Study on Zinc Oxide-Based Electrolytes in Low-Temperature Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
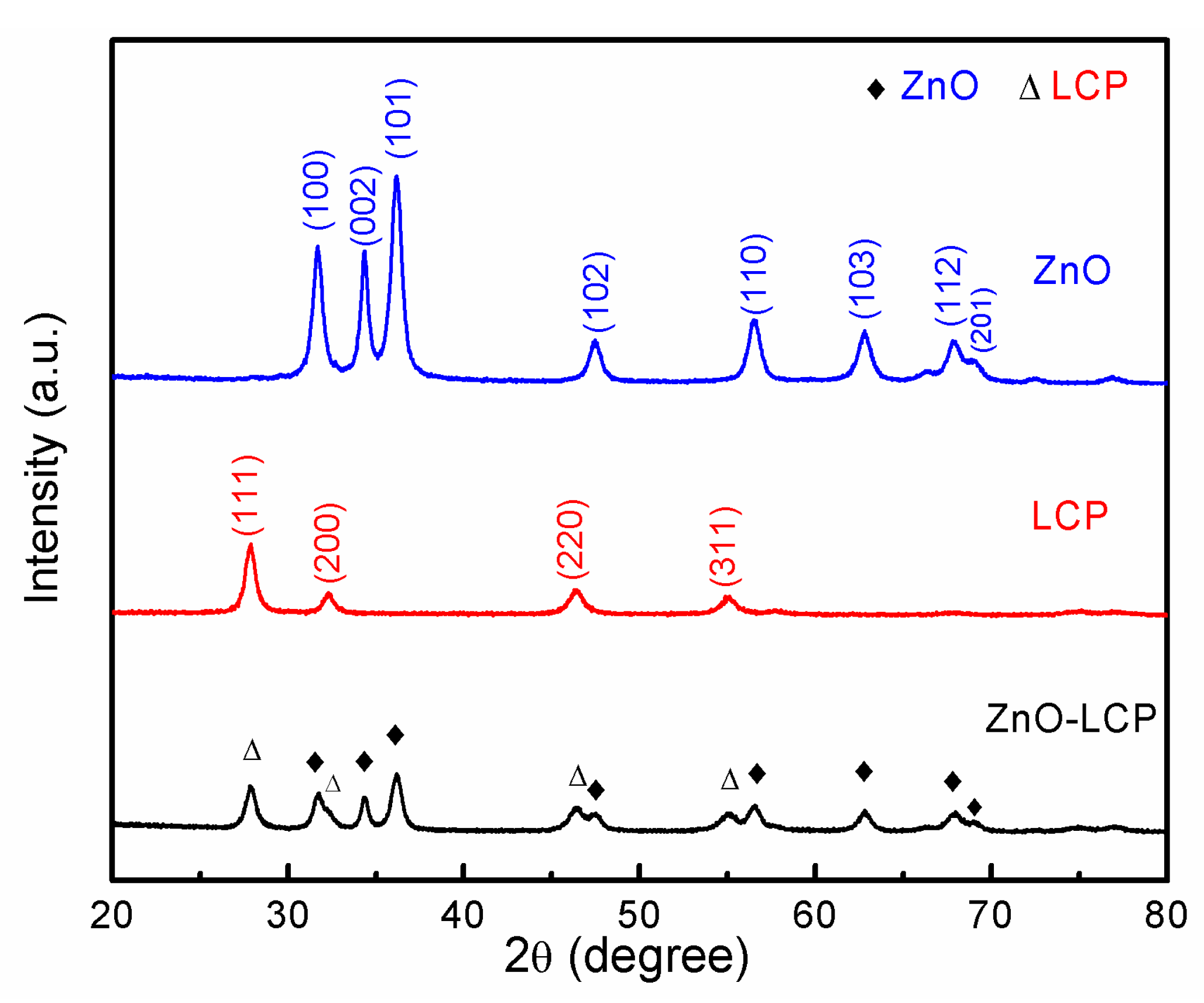
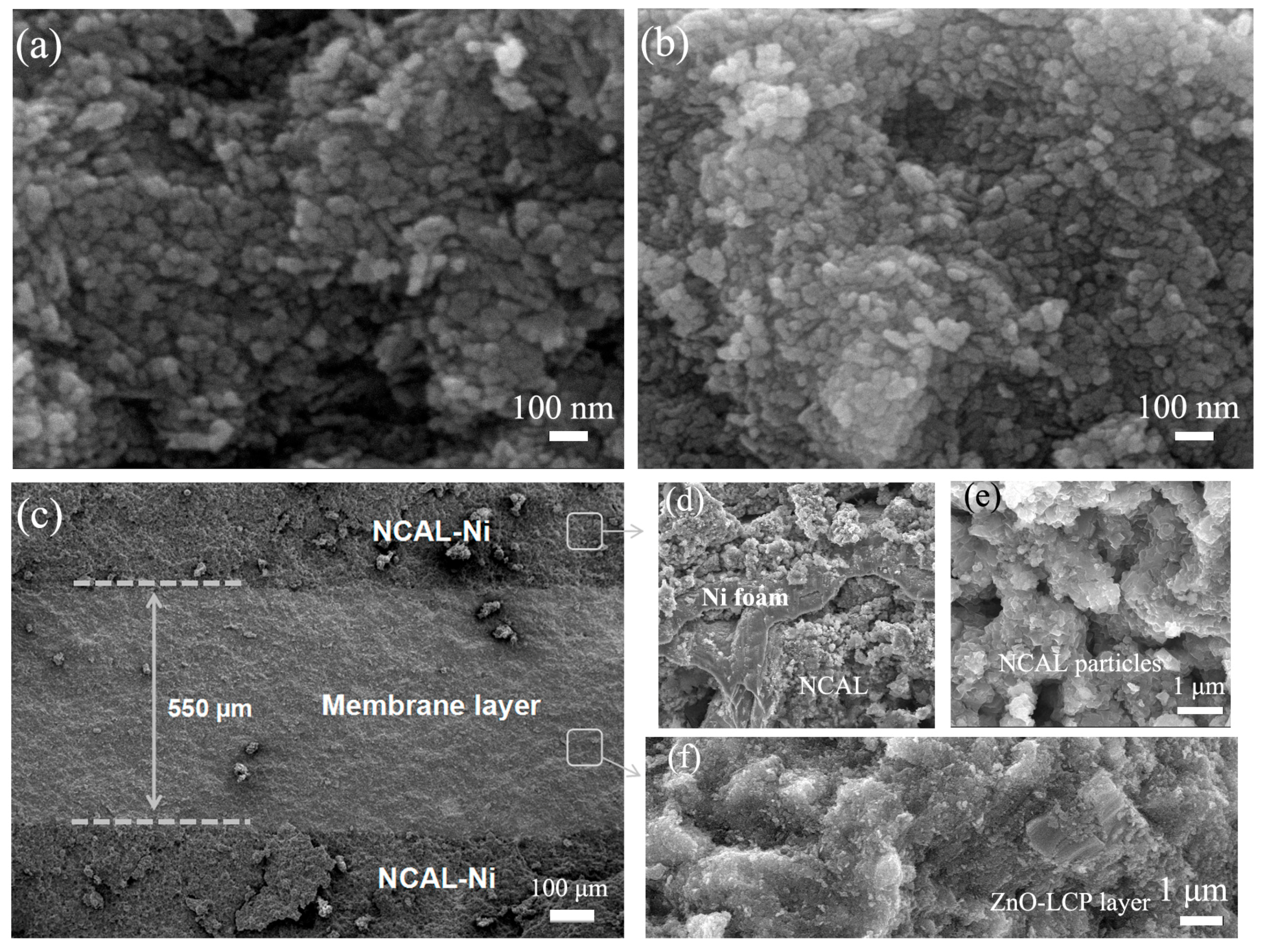
3.1. Crystalline Structure and Morphology
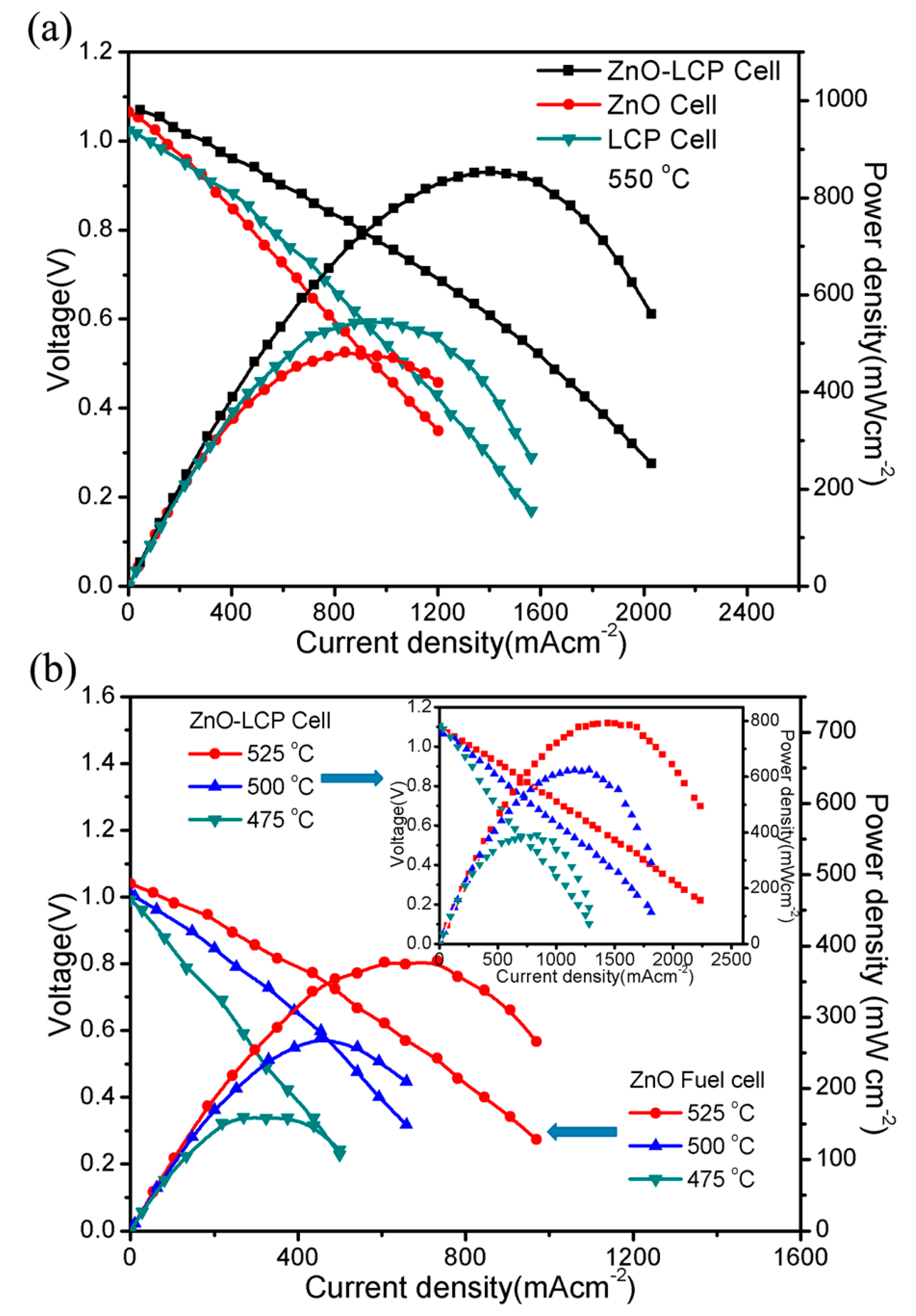
3.2. Electrochemical Performance
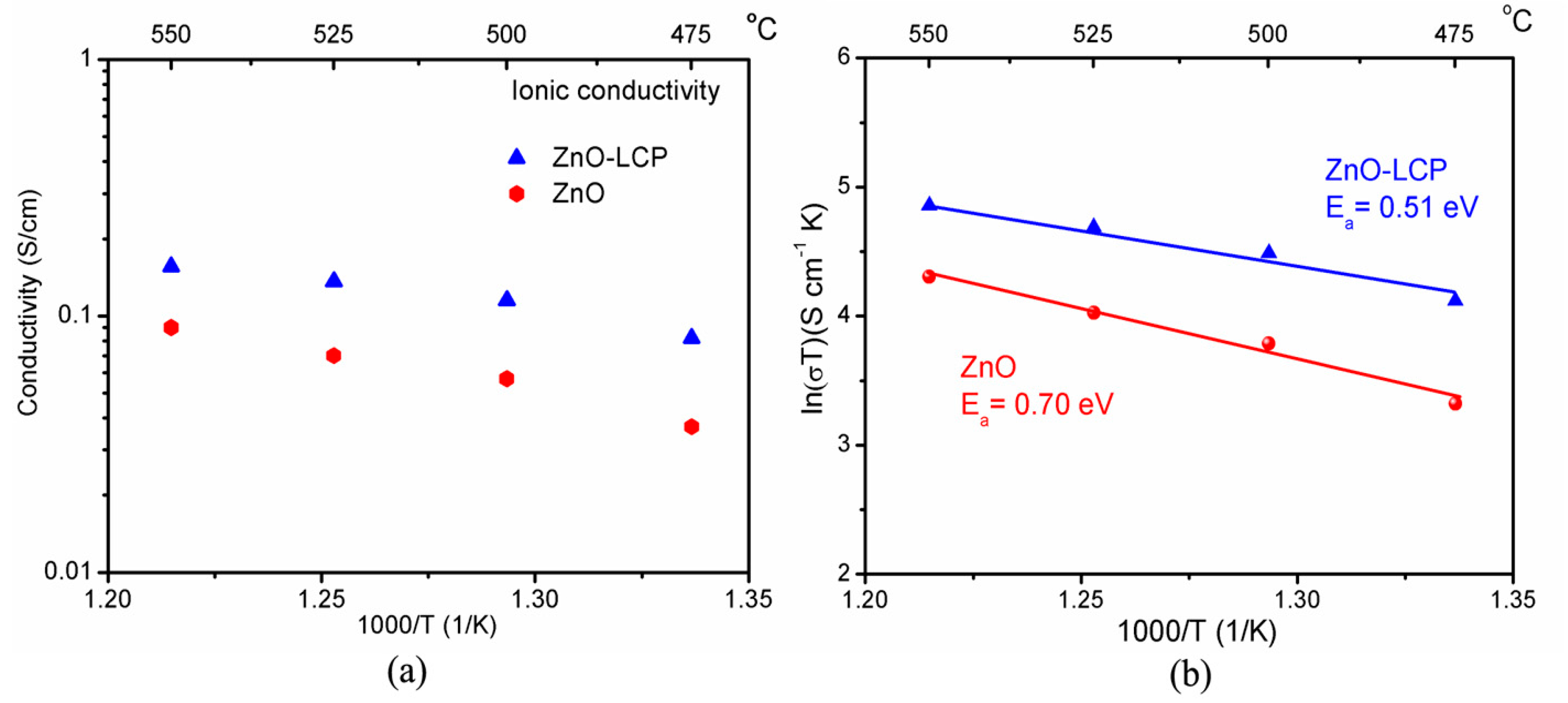
3.3. Electrical Conductivity
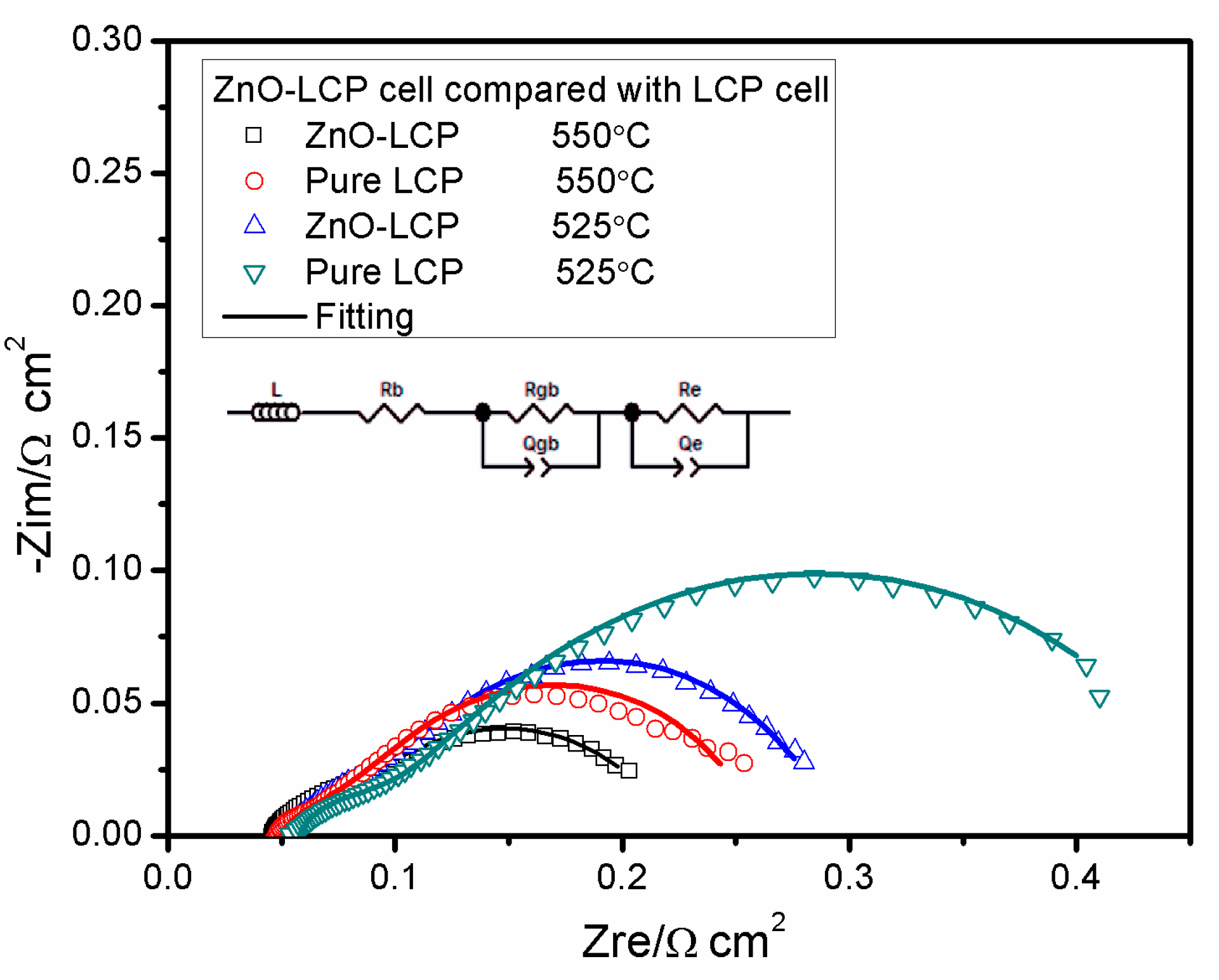
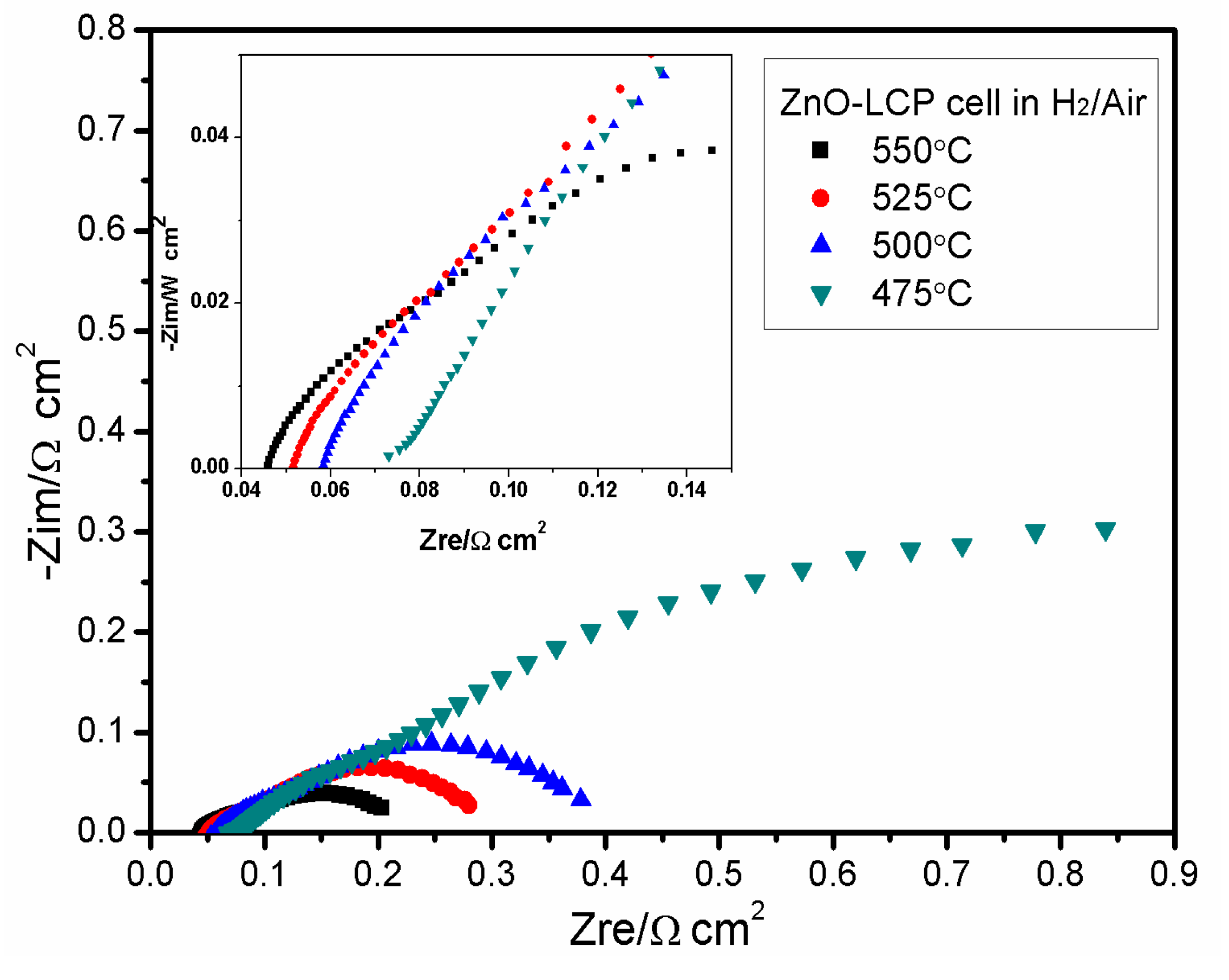
3.4. Impedance Spectroscopy Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Dyer, C.K. Replacing the battery in portable electronics. Sci. Am. 1999, 281, 88–93. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Wu, Y.; Ran, R.; Shao, Z. Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 2013, 113, 8104–8151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, W.; Ding, D.; Liu, M.; Ciucci, F.; Tade, M.; Shao, Z. Advances in cathode materials for solid oxide fuel cells: Complex oxides without alkaline earth metal elements. Adv. Energy Mater. 2015, 5, 1500537. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.H. Appraisal of Ce1−yGdyO2−y/2 Electrolytes for ITSOFC Operation at 500 °C. Solid State Ion. 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Andersson, D.A.; Simak, S.I.; Skorodumova, N.V.; Abrikosov, I.A.; Johansson, B. Optimization of ionic conductivity in doped ceria. Proc. Natl. Acad. Sci. USA 2006, 103, 3518–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Raza, R.; Abbas, G.; Singh, M. An electrolyte-free fuel cell constructed from one homogenous layer with mixed conductivity. Adv. Funct. Mater. 2011, 21, 2465–2469. [Google Scholar] [CrossRef]
- Zhu, B.; Raza, R.; Qin, H.; Liu, Q.; Fan, L. Fuel cells based on electrolyte and non-electrolyte separators. Energy Environ. Sci. 2011, 4, 2986–2992. [Google Scholar] [CrossRef]
- Wang, B.; Cai, Y.; Xia, C.; Kim, J.S.; Liu, Y.; Dong, W.; Wang, H.; Afzal, M.; Li, J.; Raza, R.; et al. Semiconductor-ionic membrane of lasrcofe-oxide-doped ceria solid oxide fuel cells. Electrochim. Acta 2017, 248, 496–504. [Google Scholar] [CrossRef]
- Zagórski, K.; Wachowski, S.; Szymczewska, D.; Mielewczyk-Gryń, A.; Jasiński, P.; Gazda, M. Performance of a single layer fuel cell based on a mixed proton-electron conducting composite. J. Power Sources 2017, 353, 230–236. [Google Scholar] [CrossRef]
- Xia, C.; Wang, B.; Ma, Y.; Cai, Y.; Afzal, M.; Liu, Y.; He, Y.; Zhang, W.; Dong, W.; Li, J.; et al. Industrial-grade rare-earth and perovskite oxide for high-performance electrolyte layer-free fuel cell. J. Power Sources 2016, 307, 270–279. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, B.; Wang, Y.; Raza, R.; Tan, W.; Kim, J.S.; van Aken, P.A.; Lund, P. Charge separation and transport in La0.6Sr0.4Co0.2Fe0.8O3-δ and ion-doping ceria heterostructure material for new generation fuel cell. Nano Energy 2017, 37, 195–202. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Fan, L.; Cai, Y.; Xia, C.; Liu, Y.; Raza, R.; van Aken, P.A.; Wang, H. Preparation and characterization of Sm and Ca co-doped ceria-La0.6Sr0.4Co0.2Fe0.8O3-δ semiconductor-ionic composites for electrolyte-layer-free fuel cells. J. Mater. Chem. A 2016, 4, 15426–15436. [Google Scholar] [CrossRef]
- Zhou, Y.; Guan, X.; Zhou, H.; Ramadoss, K.; Adam, S.; Liu, H.; Lee, S.; Shi, J.; Tsuchiya, M.; Fong, D.D.; et al. Strongly correlated perovskite fuel cells. Nature 2016, 534, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Tao, S. Novel proton conductors in the layered oxide material LixlAl0.5Co0.5O2. Adv. Energy Mater. 2014, 4, 1301683. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, C.; Zhang, W.; Yang, X.; Bao, Z.Y.; Li, J.J.; Zhu, B. Natural Hematite for Next-Generation Solid Oxide Fuel Cells. Adv. Funct. Mater. 2016, 26, 938–942. [Google Scholar] [CrossRef]
- Xia, C.; Cai, Y.; Ma, Y.; Wang, B.; Zhang, W.; Karlsson, M.; Wu, Y.; Zhu, B. Natural mineral-based solid oxide fuel cell with heterogeneous nanocomposite derived from hematite and rare-earth minerals. ACS Appl. Mater. Interfaces 2016, 8, 20748–20755. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Fan, L.; Zhao, Y.; Tan, W.; Xiong, D.; Wang, H. Functional semiconductor-ionic composite GDC-KZnAl/LiNiCuZnOx for single-component fuel cell. RSC Adv. 2014, 4, 9920–9925. [Google Scholar] [CrossRef]
- Zhu, B.; Huang, Y.; Fan, L.; Ma, Y.; Wang, B.; Xia, C.; Afzal, M.; Zhang, B.; Dong, W.; Wang, H.; et al. Novel fuel cell with nanocomposite functional layer designed by perovskite solar cell principle. Nano Energy 2016, 19, 156–164. [Google Scholar] [CrossRef]
- Li, P.; Yu, B.; Li, J.; Yao, X.; Zhao, Y.; Li, Y. A single layer solid oxide fuel cell composed of La2NiO4 and doped ceria-carbonate with H2 and methanol as fuels. Int. J. Hydrogen Energy 2016, 41, 9059–9065. [Google Scholar] [CrossRef]
- Dong, X.; Tian, L.; Li, J.; Zhao, Y.; Tian, Y.; Li, Y. Single layer fuel cell based on a composite of Ce0.8Sm0.2O2−δ-Na2CO3 and a mixed ionic and electronic conductor Sr2Fe1.5Mo0.5O6−δ. J. Power Sources 2014, 249, 270–276. [Google Scholar] [CrossRef]
- Chang, S.J.; Duan, B.G.; Hsiao, C.H.; Liu, C.W.; Young, S.J. UV enhanced emission performance of low temperature grown Ga-doped ZnO nanorods. IEEE Photonics Technol. Lett. 2014, 26, 66–69. [Google Scholar] [CrossRef]
- Carrasco, J.; Lopez, N.; Illas, F. First principles analysis of the stability and diffusion of oxygen vacancies in metal oxides. Phys. Rev. Lett. 2004, 93, 225502. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Oxygen vacancies in ZnO. Appl. Phys. Lett. 2005, 87, 122102. [Google Scholar] [CrossRef]
- Liu, Y.; Lao, L.E. Structural and electrical properties of ZnO-doped 8 mol% yttria-stabilized zirconia. Solid State Ion. 2006, 177, 159–163. [Google Scholar] [CrossRef]
- Norbya, T. Proton conduction in oxides. Solid State Ion. 1990, 40, 857–862. [Google Scholar] [CrossRef]
- Suwanboon, S.; Amornpitoksuk, P.; Haidoux, A.; Tedenac, J.C. Structural and optical properties of undoped and aluminium doped zinc oxide nanoparticles via precipitation method at low temperature. J. Alloys Compd. 2008, 462, 335–339. [Google Scholar] [CrossRef]
- Xia, C.; Wang, B.; Cai, Y.; Zhang, W.; Afzal, M.; Zhu, B. Electrochemical properties of LaCePr-oxide/K2WO4 composite electrolyte for low-temperature SOFCs. Electrochem. Commun. 2017, 77, 44–48. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, M.; Neoh, K.C.; Han, G.D.; Bae, K.; Shin, J.M.; Kim, G.T.; Shim, J.H. Slurry spin coating of thin film yttria stabilized zirconia/gadolinia doped ceria bi-layer electrolytes for solid oxide fuel cells. J. Power Sources 2016, 327, 401–407. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yin, C.Q.; Wang, L.H.; Li, D.B.; Lian, J.S.; Hu, J.D.; Guo, Z.X. Properties of a ceria-based (C6S2G2) solid oxide electrolyte sintered with Al2O3 additive. Sci. Sinter. 2008, 40, 13–20. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shimizu, S.; Suzuki, T.; Fujishiro, Y.; Awano, M. Evaluation of micro LSM-supported GDC/ScSZ bilayer electrolyte with LSM–GDC activation layer for intermediate temperature-SOFCs. J. Electrochem. Soc. 2008, 155, B423–B426. [Google Scholar] [CrossRef]
- Fan, L.; Ma, Y.; Wang, X.; Singh, M.; Zhu, B. Understanding the electrochemical mechanism of the core-shell ceria-LiZnO nanocomposite in a low temperature solid oxide fuel cell. J. Mater. Chem. A 2014, 2, 5399–5407. [Google Scholar] [CrossRef]
- Shiratori, Y.; Tietz, F.; Buchkremer, H.P.; Stöver, D. YSZ-MgO composite electrolyte with adjusted thermal expansion coefficient to other SOFC components. Solid State Ion. 2003, 164, 27–33. [Google Scholar] [CrossRef]
- Zhu, B.; Lund, P.D.; Raza, R.; Ma, Y.; Fan, L.; Afzal, M.; Patakangas, J.; He, Y.; Zhao, Y.; Tan, W.; et al. Schottky junction effect on high performance fuel cells based on nanocomposite materials. Adv. Energy Mater. 2015, 5, 1401895. [Google Scholar] [CrossRef]
- Fan, L.; Su, P.C. Layer-structured LiNi0.8Co0.2O2: A new triple (H+/O2−/e−) conducting cathode for low temperature proton conducting solid oxide fuel cells. J. Power Sources 2016, 306, 369–377. [Google Scholar] [CrossRef]
- Xia, C.; Cai, Y.; Wang, B.; Afzal, M.; Zhang, W.; Soltaninazarlou, A.; Zhu, B. Strategy towards cost-effective low-temperature solid oxide fuel cells: A mixed-conductive membrane comprised of natural minerals and perovskite oxide. J. Power Sources 2017, 342, 779–786. [Google Scholar] [CrossRef]
- Zhu, B. Using a fuel cell to study fluoride-based electrolytes. Electrochem. Commun. 1999, 1, 242–246. [Google Scholar] [CrossRef]
- Fan, L.; Wang, C.; Chen, M.; Di, J.; Zheng, J.; Zhu, B. Potential low-temperature application and hybrid-ionic conducting property of ceria-carbonate composite electrolytes for solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 9987–9993. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Zuo, C.; Zha, S.; Liu, M.; Hatano, M.; Uchiyama, M. Ba (Zr0.1Ce0.7Y0.2)O3–δ as an electrolyte for low-temperature solid-oxide fuel cells. Adv. Mater. 2006, 18, 3318–3320. [Google Scholar] [CrossRef]
- Sawant, P.; Varma, S.; Wani, B.N.; Bharadwaj, S.R. Synthesis, stability and conductivity of BaCe0.8–xZrxY0.2O3–δ as electrolyte for proton conducting SOFC. Int. J. Hydrogen Energy 2012, 37, 3848–3856. [Google Scholar] [CrossRef]
- Qian, J.; Tao, Z.; Xiao, J.; Jiang, G.; Liu, W. Performance improvement of ceria-based solid oxide fuel cells with yttria-stabilized zirconia as an electronic blocking layer by pulsed laser deposition. Int. J. Hydrogen Energy 2013, 38, 2407–2412. [Google Scholar] [CrossRef]
- Sun, W.; Shi, Z.; Wang, Z.; Liu, W. Bilayered BaZr0.1Ce0.7Y0.2O3–δ/Ce0.8Sm0.2O2–δ electrolyte membranes for solid oxide fuel cells with high open circuit voltages. J. Membr. Sci. 2015, 476, 394–398. [Google Scholar] [CrossRef]
- Cai, Y.; Xia, C.; Wang, B.; Zhang, W.; Wang, Y.; Zhu, B. Bio-derived calcite as a novel electrolyte for solid oxide fuel cells: A strategy toward utilization of waste shells. ACS Sustain. Chem. Eng. 2017, 5, 10387–10395. [Google Scholar] [CrossRef]
- Wang, B.; Cai, Y.; Xia, C.; Liu, Y.; Muhammad, A.; Wang, H.; Zhu, B. CoFeZrAl-oxide based composite for advanced solid oxide fuel cells. Electrochem. Commun. 2016, 73, 15–19. [Google Scholar] [CrossRef]
- Ortiz-Vitoriano, N.; De Larramendi, I.R.; De Muro, I.G.; De Larramendi, J.R.; Rojo, T. Nanoparticles of La0.8Ca0.2Fe0.8Ni0.2O3-δ perovskite for solid oxide fuel cell application. Mater. Res. Bull. 2010, 45, 1513–1519. [Google Scholar] [CrossRef]
- Kant, K.M.; Esposito, V.; Pryds, N. Enhanced conductivity in pulsed laser deposited Ce0.9Gd0.1O2–δ/SrTiO3 heterostructures. Appl. Phys. Lett. 2010, 97, 143110. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Fang, S.; Su, D.; Brinkman, K.S.; Chen, F. Enhancing grain boundary ionic conductivity in mixed ionic-electronic conductors. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]


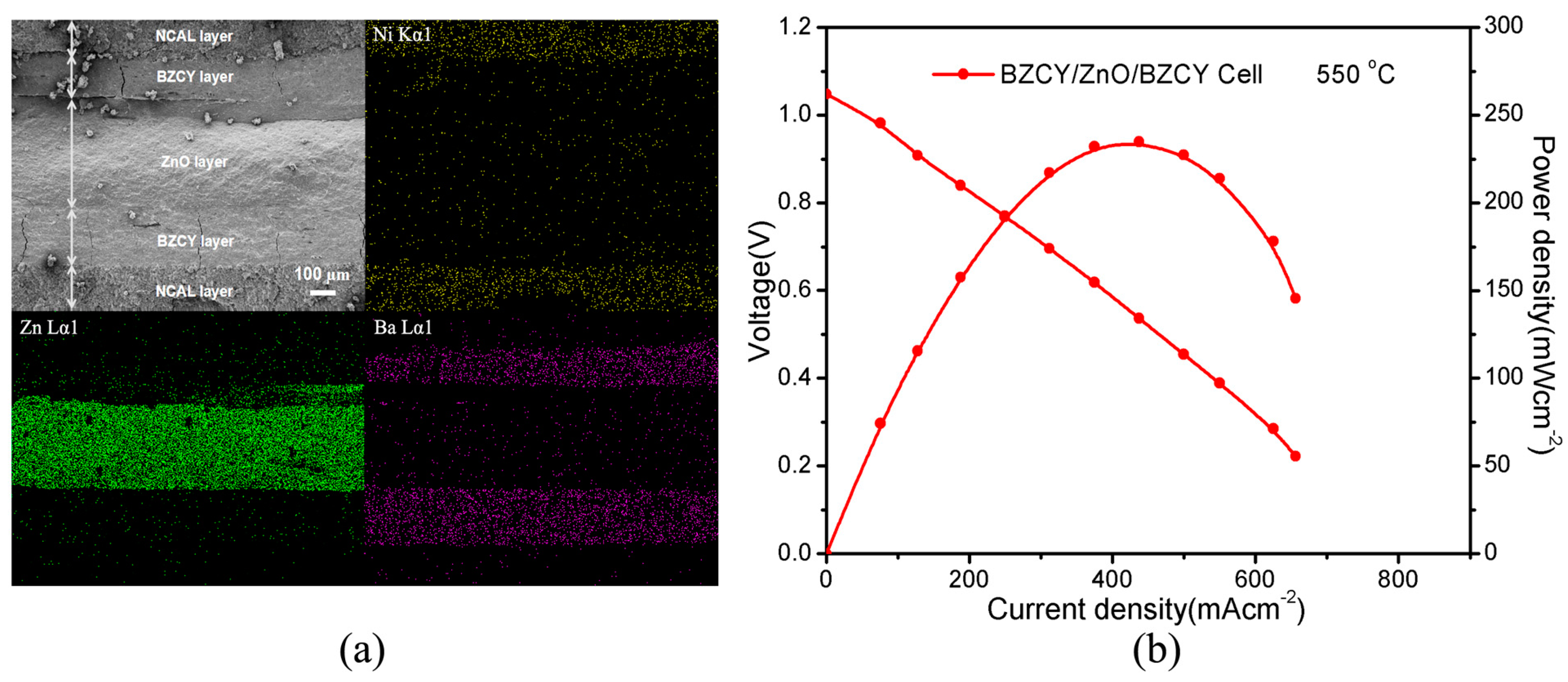
| Sample | d Spacing (nm) | Lattice Constant (nm) |
|---|---|---|
| ZnO | 0.2485 (101) plane 0.2612 (002) plane | a = b = 0.3243 c = 0.5205 |
| LCP | 0.3203 (111) plane | a = b = c = 0.5470 |
| Sample | T | Rb | Rgb | Qgb | n | Re | Qe | n | Chi Squared |
|---|---|---|---|---|---|---|---|---|---|
| ZnO-LCP | 550 °C | 0.046 | 0.034 | 0.610 | 0.6362 | 0.144 | 2.810 | 0.6307 | 1.675 × 10−4 |
| LCP | 0.048 | 0.042 | 0.820 | 0.506 | 0.173 | 1.650 | 0.7092 | 6.063 × 10−4 | |
| ZnO-LCP | 525 °C | 0.052 | 0.045 | 0.472 | 0.6312 | 0.197 | 1.325 | 0.7296 | 1.696 × 10−4 |
| LCP | 0.054 | 0.054 | 0.277 | 0.5322 | 0.370 | 1.164 | 0.6196 | 8.338 × 10−4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, C.; Qiao, Z.; Feng, C.; Kim, J.-S.; Wang, B.; Zhu, B. Study on Zinc Oxide-Based Electrolytes in Low-Temperature Solid Oxide Fuel Cells. Materials 2018, 11, 40. https://doi.org/10.3390/ma11010040
Xia C, Qiao Z, Feng C, Kim J-S, Wang B, Zhu B. Study on Zinc Oxide-Based Electrolytes in Low-Temperature Solid Oxide Fuel Cells. Materials. 2018; 11(1):40. https://doi.org/10.3390/ma11010040
Chicago/Turabian StyleXia, Chen, Zheng Qiao, Chu Feng, Jung-Sik Kim, Baoyuan Wang, and Bin Zhu. 2018. "Study on Zinc Oxide-Based Electrolytes in Low-Temperature Solid Oxide Fuel Cells" Materials 11, no. 1: 40. https://doi.org/10.3390/ma11010040





