Organic Thin Film Transistors Incorporating Solution Processable Thieno[3,2-b]thiophene Thienoacenes
Abstract
:1. Introduction
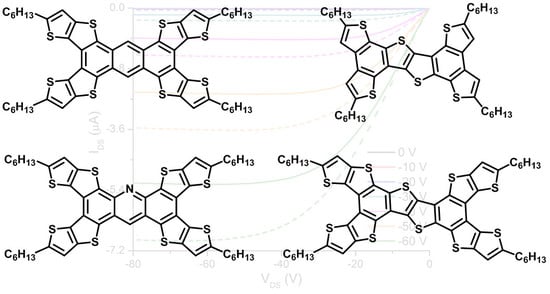
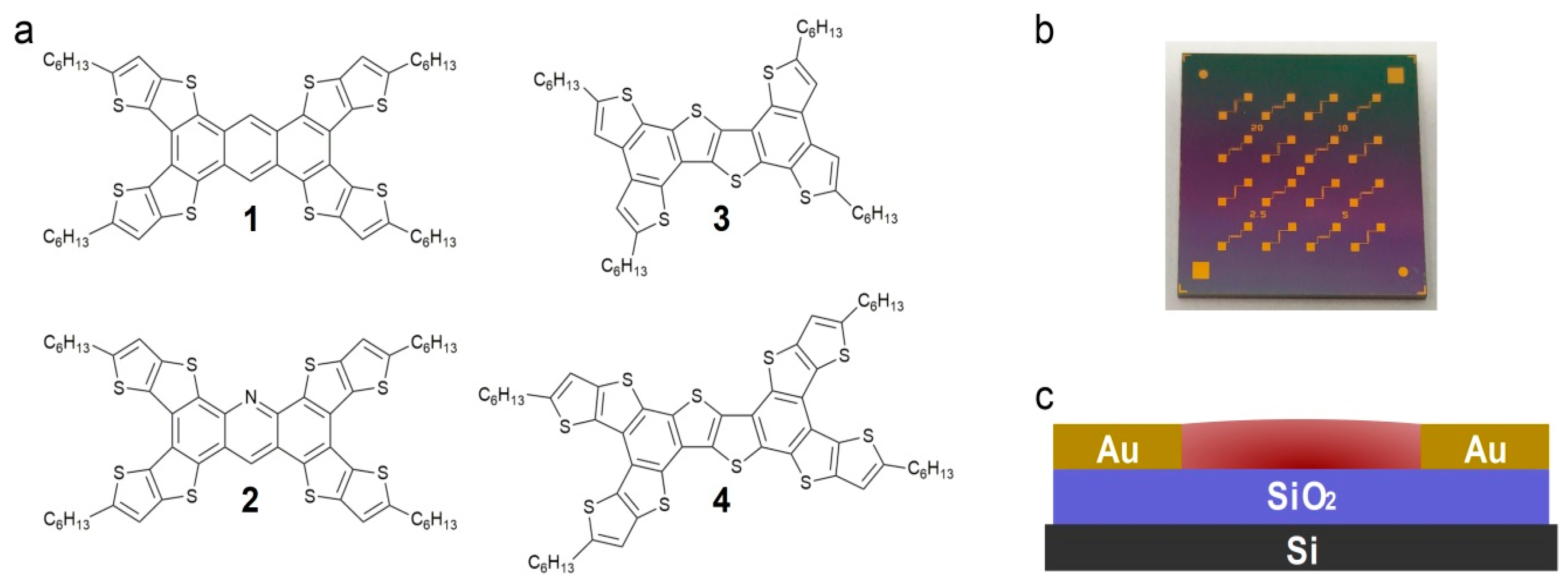
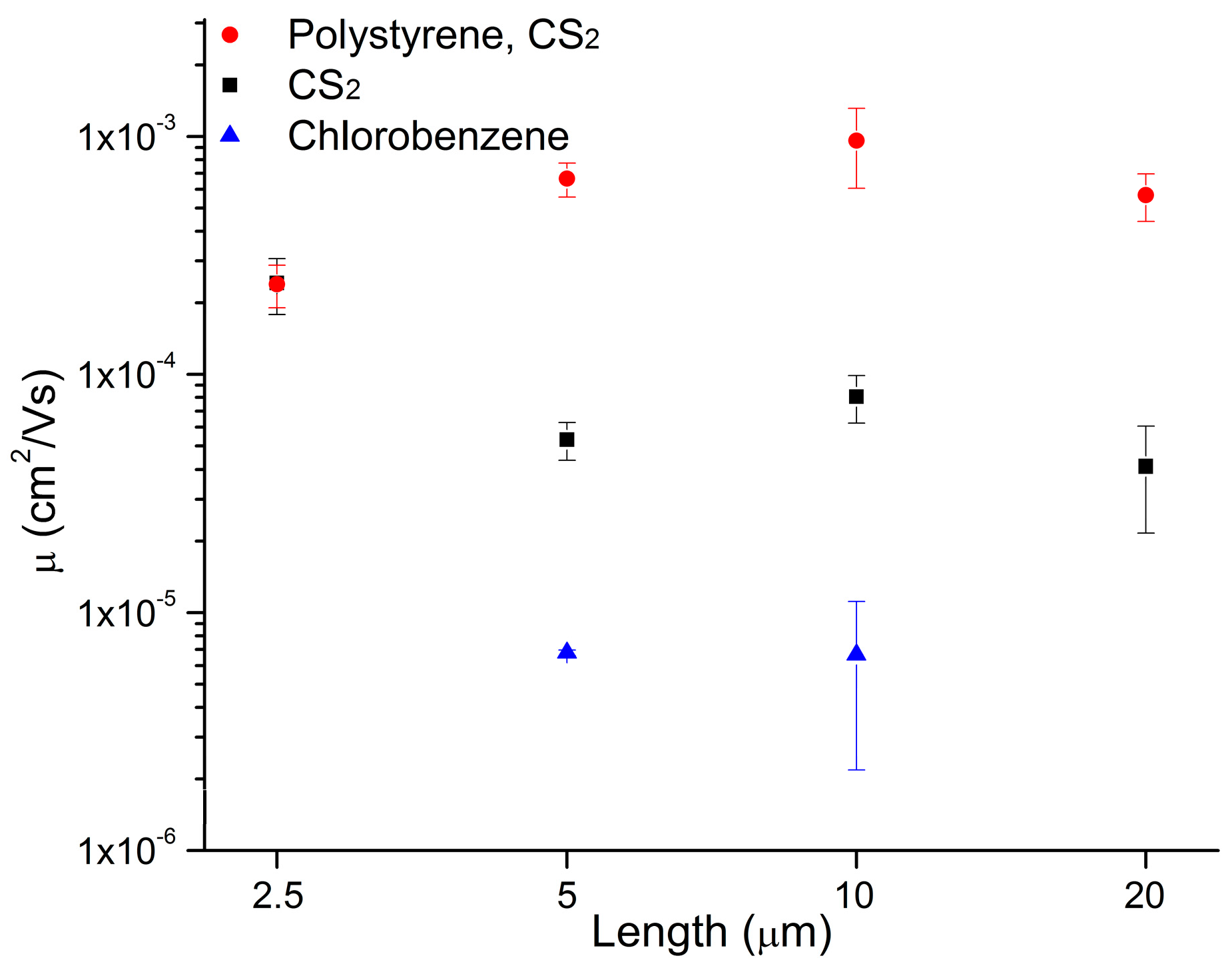
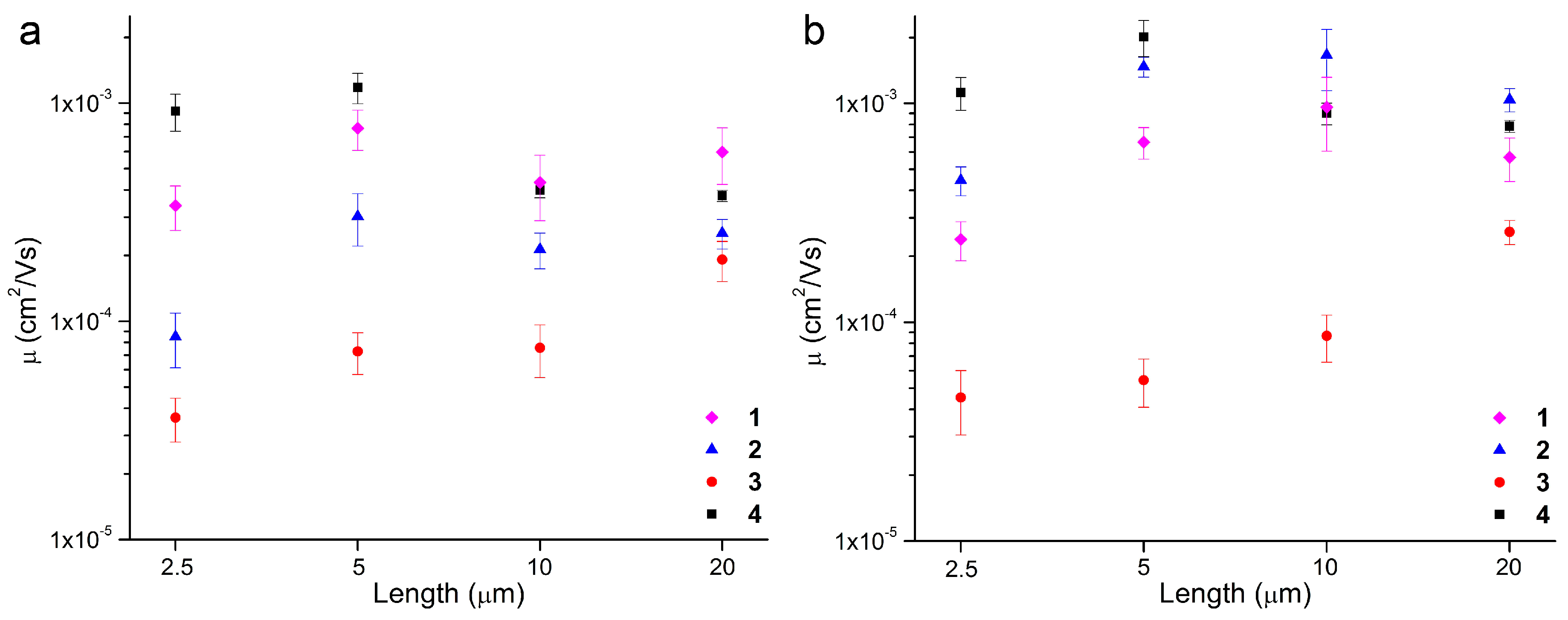
2. Results
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ma, C.Q.; Bäuerle, P. Functional oligothiophenes: Molecular design for multidimensional nanoarchitectures and their applications. Chem. Rev. 2009, 109, 1141–1176. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Diao, Y.; Appleton, A.L.; Fang, L.; Bao, Z. Integrated materials design of organic semiconductors for field-effect transistors. J. Am. Chem. Soc. 2013, 135, 6724–6746. [Google Scholar] [CrossRef] [PubMed]
- Melville, O.A.; Lessard, B.H.; Bender, T.P. Phthalocyanine-Based Organic Thin-Film Transistors: A Review of Recent Advances. ACS Appl. Mater. Interfaces 2015, 7, 13105–13118. [Google Scholar] [CrossRef] [PubMed]
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; Bässler, H. The Electronic Structure of Organic Semiconductors. In Electronic Processes in Organic Semiconductors; Wiley-VCH Verlag GmbH: Hoboken, NJ, USA, 2015; pp. 1–86. ISBN 978-352-768-5172. [Google Scholar]
- El-Kareh, B.; Hutter, L.N. Review of Single-Crystal Silicon Properties. In Silicon Analog Components: Device Design, Process Integration, Characterization and Reliability; El-Kareh, B., Hutter, L.N., Eds.; Springer: New York, NY, USA, 2015; pp. 25–63. ISBN 978-1-4939-2750-0. [Google Scholar]
- Pfeiffer, M. Controlled p-doping of pigment layers by cosublimation: Basic mechanisms and implications for their use in organic photovoltaic cells. Sol. Energy Mater. Sol. Cells 2000, 63, 83–99. [Google Scholar] [CrossRef]
- Méndez, H.; Heimel, G.; Winkler, S.; Frisch, J.; Opitz, A.; Sauer, K.; Wegner, B.; Oehzelt, M.; Röthel, C.; Duhm, S.; et al. Charge-transfer crystallites as molecular electrical dopants. Nat. Commun. 2015, 6, 8560. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, I.; Heimel, G.; Oehzelt, M.; Winkler, S.; Koch, N. Molecular Electrical Doping of Organic Semiconductors: Fundamental Mechanisms and Emerging Dopant Design Rules. Acc. Chem. Res. 2016, 49, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Yoo, S.-J.; Moon, C.-K.; Sim, B.; Lee, J.-H.; Lim, H.; Kim, J.W.; Kim, J.-J. N-Type Molecular Doping in Organic Semiconductors: Formation and Dissociation Efficiencies of a Charge Transfer Complex. J. Phys. Chem. C 2016, 120, 9475–9481. [Google Scholar] [CrossRef]
- Riede, M.; Lüssem, B.; Leo, K. Organic Semiconductors. In Comprehensive Semiconductor Science and Technology; Schwoerer, M., Wolf, H.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 448–507. ISBN 978-352-740-5404. [Google Scholar]
- Balaji, G.; Della Pelle, A.M.; Popere, B.C.; Chandrasekaran, A.; Thayumanavan, S. Synthesis and properties of thienopyrrole based heteroacenes—Indolodibenzothienopyrrole and dicarbazolodithienopyrrole. Org. Biomol. Chem. 2012, 10, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-X.; Zhao, G.-J. Modification of n-Type Organic Semiconductor Performance of Perylene Diimides by Substitution in Different Positions: Two-Dimensional π-Stacking and Hydrogen Bonding. ChemSusChem 2012, 5, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Kawasaki, N.; Kaji, Y.; Kubozono, Y.; Fujiwara, A.; Yamaji, M. Air-assisted High-performance Field-effect Transistor with Thin Films of Picene. J. Am. Chem. Soc. 2008, 130, 10470–10471. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Kakinuma, K.; Yoshimura, M.; Ishii, K.; Yamazaki, S.; Kobori, T.; Okuyama, H.; Kobayashi, H.; Tada, H. Charge carrier transport in high purity perylene single crystal studied by time-of-flight measurements and through field effect transistor characteristics. Chem. Phys. 2006, 325, 160–169. [Google Scholar] [CrossRef]
- Bunz, U.H.F. The Larger Linear N-Heteroacenes. Acc. Chem. Res. 2015, 48, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Juárez, R.; Ramos, M.; Seoane, C. Hexaazatriphenylene (HAT) derivatives: From synthesis to molecular design, self-organization and device applications. Chem. Soc. Rev. 2015, 44, 6850–6885. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q. N-heteropentacenes and N-heteropentacenequinones: From molecules to semiconductors. Synlett 2012, 2012, 326–336. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gao, J.; Jiang, L.; Qi, X.; Hao, W.; Zou, S.; Zhang, H.; Li, H.; Hu, W. Easily solution-processed, high-performance microribbon transistors based on a 2D condensed benzothiophene derivative. Chem. Commun. 2014, 50, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, Y.; Zhang, Y.; Liu, R.; Li, Q.; Zhan, X.; Liu, Y.; Hu, W. Synthesis, self-assembly, and solution-processed nanoribbon field-effect transistor of a fused-nine-ring thienoacene. Chem. Commun. 2010, 46, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, S.; Gao, J.; Zhang, H.; Lai, G.; Yang, C.; Xie, H.; Fang, R.; Li, H.; Hu, W. High-performance organic field-effect transistors based on single-crystalline microribbons of a two-dimensional fused heteroarene semiconductor. Chem. Commun. 2015, 51, 11961–11963. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Zhou, P.; Zhong, J.; Fan, J.; Wang, Z.; Wang, Y.; Pei, Y.; Bao, X.; Yang, R.; Hu, W.; et al. Novel wide band-gap polymer utilizing fused hetero-aromatic unit for efficient polymer solar cells and field-effect transistors. Polymer 2014, 55, 6708–6716. [Google Scholar] [CrossRef]
- Leitch, A.A.; Mansour, A.; Stobo, K.A.; Korobkov, I.; Brusso, J.L. Functionalized Tetrathienoanthracene: Enhancing π–π Interactions Through Expansion of the π-Conjugated Framework. Cryst. Growth Des. 2012, 12, 1416–1421. [Google Scholar] [CrossRef]
- Leitch, A.A.; Stobo, K.A.; Hussain, B.; Ghoussoub, M.; Ebrahimi-Takalloo, S.; Servati, P.; Korobkov, I.; Brusso, J.L. Oligothiophene-functionalized benzene and tetrathienoanthracene: Effect of enhanced π-conjugation on optoelectronic properties, self-assembly and device performance. Eur. J. Org. Chem. 2013, 2013, 5854–5863. [Google Scholar] [CrossRef]
- Robertson, S.F.; Leitch, A.A.; Korobkov, I.; Soldatov, D.V.; Brusso, J.L. Functionalized thienoacridines: Synthesis, optoelectronic, and structural properties. Can. J. Chem. 2014, 92, 1106–1110. [Google Scholar] [CrossRef]
- Brusso, J.L.; Hirst, O.D.; Dadvand, A.; Ganesan, S.; Cicoira, F.; Robertson, C.M.; Oakley, R.T.; Rosei, F.; Perepichka, D.F. Two-Dimensional Structural Motif in Thienoacene Semiconductors: Synthesis, Structure, and Properties of Tetrathienoanthracene Isomers. Chem. Mater. 2008, 20, 2484–2494. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Gong, X.; Heeger, A.J. Low bandgap semiconducting polymers for polymeric photovoltaics. Chem. Soc. Rev. 2016, 45, 4825–4846. [Google Scholar] [CrossRef] [PubMed]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef] [PubMed]
- Magnan, F.; Gabidullin, B.; Brusso, J.L. Applying thieno[3,2-b]thiophene as a building block in the design of rigid extended thienoacenes. RSC Adv. 2016, 6, 97420–97429. [Google Scholar] [CrossRef]
- Melville, O.A.; Rice, N.A.; Therrien, I.; Lessard, B.H. Organic thin-film transistors incorporating a commercial pigment (Hostasol Red GG) as a low-cost semiconductor. Dye Pigment 2018, 149, 449–455. [Google Scholar] [CrossRef]
- Smith, J.; Hamilton, R.; McCulloch, I.; Stingelin-Stutzmann, N.; Heeney, M.; Bradley, D.D.C.; Anthopoulos, T.D. Solution-processed organic transistors based on semiconducting blends. J. Mater. Chem. 2010, 20, 2562–2574. [Google Scholar] [CrossRef]
- Niazi, M.R.; Li, R.; Li, E.Q.; Kirmani, A.R.; Abdelsamie, M.; Wang, Q.; Pan, W.; Payne, M.M.; Anthony, J.E.; Smilgies, D.-M.; et al. Solution-printed organic semiconductor blends exhibiting transport properties on par with single crystals. Nat. Commun. 2015, 6, 8598. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Shin, N.; Jang, D.Y.; Prabhu, V.M.; Yoon, D.Y. Structure and Properties of Small Molecule-Polymer Blend Semiconductors for Organic Thin Film Transistors. J. Am. Chem. Soc. 2008, 130, 12273–12275. [Google Scholar] [CrossRef] [PubMed]

| Name | Vacuum | Air | ||||||
|---|---|---|---|---|---|---|---|---|
| Average µ (cm2/Vs) × 10−4 | Highest µ (cm2/Vs) | Ion/off | VT (V) | Average µ (cm2/Vs) × 10−4 | Highest µ (cm2/Vs) | Ion/off | VT (V) | |
| 1 | 7.7 ± 1.6 | 2.3 × 10−3 | 10–103 | −8 ± 2 | 6.7 ± 1.1 | 1.4 × 10−3 | 10–102 | −0.96 ± 4 |
| 2 | 3.0 ± 0.82 | 2.5 × 10−3 | 102–104 | −23 ± 0.9 | 15 ± 1.5 | 3.9 × 10−3 | 102–103 | −16 ± 0.6 |
| 3 | 0.73 ± 0.16 | 2.2 × 10−4 | 10–103 | −16 ± 2 | 0.54 ± 0.14 | 2.4 × 10−4 | 10–103 | −13 ± 3 |
| 4 | 12 ± 1.7 | 3.1 × 10−3 | 103–104 | −32 ± 0.6 | 20 ± 3.8 | 9.2 × 10−3 | 103–104 | −28 ± 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rice, N.A.; Magnan, F.; Melville, O.A.; Brusso, J.L.; Lessard, B.H. Organic Thin Film Transistors Incorporating Solution Processable Thieno[3,2-b]thiophene Thienoacenes. Materials 2018, 11, 8. https://doi.org/10.3390/ma11010008
Rice NA, Magnan F, Melville OA, Brusso JL, Lessard BH. Organic Thin Film Transistors Incorporating Solution Processable Thieno[3,2-b]thiophene Thienoacenes. Materials. 2018; 11(1):8. https://doi.org/10.3390/ma11010008
Chicago/Turabian StyleRice, Nicole A., François Magnan, Owen A. Melville, Jaclyn L. Brusso, and Benoît H. Lessard. 2018. "Organic Thin Film Transistors Incorporating Solution Processable Thieno[3,2-b]thiophene Thienoacenes" Materials 11, no. 1: 8. https://doi.org/10.3390/ma11010008
APA StyleRice, N. A., Magnan, F., Melville, O. A., Brusso, J. L., & Lessard, B. H. (2018). Organic Thin Film Transistors Incorporating Solution Processable Thieno[3,2-b]thiophene Thienoacenes. Materials, 11(1), 8. https://doi.org/10.3390/ma11010008