Cellulose-Based Smart Fluids under Applied Electric Fields
Abstract
:1. Introduction
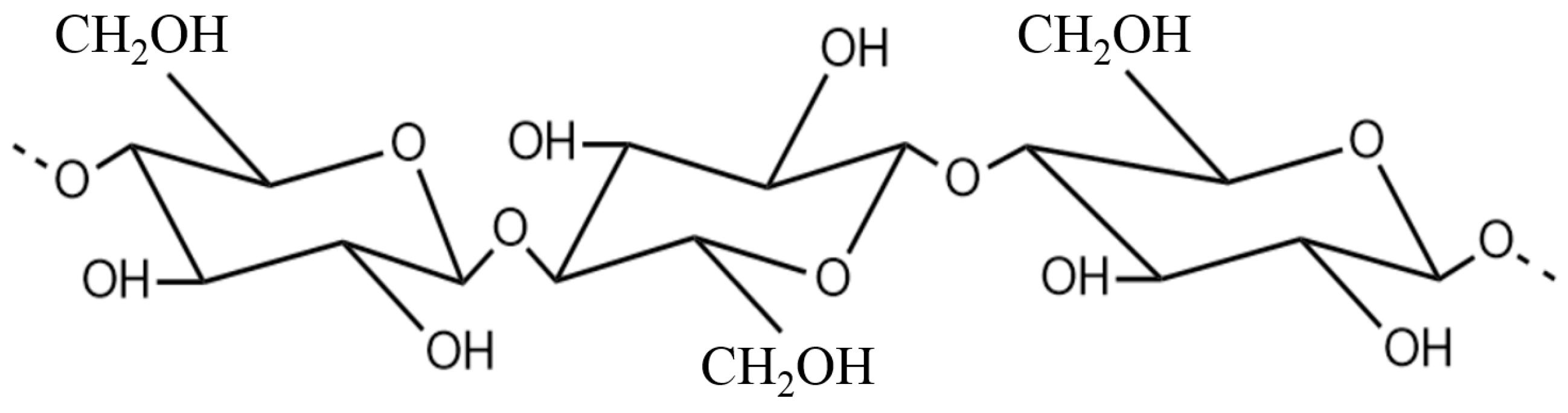
2. Cellulose
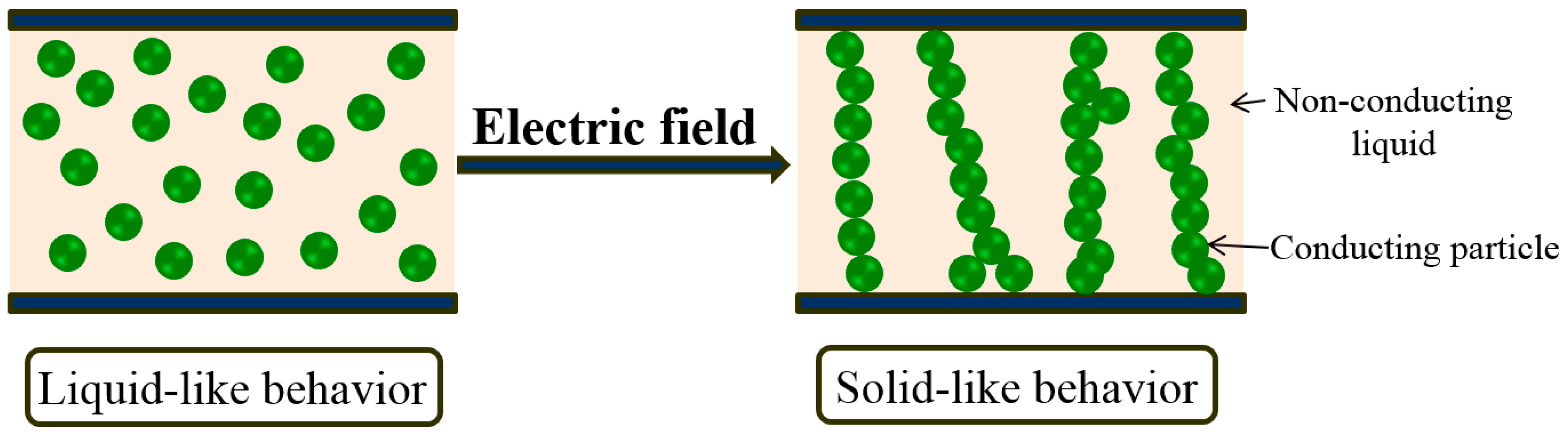
3. Electrorheological Fluids
4. Cellulose Based ER Fluids
5. Cellulose Derivatives and Composite Based ER Fluids
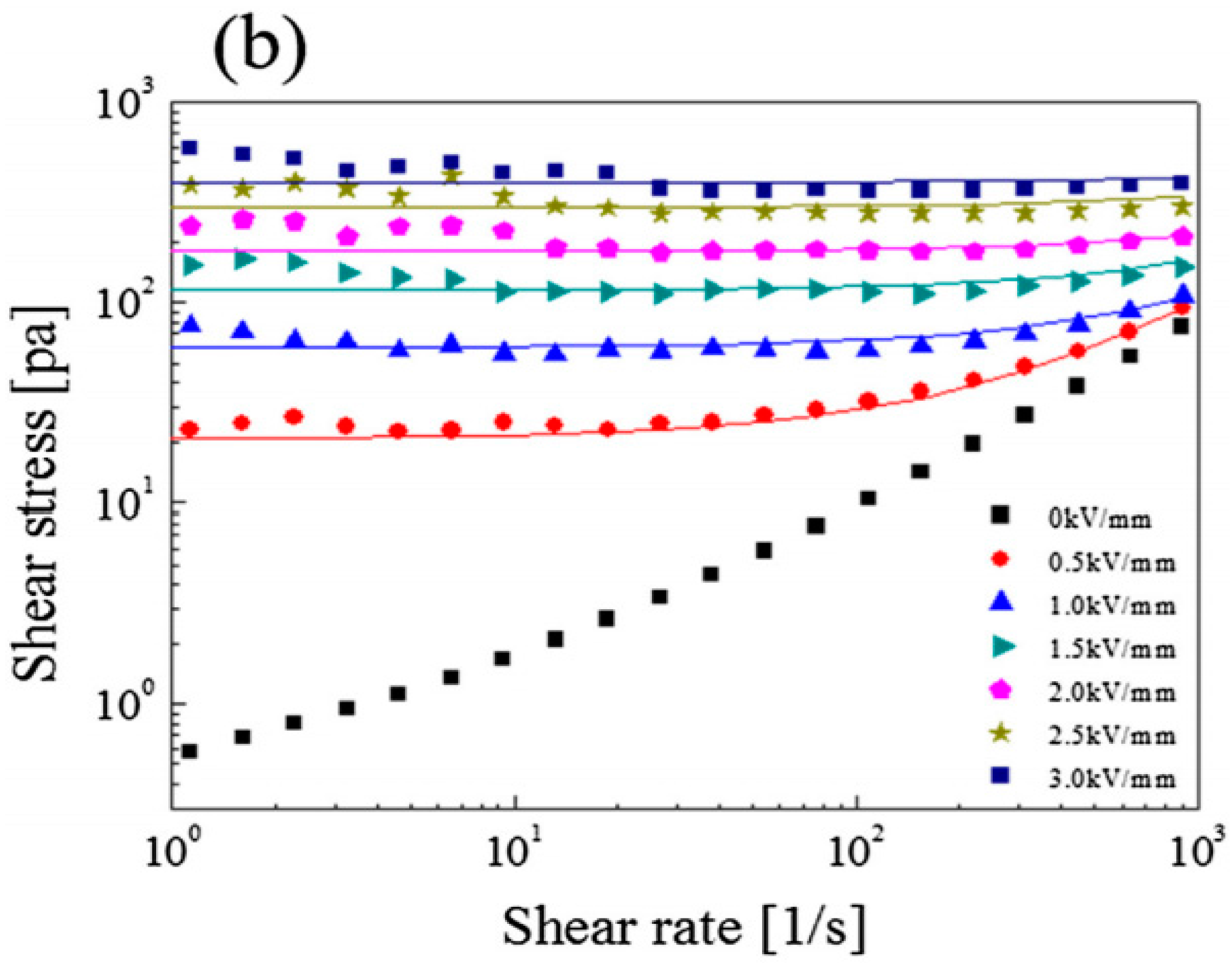
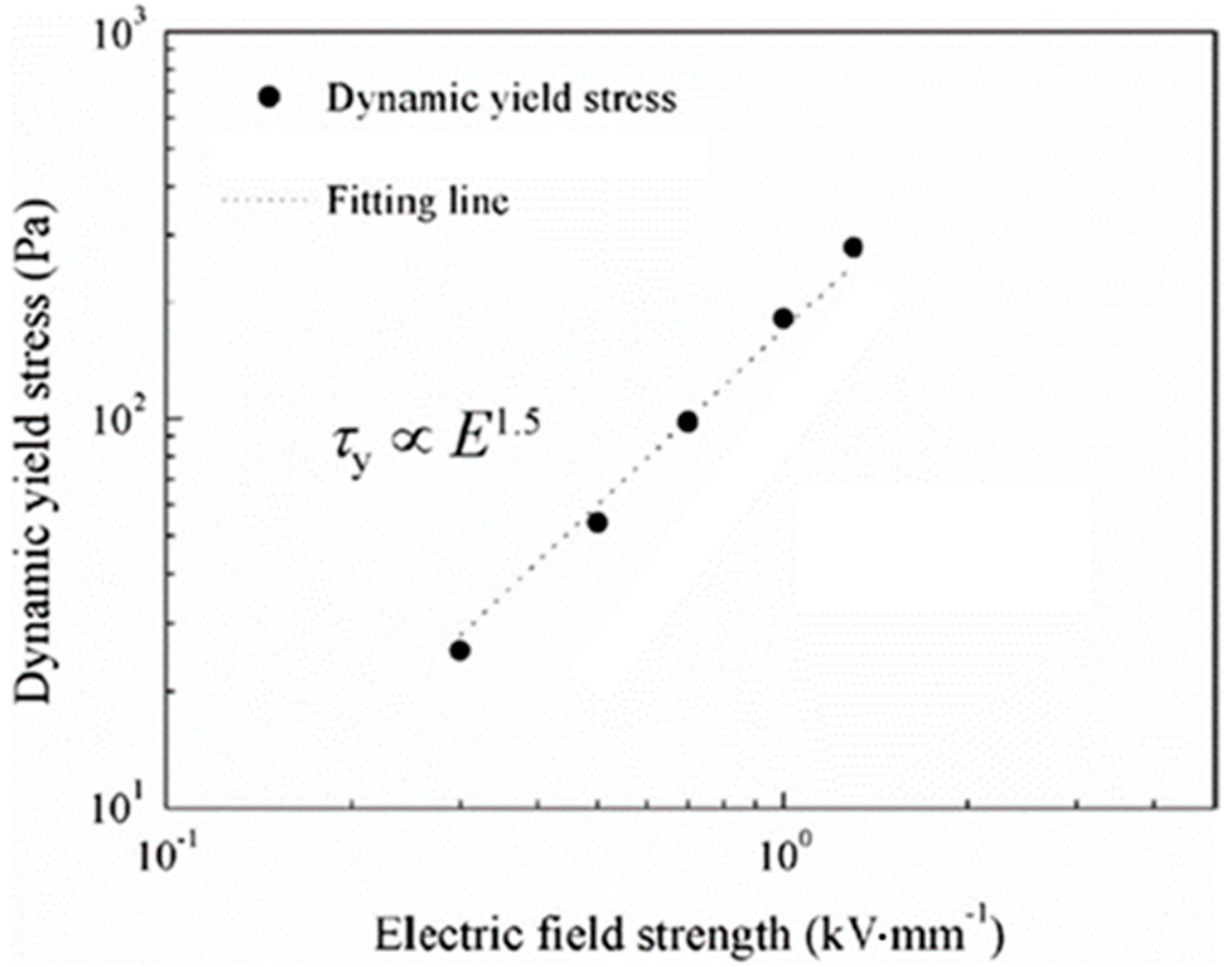
6. ER Characteristics of Cellulose and Its Derivative Materials
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, F.; Morreale, M. Green composites: A brief review. Compos. Part A Appl. Sci. Manuf. 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, D.; de Morais Teixeira, E.; da Silva Curvelo, A.A.; Belgacem, M.N.; Dufresne, A. Extraction of cellulose whiskers from cassava bagasse and their applications as reinforcing agent in natural rubber. Ind. Crops Prod. 2010, 32, 486–490. [Google Scholar] [CrossRef]
- Rosa, M.; Medeiros, E.; Malmonge, J.; Gregorski, K.; Wood, D.; Mattoso, L.; Glenn, G.; Orts, W.; Imam, S. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Aytekin, A.Ö.; Demirbağ, D.D.; Bayrakdar, T. The statistical optimization of bacterial cellulose production via semi-continuous operation mode. J. Ind. Eng. Chem. 2016, 37, 243–250. [Google Scholar] [CrossRef]
- Qu, P.; Gao, Y.; Wu, G.; Zhang, L. Nanocomposites of poly(lactic acid) reinforced with cellulose nanofibrils. BioResources 2010, 5, 1811–1823. [Google Scholar]
- Rånby, B.G. Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss. Faraday Soc. 1951, 11, 158–164. [Google Scholar] [CrossRef]
- Battista, O.A. Hydrolysis and crystallization of cellulose. Ind. Eng. Chem. 1950, 42, 502–507. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulose whiskers versus microfibrils: Influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 2008, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.G. Electrorheological properties of phosphoric ester cellulose electrorheological suspensions with activation of dispersed particles. Polym. J. 2003, 35, 23–29. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.W.; Jang, W.H.; Choi, H.J.; Jhon, M.S. Electrorheological characteristics of phosphate cellulose-based suspensions. Polymer 2001, 42, 5005–5012. [Google Scholar] [CrossRef]
- Tilki, T.; Yavuz, M.; Karabacak, Ç.; Çabuk, M.; Ulutürk, M. Investigation of electrorheological properties of biodegradable modified cellulose/corn oil suspensions. Carbohydr. Res. 2010, 345, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Winter, W.T.; Stipanovic, A.J. Water-activated cellulose-based electrorheological fluids. Cellulose 2005, 12, 135–144. [Google Scholar] [CrossRef]
- Min, T.H.; Choi, H.J. Synthesis of poly(methyl methacrylate)/graphene oxide nanocomposite particles via pickering emulsion polymerization and their viscous response under an electric field. Macromol. Res. 2017, 25, 565–571. [Google Scholar] [CrossRef]
- Trlica, J.; Sáha, P.; Quadrat, O.; Stejskal, J. Electrorheological activity of polyphenylenediamine suspensions in silicone oil. Phys. A Stat. Mech. Appl. 2000, 283, 337–348. [Google Scholar] [CrossRef]
- Wu, J.; Jin, T.; Liu, F.; Guo, J.; Cui, P.; Cheng, Y.; Xu, G. Preparation of rod-like calcium titanyl oxalate with enhanced electrorheological activity and their morphological effect. J. Mater. Chem. C 2014, 2, 5629–5635. [Google Scholar] [CrossRef]
- Coulter, J.P.; Weiss, K.D.; Carlson, J.D. Engineering applications of electrorheological materials. J. Intell. Mater. Syst. Struct. 1993, 4, 248–259. [Google Scholar] [CrossRef]
- Eshaghi, M.; Sedaghati, R.; Rakheja, S. Dynamic characteristics and control of magnetorheological/electrorheological sandwich structures: A state-of-the-art review. J. Intell. Mater. Syst. Struct. 2016, 27, 2003–2037. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, Y.; Tam, H.Y.; Wen, Y. Electrorheological finishing for glasses by using an integrated-electrodes tool. Nanosci. Nanotechnol. Lett. 2011, 3, 666–668. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, X.; Wen, W. Manipulation of microfluidic droplets by electrorheological fluid. Electrophoresis 2009, 30, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Han, Y.M.; Song, H.J.; Sohn, J.W.; Choi, H.J. Field test on vibration control of vehicle suspension system featuring er shock absorbers. J. Intell. Mater. Syst. Struct. 2007, 18, 1169–1174. [Google Scholar] [CrossRef]
- Nikitczuk, J.; Weinberg, B.; Mavroidis, C. Control of electro-rheological fluid based resistive torque elements for use in active rehabilitation devices. Smart Mater. Struct. 2007, 16, 418–428. [Google Scholar] [CrossRef]
- Sung, J.H.; Choi, H.J. Biocompatible polysaccharide-based electrorheological suspensions. J. Macromol. Sci. Part B Phys. 2005, 44, 573–581. [Google Scholar] [CrossRef]
- Abdul Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Haafiz, M.M.; Eichhorn, S.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Sakurada, I.; Nukushina, Y.; Ito, T. Experimental determination of the elastic modulus of crystalline regions in oriented polymers. J. Polym. Sci. Part A Polym. Chem. 1962, 57, 651–660. [Google Scholar] [CrossRef]
- Page, D.; El-Hosseiny, F.; Winkler, K. Behaviour of single wood fibres under axial tensile strain. Nature 1971, 229, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Pasanovic-Zujo, V.; Gupta, R.K.; Cser, F.; Bhattacharya, S.N. Morphology of eva based nanocomposites under shear and extensional flow. Polym. Eng. Sci. 2004, 44, 1220–1230. [Google Scholar] [CrossRef]
- Patel, M.; Karera, A.; Prasanna, P. Effect of thermal and chemical treatments on carbon and silica contents in rice husk. J. Mater. Sci. 1987, 22, 2457–2464. [Google Scholar] [CrossRef]
- Reid, J.D.; Mazzeno, L.W. Preparation and properties of cellulose phosphates. Ind. Eng. Chem. 1949, 41, 2828–2831. [Google Scholar] [CrossRef]
- Granja, P.; Barbosa, M.; Pouységu, L.; De Jéso, B.; Rouais, F.; Baquey, C. Cellulose phosphates as biomaterials. Mineralization of chemically modified regenerated cellulose hydrogels. J. Mater. Sci. 2001, 36, 2163–2172. [Google Scholar] [CrossRef]
- Leone, G.; Torricelli, P.; Giardino, R.; Barbucci, R. New phosphorylated derivatives of carboxymethylcellulose with osteogenic activity. Polym. Adv. Technol. 2008, 19, 824–830. [Google Scholar] [CrossRef]
- Heinze, T. New ionic polymers by cellulose functionalization. Macromol. Chem. Phys. 1998, 199, 2341–2364. [Google Scholar] [CrossRef]
- Nada, A.A.; Hassan, M.L. Phosphorylated cation-exchangers from cotton stalks and their constituents. J. Appl. Polym. Sci. 2003, 89, 2950–2956. [Google Scholar] [CrossRef]
- Tobyn, M.J.; McCarthy, G.P.; Staniforth, J.N.; Edge, S. Physicochemical comparison between microcrystalline cellulose and silicified microcrystalline cellulose. Int. J. Pharm. 1998, 169, 183–194. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sain, M. Processing of cellulose nanofiber-reinforced composites. J. Reinf. Plast. Compos. 2005, 24, 1259–1268. [Google Scholar] [CrossRef]
- Park, D.P.; Hwang, J.Y.; Choi, H.J.; Kim, C.A.; Jhon, M.S. Synthesis and characterization of polysaccharide phosphates based electrorheological fluids. Mater. Res. Innov. 2003, 7, 161–166. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, M.S.; Choi, H.J.; Jhon, M.S. Effect of polymerization temperature on polyaniline based electrorheological suspensions. Colloid Polym. Sci. 1999, 277, 73–76. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 2016, 135, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Piao, S.H.; Choi, H.J.; Zakaria, S.; Chia, C.H. Synthesis of kenaf cellulose carbamate and its smart electric stimuli-response. Carbohydr. Polym. 2016, 137, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Cho, M.S.; Choi, H.J.; Jhon, M.S. Electrorheological characterization of polyaniline-coated poly(methyl methacrylate) suspensions. Colloid Polym. Sci. 2002, 280, 1062–1066. [Google Scholar]
- Liu, B.; Shaw, M.T. Electrorheology of filled silicone elastomers. J. Rheol. 2001, 45, 641–657. [Google Scholar] [CrossRef]
- Ikazaki, F.; Kawai, A.; Uchida, K.; Kawakami, T.; Edamura, K.; Sakurai, K.; Anzai, H.; Asako, Y. Mechanisms of electrorheology: The effect of the dielectric property. J. Phys. D Appl. Phys. 1998, 31, 336–347. [Google Scholar] [CrossRef]
- Yavuz, M.; Tilki, T.; Karabacak, C.; Erol, O.; Unal, H.I.; Uluturk, M.; Cabuk, M. Electrorheological behavior of biodegradable modified corn starch/corn oil suspensions. Carbohydr. Polym. 2010, 79, 318–324. [Google Scholar] [CrossRef]
- Yilmaz, H.; Zengin, H.; Unal, H.I. Synthesis and electrorheological properties of polyaniline/silicon dioxide composites. J. Mater. Sci. 2012, 47, 5276–5286. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, X. Electrorheology of nanofiber suspensions. Nanoscale Res. Lett. 2011, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Lee, J.Y.; Sung, J.H.; Choi, H.J. Synthesis and electrorheological characteristics of polyaniline-titanium dioxide hybrid suspension. Synth. Met. 2005, 152, 173–176. [Google Scholar] [CrossRef]
- Cho, M.S.; Choi, H.J.; Jhon, M.S. Shear stress analysis of a semiconducting polymer based electrorheological fluid system. Polymer 2005, 46, 11484–11488. [Google Scholar] [CrossRef]
- Seo, Y.P.; Seo, Y. Modeling and analysis of electrorheological suspensions in shear flow. Langmuir 2012, 28, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J.; Kim, S.G. Electrorheology of graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kobayashi, K.; Sato, M. Static yield stress of an electro-rheological fluid. J. Colloid Interface Sci. 1996, 180, 315–322. [Google Scholar] [CrossRef]
- Cho, M.S.; Choi, H.J.; To, K. Effect of ionic pendent groups on a polyaniline-based electrorheological fluid. Macromol. Rapid Commun. 1998, 19, 271–273. [Google Scholar] [CrossRef]
- Yin, J.; Xia, X.; Zhao, X. Conductivity, polarization and electrorheological activity of polyaniline nanotubes during thermo-oxidative treatment. Polym. Degrad. Stab. 2012, 97, 2356–2363. [Google Scholar] [CrossRef]
- Sedlačík, M.; Mrlík, M.; Pavlínek, V.; Sáha, P.; Quadrat, O. Electrorheological properties of suspensions of hollow globular titanium oxide/polypyrrole particles. Colloid Polym. Sci. 2012, 290, 41–48. [Google Scholar] [CrossRef]
- Oz, K.; Yavuz, M.; Yilmaz, H.; Unal, H.I.; Sari, B. Electrorheological properties and creep behavior of polyindole/poly(vinyl acetate) composite suspensions. J. Mater. Sci. 2008, 43, 1451–1459. [Google Scholar] [CrossRef]
- Cheng, Q.; Pavlinek, V.; Li, C.; Lengalova, A.; He, Y.; Saha, P. Synthesis and characterization of new mesoporous material with conducting polypyrrole confined in mesoporous silica. Mater. Chem. Phys. 2006, 98, 504–508. [Google Scholar] [CrossRef]
- Park, D.P.; Sung, J.H.; Kim, C.A.; Choi, H.J.; Jhon, M.S. Synthesis and electrorheology of potato starch phosphate. J. Appl. Polym. Sci. 2004, 91, 1770–1773. [Google Scholar] [CrossRef]
- Sung, J.H.; Choi, H.J.; Sohn, J.I.; Jhon, M.S. Electrorheology of chitosan polysaccharide suspensions in soybean oil. Colloid Polym. Sci. 2003, 281, 1196–1200. [Google Scholar] [CrossRef]
- Ko, Y.G.; Choi, U.S.; Sung, B.H. Chemical structure designing to enhance the yield stress of electrorheological fluids based on modified chitosan compounds. J. Appl. Polym. Sci. 2004, 93, 1559–1566. [Google Scholar] [CrossRef]
- Kim, S.G.; Choi, H.J.; Jhon, M.S. Preparation and characterization of phosphate cellulose-based electrorheological fluids. Macromol. Chem. Phys. 2001, 202, 521–526. [Google Scholar] [CrossRef]
- See, H.; Kawai, A.; Ikazaki, F. Differences in the electrorheological response of a particle suspension under direct current and alternating current electric fields. Colloid Polym. Sci. 2002, 280, 24–29. [Google Scholar] [CrossRef]
- Cho, M.S.; Choi, H.J.; Kim, K.Y.; Ahn, W.S. Synthesis and characterization of polyaniline/mesoporous sba-15 nanocomposite. Macromol. Rapid Commun. 2002, 23, 713–716. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, X. Enhancing electrorheological behaviors with formation of β-cyclodextrin supramolecular complex. Polymer 2003, 44, 4519–4526. [Google Scholar] [CrossRef]
- Bae, D.H.; Choi, H.J.; Choi, K.; Nam, J.D.; Islam, M.S.; Kao, N. Fabrication of phosphate microcrystalline rice husk based cellulose particles and their electrorheological response. Carbohydr. Polym. 2017, 165, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Lee, H.J.; Shin, S.S.; Choi, U.S. Dipolar-molecule complexed chitosan carboxylate, phosphate, and sulphate dispersed electrorheological suspensions. Soft Matter 2012, 8, 6273–6279. [Google Scholar] [CrossRef]
- Cabuk, M.; Yavuz, M.; Unal, H.I. Electrokinetic, electrorheological and viscoelastic properties of Polythiophene-graft-Chitosan copolymer particles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 231–238. [Google Scholar] [CrossRef]
- Kim, Y.D.; Yoon, D.J. Electrorheological fluids of polypyrrole-tin oxide nanocomposite particles. Korea-Aust. Rheol. J. 2016, 28, 275–279. [Google Scholar] [CrossRef]
- Ko, Y.G.; Lee, H.J.; Chun, Y.J.; Choi, U.S.; Yoo, K.P. Positive and negative electrorheological response of alginate salts dispersed suspensions under electric field. ACS Appl. Mater. Interfaces 2013, 5, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tejada, M.; Arroyo, F.; Delgado, A. Negative electrorheological behavior in suspensions of inorganic particles. Langmuir 2010, 26, 16833–16840. [Google Scholar] [CrossRef] [PubMed]
- Mitsumata, T.; Sugitani, K. Negative electrorheological effect of silicone gels containing barium titanate. Macromol. Rapid Commun. 2004, 25, 848–852. [Google Scholar] [CrossRef]
- Pavlínek, V.; Sáha, P.; Quadrat, O.; Stejskal, J. Rheological behavior of poly(methyl methacrylate) dispersions stabilized by a diblock copolymer. 2. Positive and negative electrorheological effect. Langmuir 2000, 16, 1447–1449. [Google Scholar] [CrossRef]
- Komoda, Y.; Sakai, N.; Rao, T.N.; Tryk, D.; Fujishima, A. Photoelectrorheological phenomena involving tio2 particle suspensions. Langmuir 1998, 14, 1081–1091. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, J.S.; Choi, H.J.; Choi, S.B.; Kim, G.W. Ultraviolet light-responsive photorheological fluids: as a new class of smart fluids. Smart Mater. Struct. 2017, 26, 054007. [Google Scholar] [CrossRef]
- Qian, B.; McKinley, G.H.; Hosoi, A. Structure evolution in electrorheological fluids flowing through microchannels. Soft Matter 2013, 9, 2889–2898. [Google Scholar] [CrossRef]
- Mohanty, P.S.; Yethiraj, A.; Schurtenberger, P. Deformable particles with anisotropic interactions: Unusual field-induced structural transitions in ultrasoft ionic microgel colloids. Soft Matter 2012, 8, 10819–10822. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.F.; Zahn, M.; Lemaire, E. Continuum modeling of micro-particle electrorotation in couette and poiseuille flows—The zero spin viscosity limit. J. Electrost. 2010, 68, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, E.; Lobry, L.; Pannacci, N.; Peters, F. Viscosity of an electro-rheological suspension with internal rotations. J. Rheol. 2008, 52, 769–783. [Google Scholar] [CrossRef]
- Lee, S.R.; Uhm, C.H.; Seong, M.S.; Oh, J.S.; Choi, S.B. Repulsive force control of minimally invasive surgery robot associated with three degrees of freedom electrorheological fluid-based haptic master. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2014, 228, 1606–1621. [Google Scholar] [CrossRef]
- Stanway, R.; Sproston, J.; El-Wahed, A. Applications of electro-rheological fluids in vibration control: A survey. Smart Mater. Struct. 1996, 5, 464. [Google Scholar] [CrossRef]
- Tan, K.; Johnson, A.; Stanway, R.; Bullough, W. Model validation of the output reciprocating dynamic responses of a twin electro-rheological (ER) clutch mechanism. Mech. Mach. Theory 2007, 42, 1547–1562. [Google Scholar] [CrossRef]
- Bouzidane, A.; Thomas, M. An electrorheological hydrostatic journal bearing for controlling rotor vibration. Comput. Struct. 2008, 86, 463–472. [Google Scholar] [CrossRef]
- Pecheux, B.; Bonneau, O.; Frêne, J. Investigation about electrorheological squeeze film damper applied to active control of rotor dynamic. Int. J. Rotating Mach. 1997, 3, 53–60. [Google Scholar] [CrossRef]
- Gindl, W.; Emsenhuber, G.; Maier, G.; Keckes, J. Cellulose in never-dried gel oriented by an ac electric field. Biomacromolecules 2009, 10, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.P.; Weigel, P.; Purz, H.; Ganster, J. Structure formation of regenerated cellulose materials from nmmo-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Stangroom, J.E. Electrorheological fluids. Phys. Technol. 1983, 14, 290–296. [Google Scholar] [CrossRef]
- Stipanovic, A.J.; Schoonmaker, J.P. The impact of crystalline phase morphology on the water-promoted electrorheological effect of polysaccharides. In Progress in Electrorheology; Havelka, K.O., Filisko, F.E., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 195–205. [Google Scholar]
- Sim, B.; Bae, D.H.; Choi, H.J.; Choi, K.; Islam, M.S.; Kao, N. Fabrication and stimuli response of rice husk-based microcrystalline cellulose particle suspension under electric fields. Cellulose 2016, 23, 185–197. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Nilsson, M.; Mihranyan, A.; Valizadeh, S.; Strømme, M. Mesopore structure of microcrystalline cellulose tablets characterized by nitrogen adsorption and sem: The influence on water-induced ionic conduction. J. Phys. Chem. B 2006, 110, 15776–15781. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Davies, J.; Blagbrough, I.; Staniforth, J. Electrorheological behaviour at low applied electric fields of microcrystalline cellulose in BP oils. Chem. Commun. 1998, 2157–2158. [Google Scholar] [CrossRef]
- Yatsuzuka, K.; Miura, K.; Kuramoto, N.; Asano, K. Observation of the electrorheological effect of silicone oil/polymer particles suspension. IEEE Trans. Ind. Appl. 1995, 31, 457–463. [Google Scholar] [CrossRef]
- Misono, Y.; Negita, K. Shear-induced particle rotation and its effect on electrorheological and dielectric properties in cellulose suspension. Phys. Rev. E 2004, 70, 061412. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Phillips, G.O.; Yang, G. Nanocellulose electroconductive composites. Nanoscale 2013, 5, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Kraev, A.S.; Agafonov, A.V.; Davydova, O.I.; Nefedova, T.A.; Trusova, T.A.; Zakharov, A.G. Sol–gel synthesis of titanium dioxide and titanium dioxide–hydroxypropyl cellulose hybrid material and electrorheological characteristics of their dispersions in poly(dimethylsiloxane). Colloid J. 2007, 69, 620–626. [Google Scholar] [CrossRef]
- Pandey, J.K.; Takagi, H.; Nakagaito, A.N.; Saini, D.R.; Ahn, S.-H. An overview on the cellulose based conducting composites. Compos. Part B Eng. 2012, 43, 2822–2826. [Google Scholar] [CrossRef]
- Nada, A.; Dawy, M.; Salama, A. Dielectric properties and ac-conductivity of cellulose polyethylene glycol blends. Mater. Chem. Phys. 2004, 84, 205–215. [Google Scholar] [CrossRef]
- Liang, X.H.; Guo, Y.Q.; Gu, L.Z.; Ding, E.Y. Crystalline-amorphous phase transition of poly(ethylene glycol)/cellulose blend. Macromolecules 1995, 28, 6551–6555. [Google Scholar] [CrossRef]
- Ge, D.; Ru, X.; Hong, S.; Jiang, S.; Tu, J.; Wang, J.; Zhang, A.; Ji, S.; Linkov, V.; Ren, B. Coating metals on cellulose–polypyrrole composites: A new route to self-powered drug delivery system. Electrochem. Commun. 2010, 12, 1367–1370. [Google Scholar] [CrossRef]
- Galkina, O.L.; Ivanov, V.K.; Agafonov, A.V.; Seisenbaeva, G.A.; Kessler, V.G. Cellulose nanofiber–titania nanocomposites as potential drug delivery systems for dermal applications. J. Mater. Chem. B 2015, 3, 1688–1698. [Google Scholar] [CrossRef]
- Yin, C.; Shen, X. Synthesis of cellulose carbamate by supercritical CO2-assisted impregnation: Structure and rheological properties. Eur. Polym. J. 2007, 43, 2111–2116. [Google Scholar] [CrossRef]
- Kim, J.W.; Liu, F.; Choi, H.J. Polypyrrole/clay nanocomposite and its electrorheological characteristics. J. Ind. Eng. Chem. 2002, 8, 399–403. [Google Scholar]
- Lee, J.H.; Cho, M.S.; Choi, H.J.; Jhon, M.S. Effect of polymerization temperature on polyaniline based electrorheological suspensions. Colloid Polym. Sci. 1999, 277, 73–76. [Google Scholar] [CrossRef]
- Kwon, S.H.; Liu, Y.D.; Choi, H.J. Monodisperse poly(2-methylaniline) coated polystyrene core–shell microspheres fabricated by controlled releasing process and their electrorheological stimuli-response under electric fields. J. Colloid Interface Sci. 2015, 440, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Choi, H.J. Fast and facile fabrication of a graphene oxide/titania nanocomposite and its electro-responsive characteristics. Chem. Commun. 2011, 47, 12286–12288. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, M.; Klingenberg, D.J. Electrorheology: Mechanisms and models. Mater. Sci. Eng. R Rep. 1996, 17, 57–103. [Google Scholar] [CrossRef]
- Klingenberg, D.; van Swol, F.; Zukoski, C. The small shear rate response of electrorheological suspensions. I. Simulation in the point–dipole limit. J. Chem. Phys. 1991, 94, 6160–6169. [Google Scholar] [CrossRef]
- Yin, J.; Xia, X.; Xiang, L.; Qiao, Y.; Zhao, X. The electrorheological effect of polyaniline nanofiber, nanoparticle and microparticle suspensions. Smart Mater. Struct. 2009, 18, 095007. [Google Scholar] [CrossRef]
- Kim, M.W.; Moon, I.J.; Choi, H.J.; Seo, Y. Facile fabrication of core/shell structured sio2/polypyrrole nanoparticles with surface modification and their electrorheology. RSC Adv. 2016, 6, 56495–56502. [Google Scholar] [CrossRef]
- Liu, Y.D.; Quan, X.; Hwang, B.; Kwon, Y.K.; Choi, H.J. Core–shell-structured monodisperse copolymer/silica particle suspension and its electrorheological response. Langmuir 2014, 30, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, J.W.; To, K. Electrorheological characteristics of semiconducting poly(aniline-co-o-ethoxyaniline) suspension. Polymer 1999, 40, 2163–2166. [Google Scholar] [CrossRef]





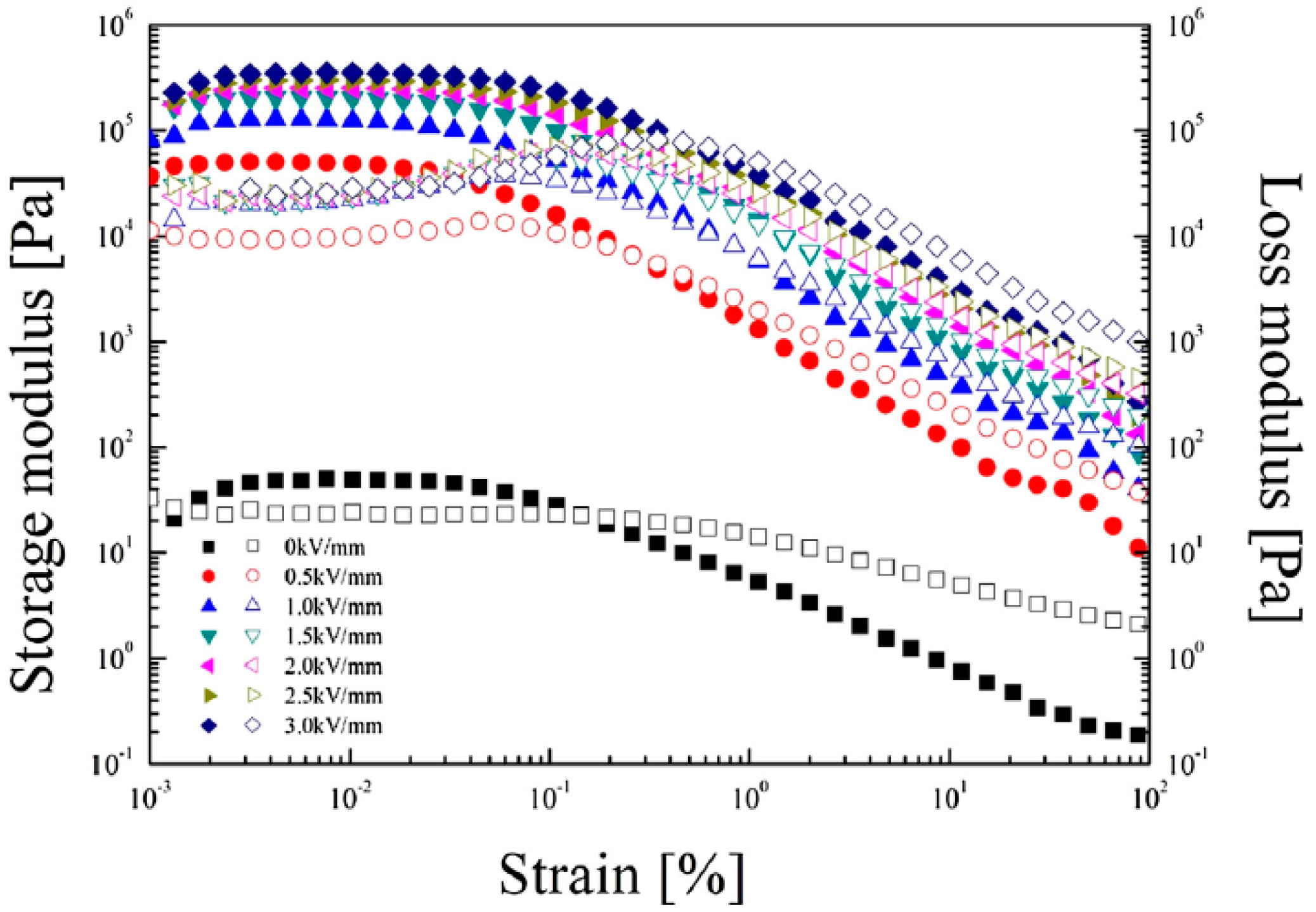
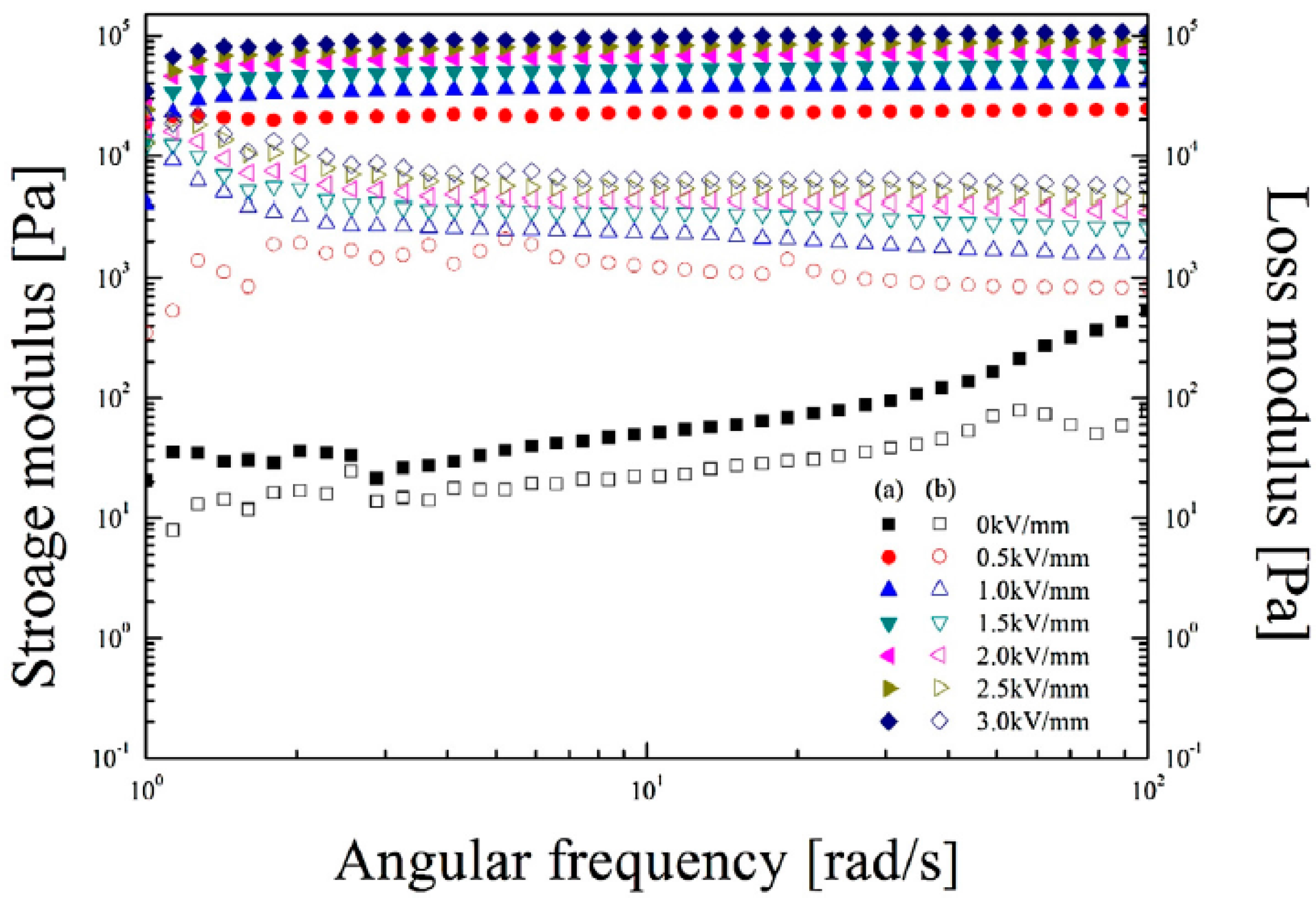
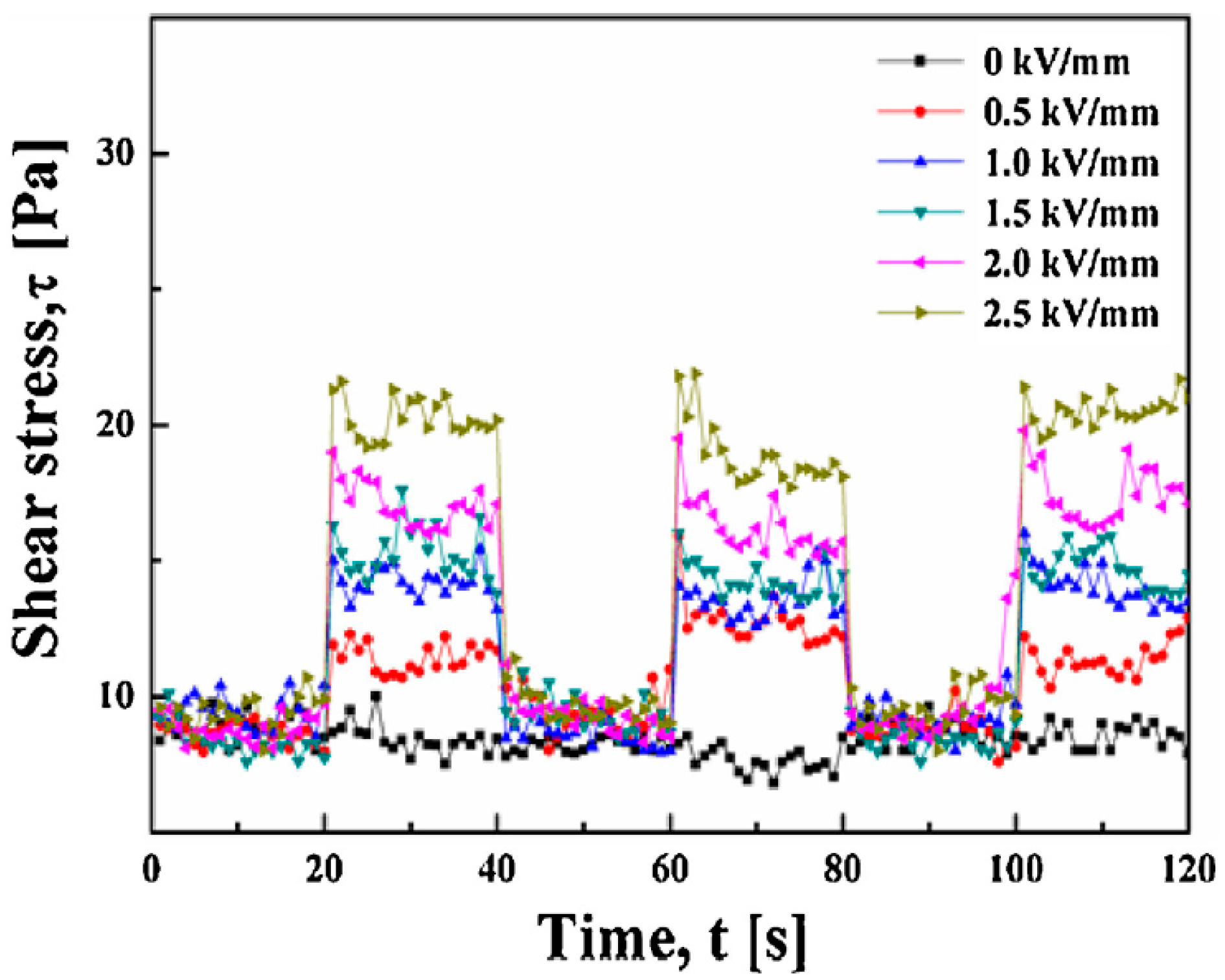
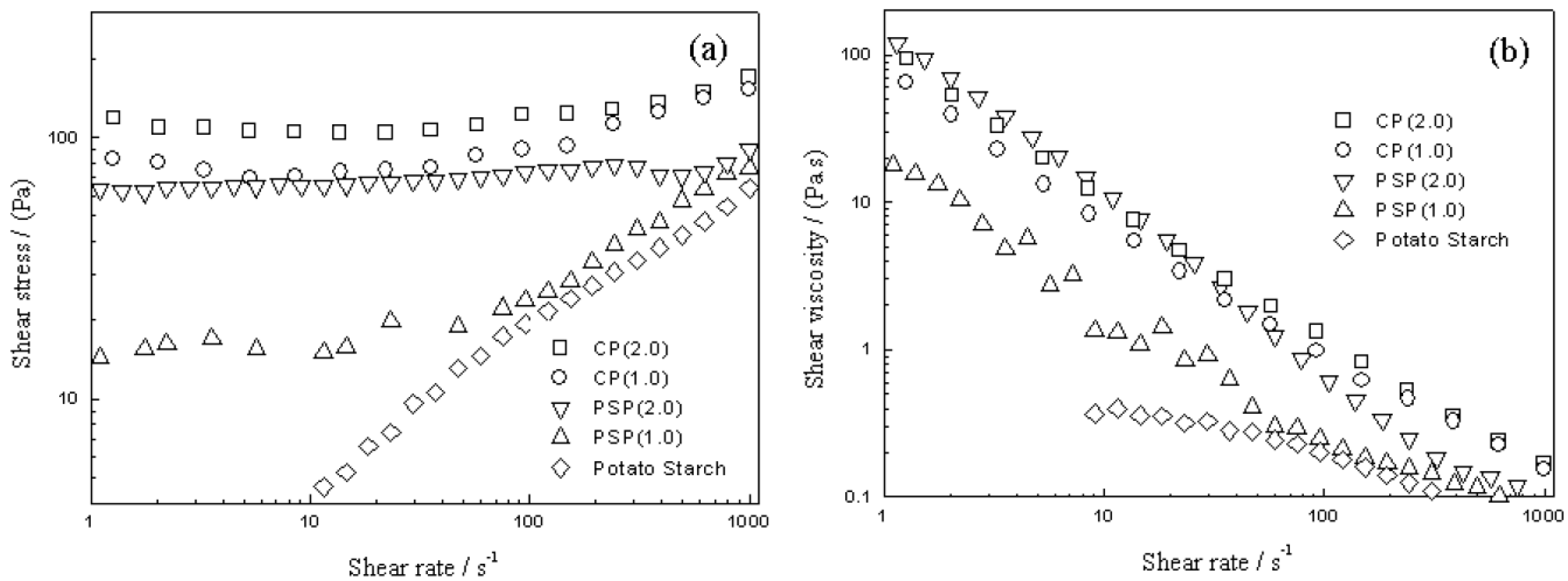

| Type of Biofiber | Composition (%) | ||||
|---|---|---|---|---|---|
| Source | Cellulose | Hemicellulose | Lignin | Extract | |
| Wood | Hardwood | 43–47 | 25–35 | 16–24 | 2–8 |
| Softwood | 40–44 | 25–29 | 25–31 | 1–5 | |
| Non-wood | Bagasse | 40 | 30 | 20 | 10 |
| Coir | 32–43 | 10–20 | 43–49 | 4 | |
| Corn cobs | 45 | 35 | 15 | 5 | |
| Corn stalks | 35 | 25 | 35 | 5 | |
| Cotton | 95 | 2 | 1 | 0.4 | |
| EFB | 50 | 30 | 17 | 3 | |
| Flax(retted) | 71 | 21 | 17 | 3 | |
| Flax(unretted) | 63 | 12 | 3 | 13 | |
| Hemp | 70 | 22 | 6 | 2 | |
| Henequen | 78 | 4–8 | 13 | 4 | |
| Istle | 73 | 4–8 | 17 | 2 | |
| Jute | 71 | 14 | 13 | 2 | |
| Kenaf | 36 | 21 | 18 | 2 | |
| Ramie | 76 | 17 | 1 | 6 | |
| Sisal | 73 | 14 | 11 | 2 | |
| Sunn | 80 | 10 | 6 | 3 | |
| Wheat straw | 30 | 50 | 15 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.; Gao, C.Y.; Nam, J.D.; Choi, H.J. Cellulose-Based Smart Fluids under Applied Electric Fields. Materials 2017, 10, 1060. https://doi.org/10.3390/ma10091060
Choi K, Gao CY, Nam JD, Choi HJ. Cellulose-Based Smart Fluids under Applied Electric Fields. Materials. 2017; 10(9):1060. https://doi.org/10.3390/ma10091060
Chicago/Turabian StyleChoi, Kisuk, Chun Yan Gao, Jae Do Nam, and Hyoung Jin Choi. 2017. "Cellulose-Based Smart Fluids under Applied Electric Fields" Materials 10, no. 9: 1060. https://doi.org/10.3390/ma10091060





