 2018; 11(10):1963. https://doi.org/10.3390/ma11101963
Chicago/Turabian StyleSensini, Alberto, and Luca Cristofolini. 2018. "Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments" Materials 11, no. 10: 1963. https://doi.org/10.3390/ma11101963