Strengthening Effects of Zn Addition on an Ultrahigh Ductility Mg-Gd-Zr Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
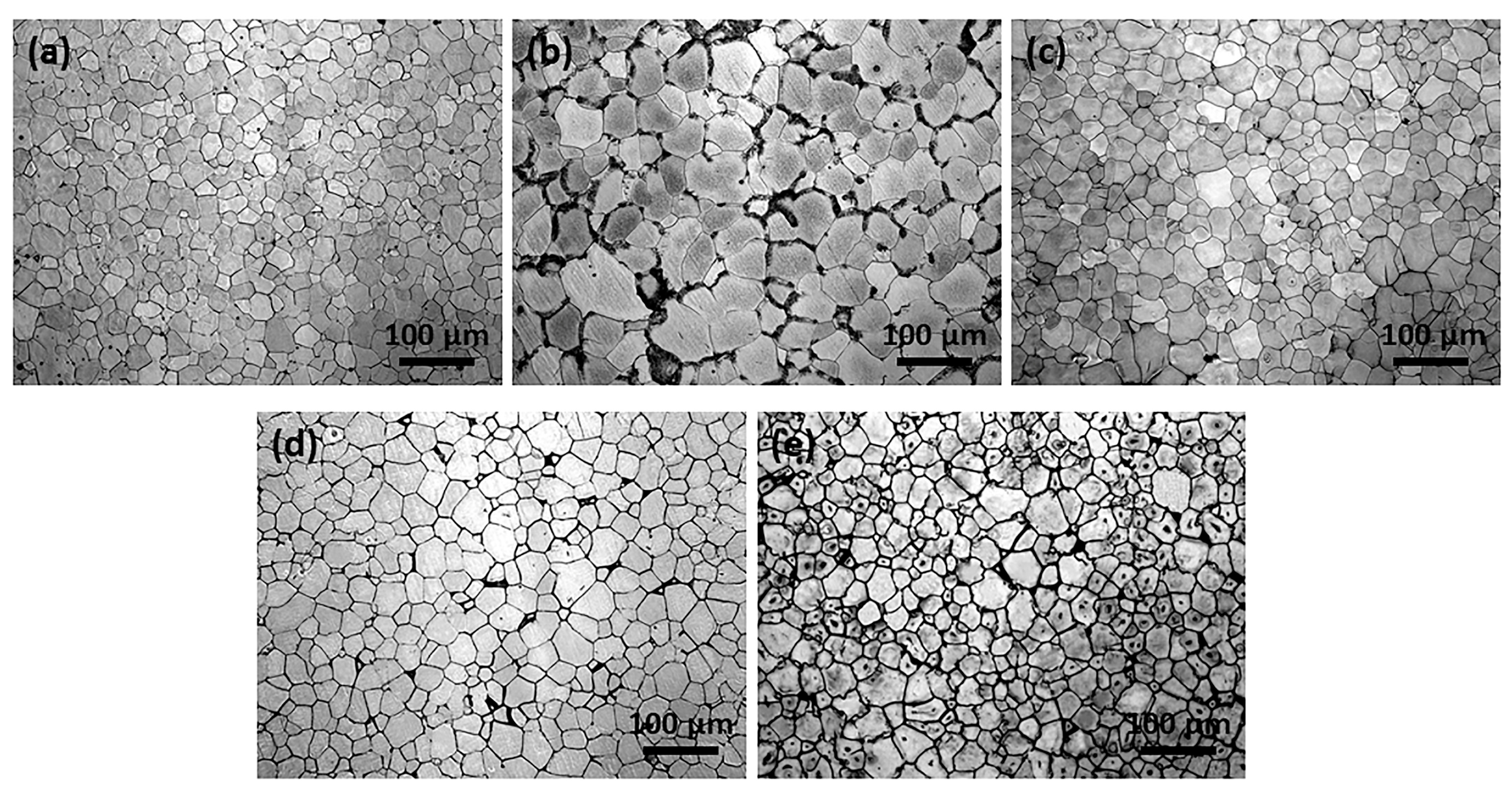
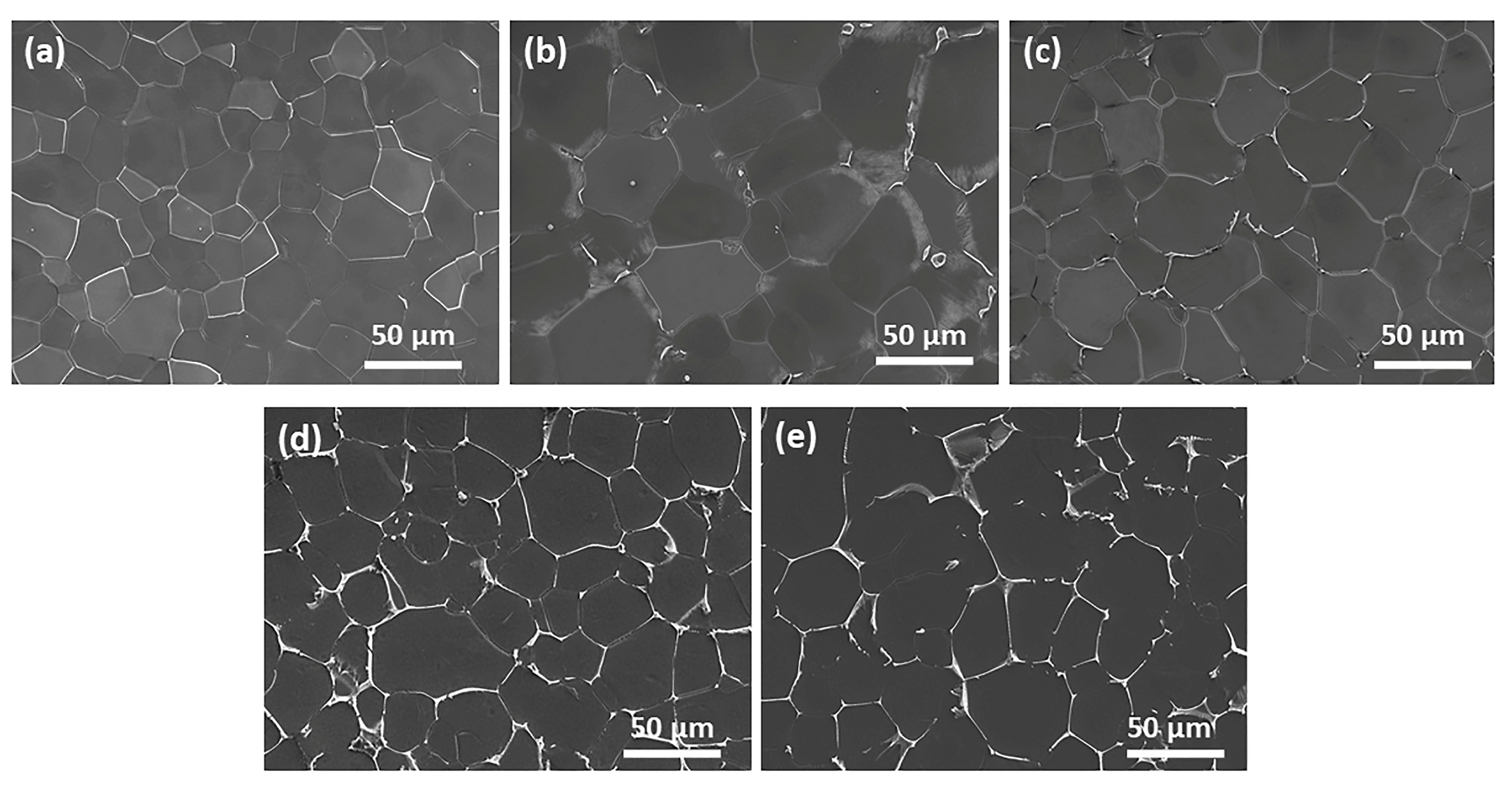
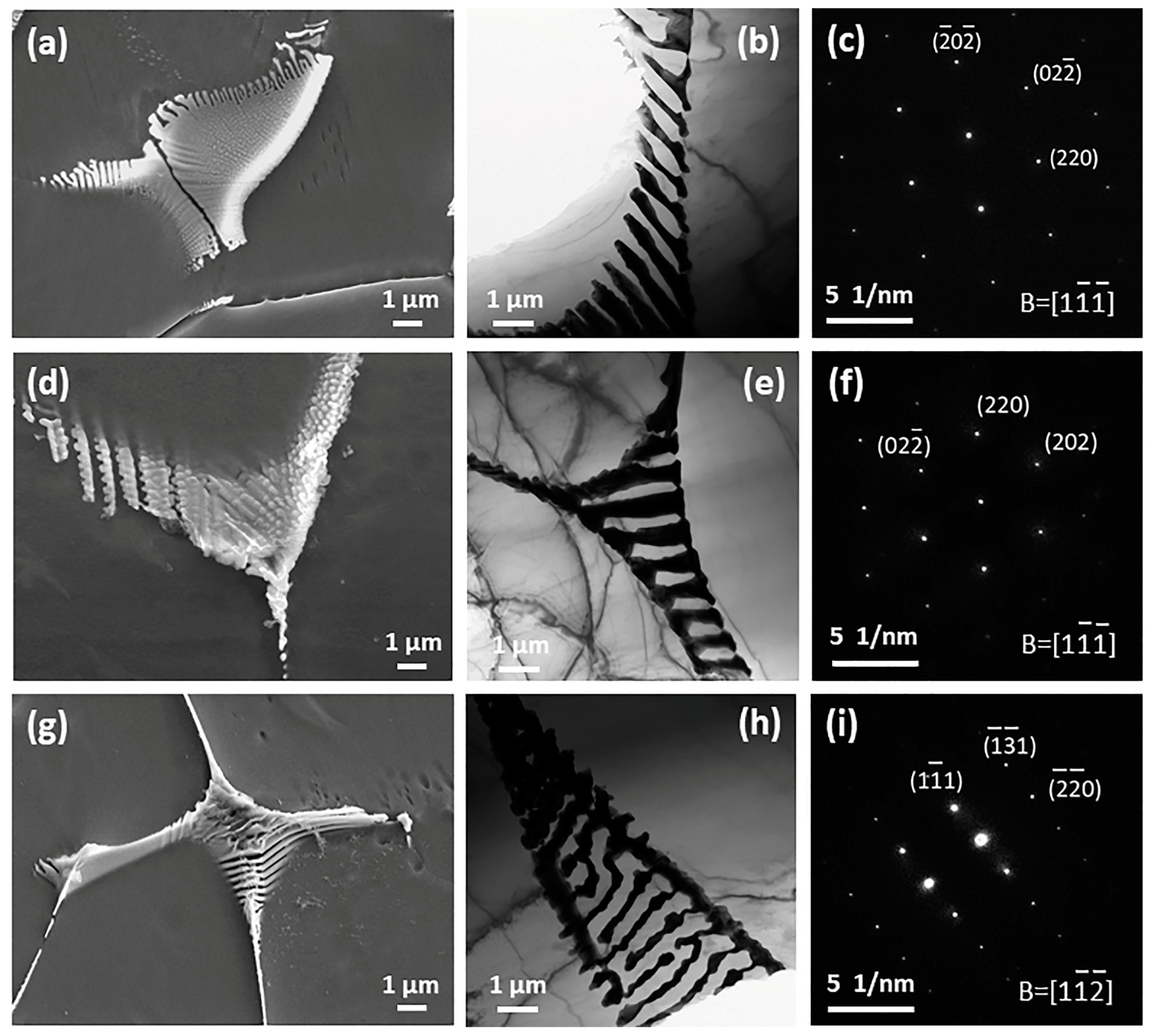
3.1. Microstructure and Phase Constitution of As-Cast and As-Extruded Alloys
3.2. Mechanical Properties of As-Extruded Alloys
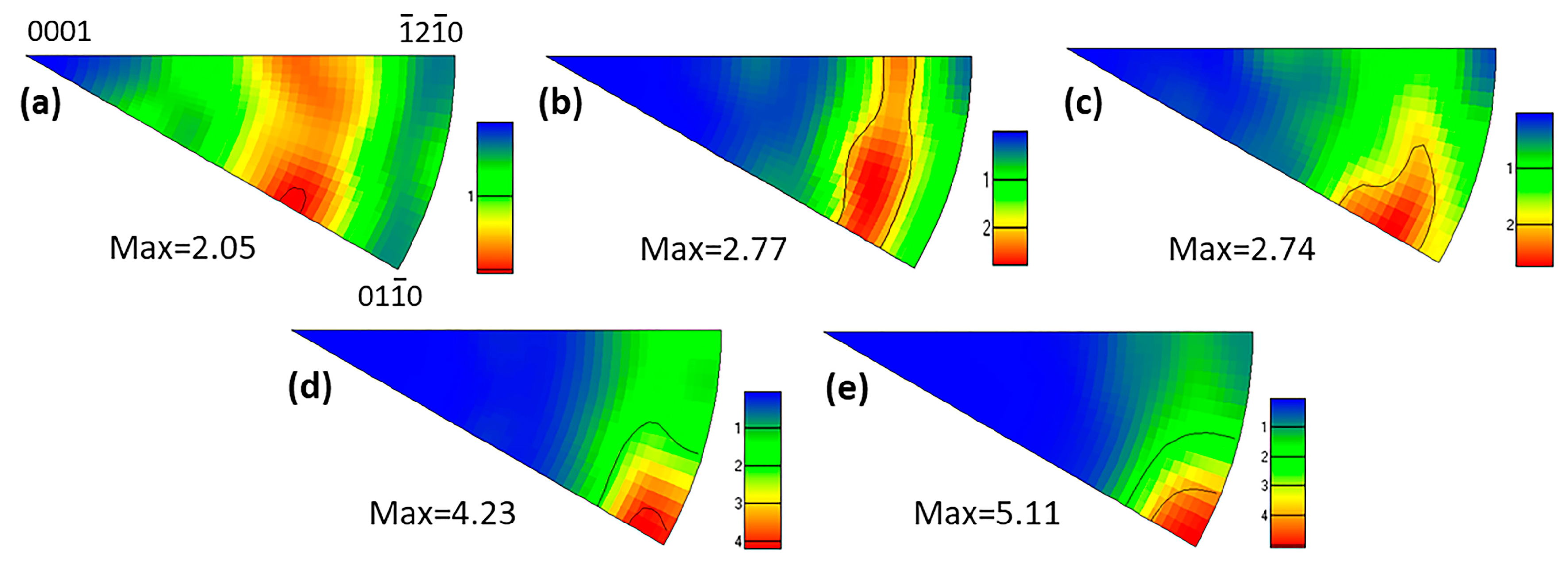
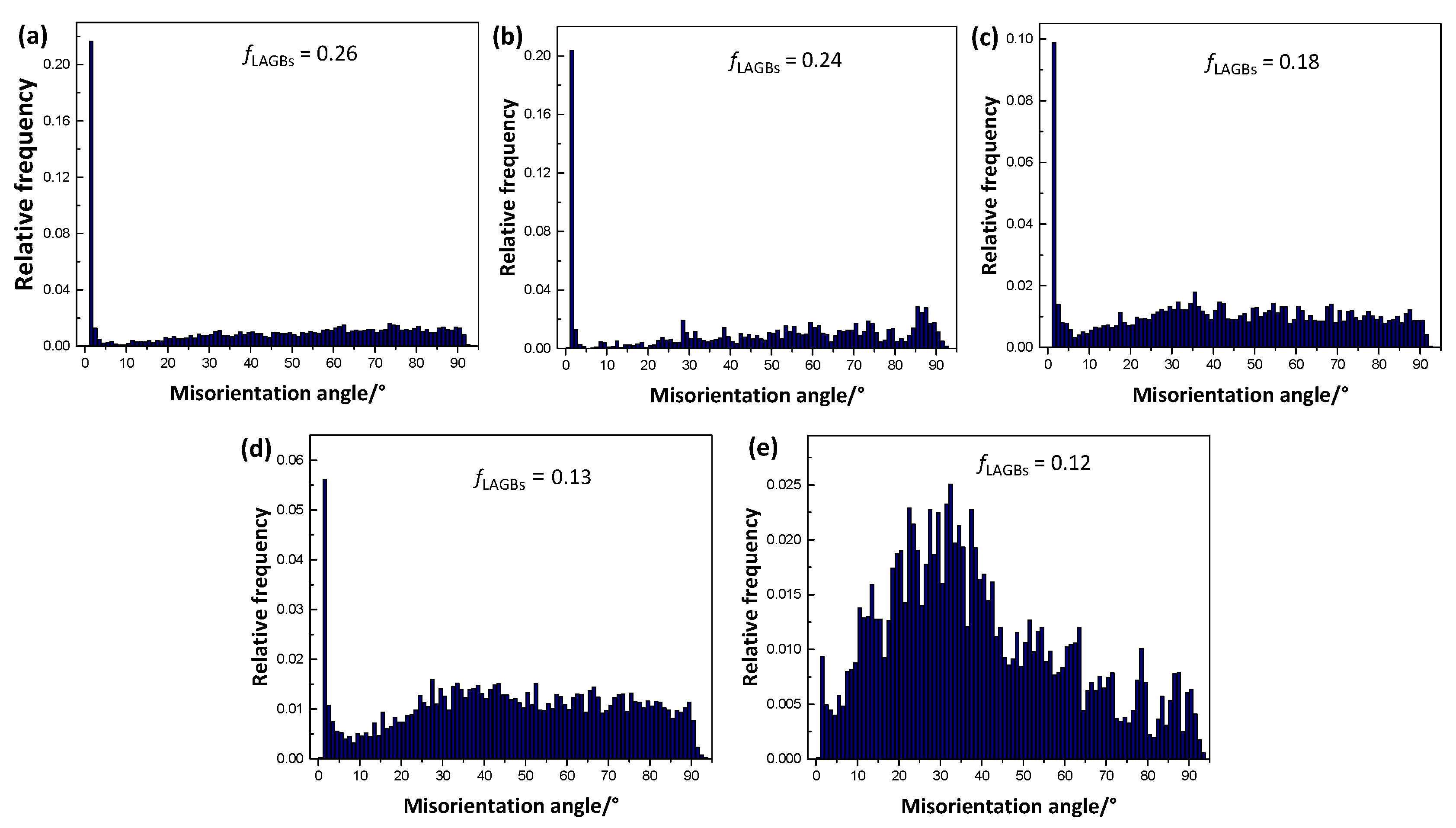
3.3. Texture of As-Extruded Alloys
4. Discussion
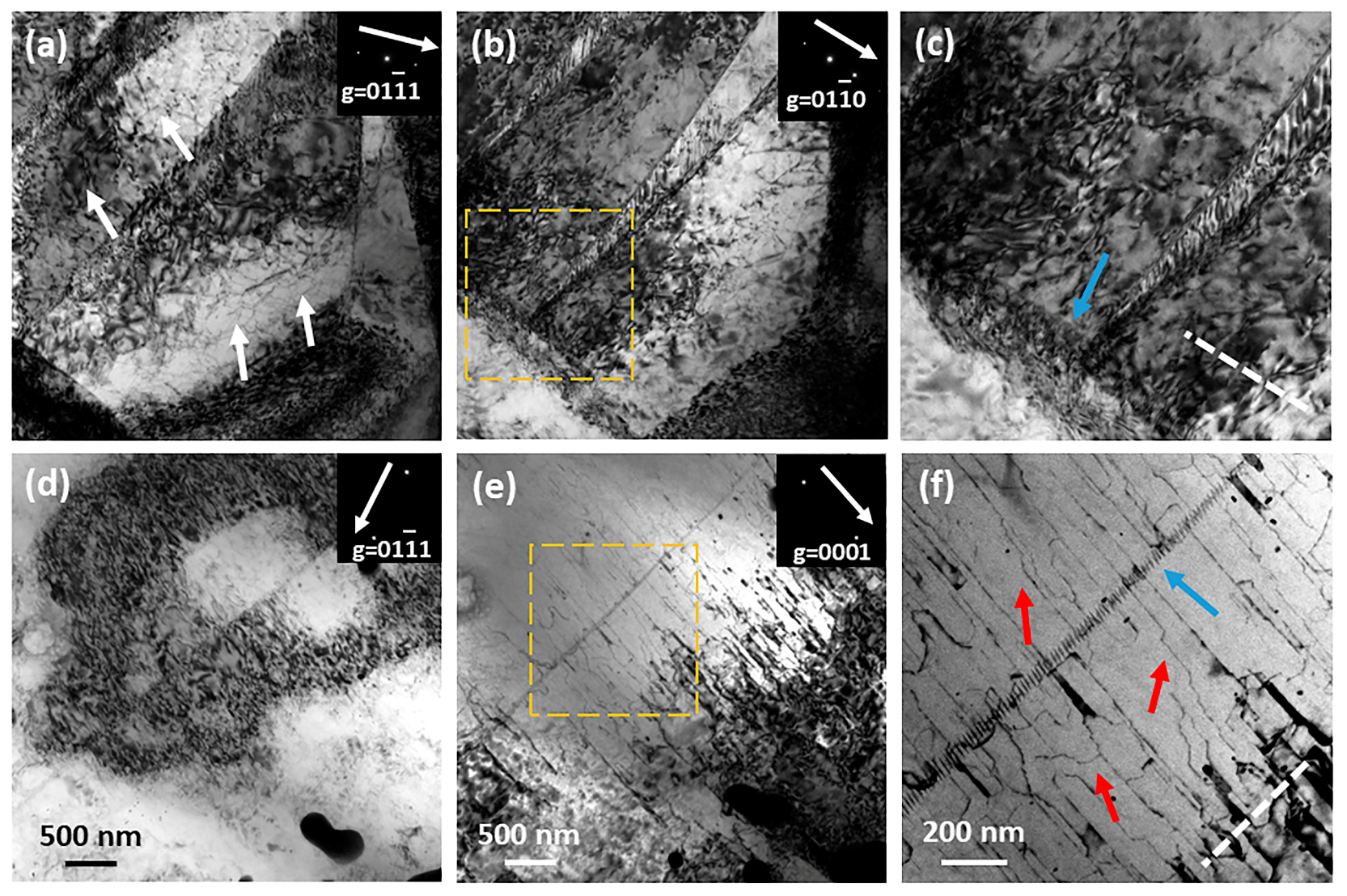
4.1. Effects of Zn Addition on Microstructure
4.2. Effects of Zn Addition on Texture
4.3. Effects of Zn Addition on Deformation Mode
5. Conclusions
- (1)
- The concentration of Zn addition has a crucial influence on the phase constitution of Mg-2Gd-0.5Zr-xZn alloys. As Zn content increases from 0.5 to 3.0 wt %, ternary phases change from 14H LPSO + (Mg,Zn)3Gd to Mg3Zn3Gd2.
- (2)
- The YS and UTS of the investigated alloys are greatly enhanced with the addition of Zn, which are attributable to grain refinement, precipitation strengthening, and texture sharpening induced by alloying with Zn.
- (3)
- Mg-2Gd-0.5Zr-3Zn exhibits well-balanced strength and ductility with YS and UTS of 285 and 314 MPa, accompanied by a tensile elongation of 24%. The comprehensive mechanical properties are much better than that of AZ31.
Author Contributions
Funding
Conflicts of Interest
References
- Sankaran, K.K.; Mishra, R.S. Magnesium Alloys. In Metallurgy and Design of Alloys with Hierarchical Microstructures; Sankaran, K.K., Mishra, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 345–383. [Google Scholar]
- Zheng, T.; Hu, Y.; Zhang, Y.; Pan, F. Formation of a hydrophobic and corrosion resistant coating on magnesium alloy via a one-step hydrothermal method. J. Colloid Interface Sci. 2017, 505, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, C.; Meng, W.; Pan, F.; Zhou, J. Microstructure, mechanical and corrosion properties of Mg-4Al-2Sn-xY-0.4Mn alloys. J. Alloys Compd. 2017, 727, 491–500. [Google Scholar] [CrossRef]
- Zeng, Z.; Nie, J.F.; Xu, S.W.; Davies, C.H.J.; Birbilis, N. Super-formable pure magnesium at room temperature. Nat. Commun. 2017, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.; Wu, R.; Hou, L.; Zhang, M. Recent developments in high-strength Mg-RE-based alloys: Focusing on Mg-Gd and Mg-Y systems. J. Magnes. Alloys 2018, 6, 277–291. [Google Scholar] [CrossRef]
- Maňák, J.; Vokoun, D. Microbending Experiments on Pure Magnesium with Nonbasal Slip Orientation. Materials 2018, 11, 1434. [Google Scholar] [CrossRef] [PubMed]
- Máthis, K.; Horváth, K.; Farkas, G.; Choe, H.; Shin, K.; Vinogradov, A. Investigation of the Microstructure Evolution and Deformation Mechanisms of a Mg-Zn-Zr-RE Twin-Roll-Cast Magnesium Sheet by In-Situ Experimental Techniques. Materials 2018, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Masoudpanah, S.M.; Mahmudi, R. The microstructure, tensile, and shear deformation behavior of an AZ31 magnesium alloy after extrusion and equal channel angular pressing. Mater. Des. 2010, 31, 3512–3517. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, A.; Wang, Q.; Gao, Z.; He, J.; She, J.; Song, K.; Pan, F. High strength and superior ductility of an ultra-fine grained magnesium–manganese alloy. Mater. Sci. Eng. A 2015, 648, 202–207. [Google Scholar] [CrossRef]
- Nakata, T.; Xu, C.; Ajima, R.; Shimizu, K.; Hanaki, S.; Sasaki, T.T.; Ma, L.; Hono, K.; Kamado, S. Strong and ductile age-hardening Mg-Al-Ca-Mn alloy that can be extruded as fast as aluminum alloys. Acta Mater. 2017, 130, 261–270. [Google Scholar] [CrossRef]
- Jiang, H.S.; Qiao, X.G.; Xu, C.; Zheng, M.Y.; Wu, K.; Kamado, S. Ultrahigh strength as-extruded Mg–10.3Zn–6.4Y–0.4Zr–0.5Ca alloy containing W phase. Mater. Des. 2016, 108, 391–399. [Google Scholar] [CrossRef]
- Sun, J.; Jin, L.; Dong, S.; Dong, J.; Zhang, Z.; Wang, F.; Ding, W.; Luo, A.A. A combined electron backscattered diffraction and visco-plastic self-consistent analysis on the anisotropic deformation behavior in a Mg-Gd-Y alloy. Mater. Des. 2017, 122, 164–171. [Google Scholar] [CrossRef]
- Al-Samman, T.; Li, X. Sheet texture modification in magnesium-based alloys by selective rare earth alloying. Mater. Sci. Eng. A 2011, 528, 3809–3822. [Google Scholar] [CrossRef]
- Basu, I.; Al-Samman, T. Twin recrystallization mechanisms in magnesium-rare earth alloys. Acta Mater. 2015, 96, 111–132. [Google Scholar] [CrossRef]
- Stanford, N. Micro-alloying Mg with Y, Ce, Gd and La for texture modification—A comparative study. Mater. Sci. Eng. A 2010, 527, 2669–2677. [Google Scholar] [CrossRef]
- Stanford, N.; Atwell, D.; Beer, A.; Davies, C.; Barnett, M.R. Effect of microalloying with rare-earth elements on the texture of extruded magnesium-based alloys. Scr. Mater. 2008, 59, 772–775. [Google Scholar] [CrossRef]
- Stanford, N.; Callaghan, M.D.; de Jong, B. The effect of rare earth elements on the behaviour of magnesium-based alloys: Part 1—Hot deformation behaviour. Mater. Sci. Eng. A 2013, 565, 459–468. [Google Scholar] [CrossRef]
- Basu, I.; Al-Samman, T.; Gottstein, G. Shear band-related recrystallization and grain growth in two rolled magnesium-rare earth alloys. Mater. Sci. Eng. A 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Imandoust, A.; Barrett, C.D.; Al-Samman, T.; Inal, K.A.; El Kadiri, H. A review on the effect of rare-earth elements on texture evolution during processing of magnesium alloys. J. Mater. Sci. 2016, 52, 1–29. [Google Scholar] [CrossRef]
- Stanford, N.; Atwell, D.; Barnett, M.R. The effect of Gd on the recrystallisation, texture and deformation behaviour of magnesium-based alloys. Acta Mater. 2010, 58, 6773–6783. [Google Scholar] [CrossRef]
- Homma, T.; Kunito, N.; Kamado, S. Fabrication of extraordinary high-strength magnesium alloy by hot extrusion. Scr. Mater. 2009, 61, 644–647. [Google Scholar] [CrossRef]
- Hu, Y.B.; Deng, J.; Zhao, C.; Pan, F.S.; Peng, J. Microstructure and mechanical properties of Mg–Gd–Zr alloys with low gadolinium contents. J. Mater. Sci. 2011, 46, 5838–5846. [Google Scholar] [CrossRef]
- Nie, J.F.; Gao, X.; Zhu, S.M. Enhanced age hardening response and creep resistance of Mg–Gd alloys containing Zn. Scr. Mater. 2005, 53, 1049–1053. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Hou, F.; Liu, S.; Peng, X.; Wang, J.; Pan, F. Enhanced strength and ductility of Mg-RE-Zn alloy simultaneously by trace Ag addition. Mater. Sci. Eng. A 2018, 728, 10–19. [Google Scholar] [CrossRef]
- Basu, I.; Al-Samman, T. Triggering rare earth texture modification in magnesium alloys by addition of zinc and zirconium. Acta Mater. 2014, 67, 116–133. [Google Scholar] [CrossRef]
- Gao, J.; Fu, J.; Zhang, N.; Chen, Y.A. Structural features and mechanical properties of Mg-Y-Zn-Sn alloys with varied LPSO phases. J. Alloys Compd. 2018, 768, 1029–1038. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Precipitation in a Mg–10Gd–3Y–0.4Zr (wt.%) alloy during isothermal ageing at 250°C. J. Alloys Compd. 2006, 421, 309–313. [Google Scholar] [CrossRef]
- Honma, T.; Ohkubo, T.; Hono, K.; Kamado, S. Chemistry of nanoscale precipitates in Mg–2.1Gd–0.6Y–0.2Zr (at.%) alloy investigated by the atom probe technique. Mater. Sci. Eng. A 2005, 395, 301–306. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Y.; Rong, W.; Wang, K.; Yuan, L.; Heng, X.; Peng, L. Effect of applied pressure on microstructures of squeeze cast Mg–15Gd–1Zn–0.4Zr alloy. J. Magnes. Alloys 2018, 6, 197–204. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, G.; Wang, G.; Zhu, G.; Zhou, W.; Wang, J.; Sun, B. Surface morphology, microstructure and properties of as-cast AZ31 magnesium alloy irradiated by high intensity pulsed ion beams. Appl. Surf. Sci. 2014, 311, 567–573. [Google Scholar] [CrossRef]
- Karparvarfard, S.M.H.; Shaha, S.K.; Behravesh, S.B.; Jahed, H.; Williams, B.W. Microstructure, texture and mechanical behavior characterization of hot forged cast ZK60 magnesium alloy. J. Mater. Sci. Technol. 2017, 33, 907–918. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, L.; Ji, Y.; Cheng, X.; Wang, N.; Zhao, Y.; Fu, Y.; Chen, L.Q.; Ding, W. The effect of low cooling rates on dendrite morphology during directional solidification in Mg–Gd alloys: In situ X-ray radiographic observation. Mater. Lett. 2016, 163, 218–221. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Gao, S.; Lu, R.; Qin, D.; Yang, W.; Pan, F. Optimization of mechanical and damping properties of Mg–0.6Zr alloy by different extrusion processing. J. Magnes. Alloys 2015, 3, 79–85. [Google Scholar] [CrossRef]
- Srinivasan, A.; Huang, Y.; Mendis, C.L.; Blawert, C.; Kainer, K.U.; Hort, N. Investigations on microstructures, mechanical and corrosion properties of Mg–Gd–Zn alloys. Mater. Sci. Eng. A 2014, 595, 224–234. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Bian, L.; Cheng, W.; Niu, X.; Xu, C.; Wu, S. Study of Mg–Gd–Zn–Zr alloys with long period stacking ordered structures. Mater. Sci. Eng. A 2013, 585, 268–276. [Google Scholar] [CrossRef]
- Wang, F.; Sandlöbes, S.; Diehl, M.; Sharma, L.; Roters, F.; Raabe, D. In situ observation of collective grain-scale mechanics in Mg and Mg–rare earth alloys. Acta Mater. 2014, 80, 77–93. [Google Scholar] [CrossRef]
- Sandlöbes, S.; Schestakow, I.; Yi, S.B.; Zaefferer, S.; Chen, J.Q.; Friák, M.; Neugebauer, J.; Raabe, D. The Relation between Shear Banding, Microstructure and Mechanical Properties in Mg and Mg-Y Alloys. Mater. Sci. Forum 2011, 690, 202–205. [Google Scholar] [CrossRef]
- Zeng, Z.R.; Zhu, Y.M.; Xu, S.W.; Bian, M.Z.; Davies, C.H.J.; Birbilis, N.; Nie, J.F. Texture evolution during static recrystallization of cold-rolled magnesium alloys. Acta Mater. 2016, 105, 479–494. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hoseini, M.; Pekguleryuz, M. Effect of Sr addition on texture evolution of Mg–3Al–1Zn (AZ31) alloy during extrusion. Mater. Sci. Eng. A 2011, 528, 3096–3104. [Google Scholar] [CrossRef]
- Bugnet, M.; Kula, A.; Niewczas, M.; Botton, G.A. Segregation and clustering of solutes at grain boundaries in Mg–rare earth solid solutions. Acta Mater. 2014, 79, 66–73. [Google Scholar] [CrossRef]
- Hadorn, J.P.; Sasaki, T.T.; Nakata, T.; Ohkubo, T.; Kamado, S.; Hono, K. Solute clustering and grain boundary segregation in extruded dilute Mg–Gd alloys. Scr. Mater. 2014, 93, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Hadorn, J.P.; Hantzsche, K.; Yi, S.; Bohlen, J.; Letzig, D.; Wollmershauser, J.A.; Agnew, S.R. Role of Solute in the Texture Modification During Hot Deformation of Mg-Rare Earth Alloys. Metall. Mater. Trans. A 2011, 43, 1347–1362. [Google Scholar] [CrossRef]
- Jain, J.; Cizek, P.; Hariharan, K. Transmission electron microscopy investigation on dislocation bands in pure Mg. Scr. Mater. 2017, 130, 133–137. [Google Scholar] [CrossRef]





| Designation | Nominal Composition | Actual Composition/wt % | |||
|---|---|---|---|---|---|
| Gd | Zr | Zn | Mg | ||
| Alloy1 | Mg-2Gd-0.5Zr | 2.20 | 0.34 | -- | Bal. |
| Alloy2 | Mg-2Gd-0.5Zr-0.5Zn | 2.13 | 0.39 | 0.56 | Bal. |
| Alloy3 | Mg-2Gd-0.5Zr-1.0Zn | 2.08 | 0.42 | 1.10 | Bal. |
| Alloy4 | Mg-2Gd-0.5Zr-2.0Zn | 1.82 | 0.50 | 1.98 | Bal. |
| Alloy5 | Mg-2Gd-0.5Zr-3.0Zn | 1.78 | 0.47 | 2.85 | Bal. |
| Condition | Average Grain Size/μm | ||||
|---|---|---|---|---|---|
| Alloy1 | Alloy2 | Alloy3 | Alloy4 | Alloy5 | |
| As-cast | 21.6 | 32.8 | 28.3 | 26.7 | 25.4 |
| As-extruded | 3.1 | 6.4 | 2.8 | 2.7 | 2.9 |
| Alloy | YS/MPa | UTS/MPa | A/% |
|---|---|---|---|
| Alloy1 | 137 (±2) | 204 (±1) | 51 (±0.4) |
| Alloy2 | 142 (±1) | 212 (±1) | 32 (±0.2) |
| Alloy3 | 164 (±3) | 246 (±2) | 28 (±0.3) |
| Alloy4 | 252 (±2) | 299 (±1) | 25 (±0.2) |
| Alloy5 | 285 (±3) | 314 (±2) | 24 (±0.4) |
| AZ31 | 209 (±3) | 246 (±4) | 14 (±0.3) |
| Alloy | Schmid Factor | |||
|---|---|---|---|---|
| Basal <a> (0001) <110> | Prismatic <a> (100) <110> | Pyramidal <a> (101) <110> | Pyramidal I <c+a> (1122) <11> | |
| Alloy1 | 0.37 | 0.33 | 0.42 | 0.43 |
| Alloy2 | 0.27 | 0.34 | 0.43 | 0.44 |
| Alloy3 | 0.27 | 0.39 | 0.43 | 0.45 |
| Alloy4 | 0.21 | 0.43 | 0.42 | 0.45 |
| Alloy5 | 0.19 | 0.43 | 0.42 | 0.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhang, C.; Zheng, T.; Pan, F.; Tang, A. Strengthening Effects of Zn Addition on an Ultrahigh Ductility Mg-Gd-Zr Magnesium Alloy. Materials 2018, 11, 1942. https://doi.org/10.3390/ma11101942
Hu Y, Zhang C, Zheng T, Pan F, Tang A. Strengthening Effects of Zn Addition on an Ultrahigh Ductility Mg-Gd-Zr Magnesium Alloy. Materials. 2018; 11(10):1942. https://doi.org/10.3390/ma11101942
Chicago/Turabian StyleHu, Yaobo, Chao Zhang, Tianxu Zheng, Fusheng Pan, and Aitao Tang. 2018. "Strengthening Effects of Zn Addition on an Ultrahigh Ductility Mg-Gd-Zr Magnesium Alloy" Materials 11, no. 10: 1942. https://doi.org/10.3390/ma11101942




