Release of VEGF from Dental Implant Improves Osteogenetic Process: Preliminary In Vitro Tests
Abstract
:1. Introduction
2. Results
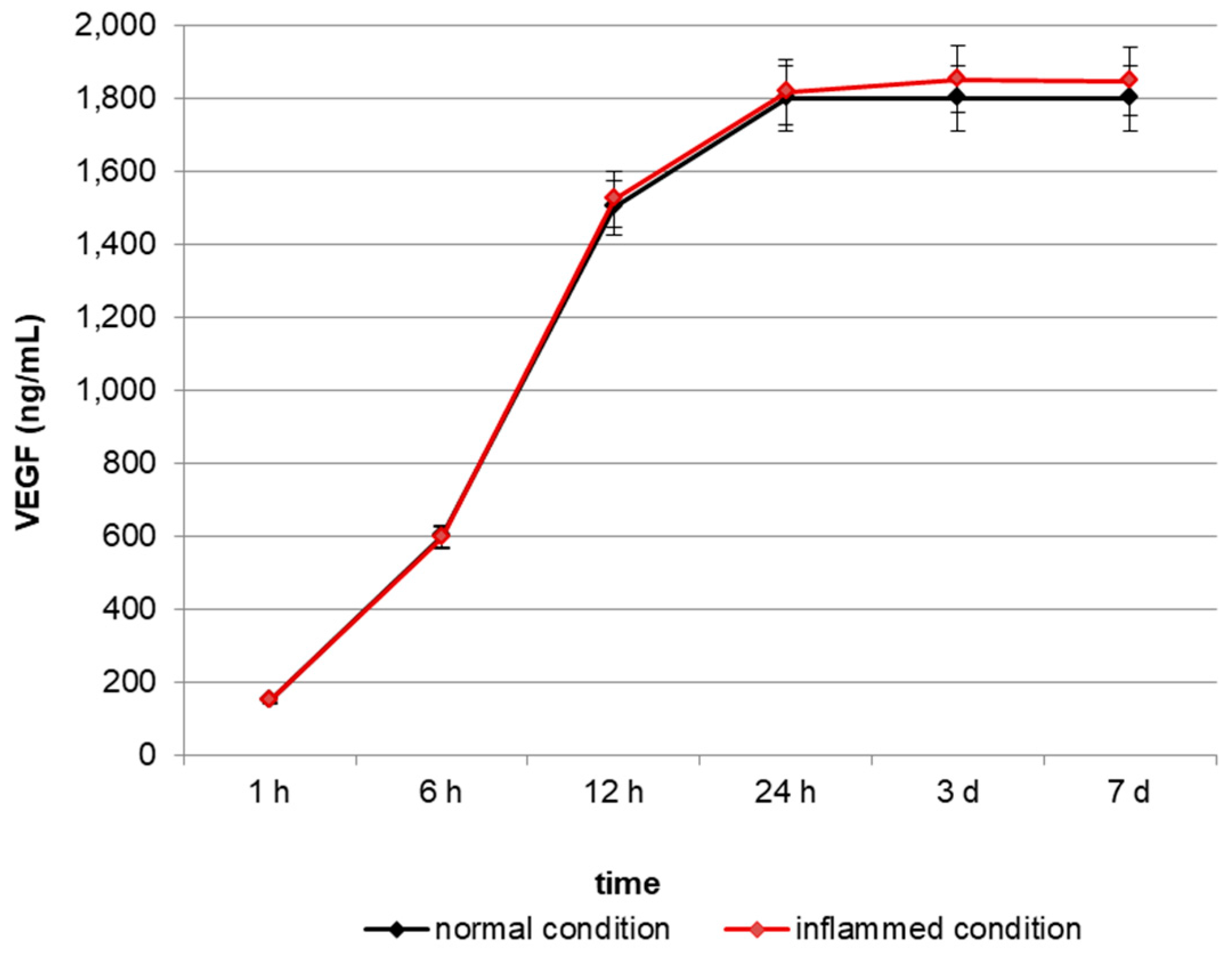
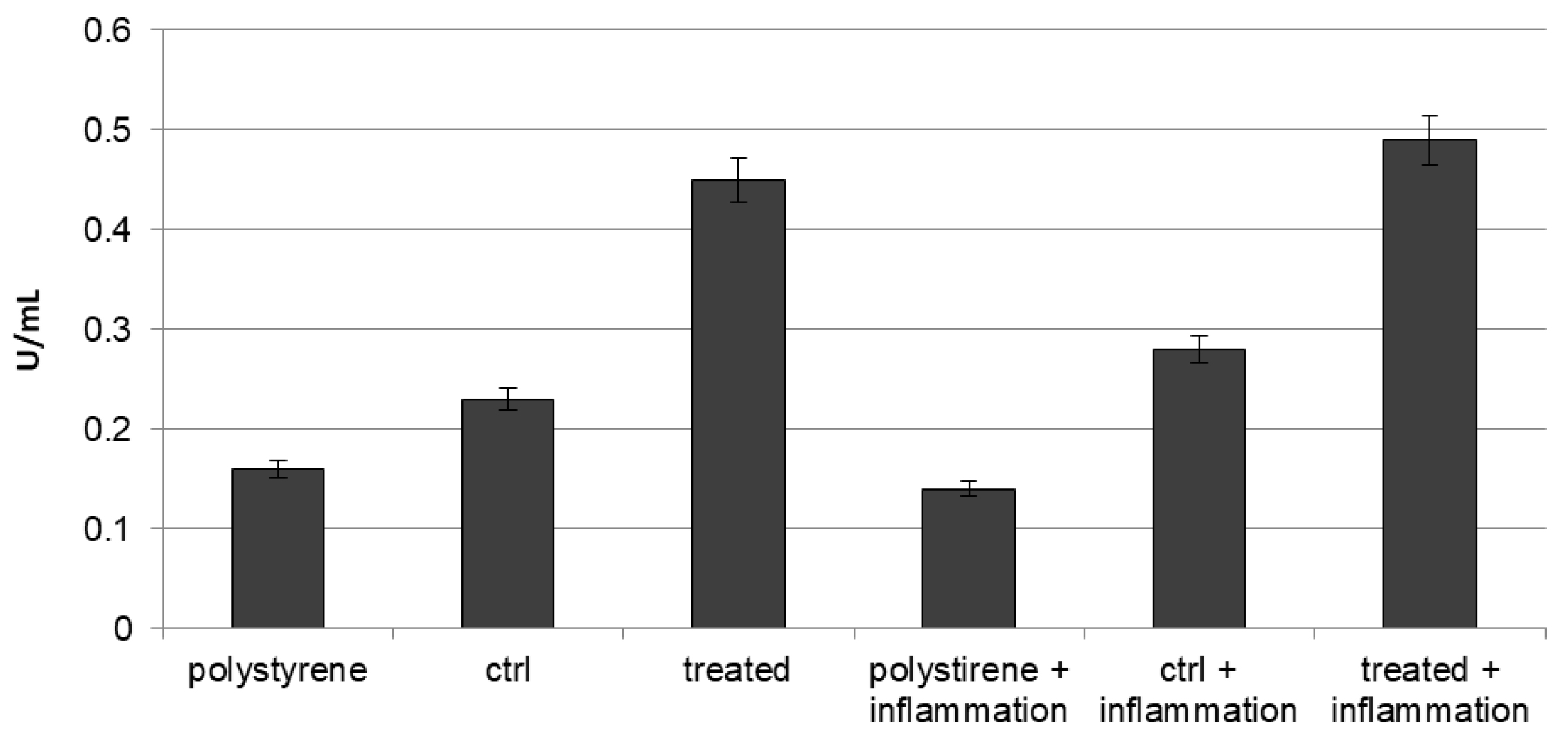
2.1. In Vitro Release of VEGF
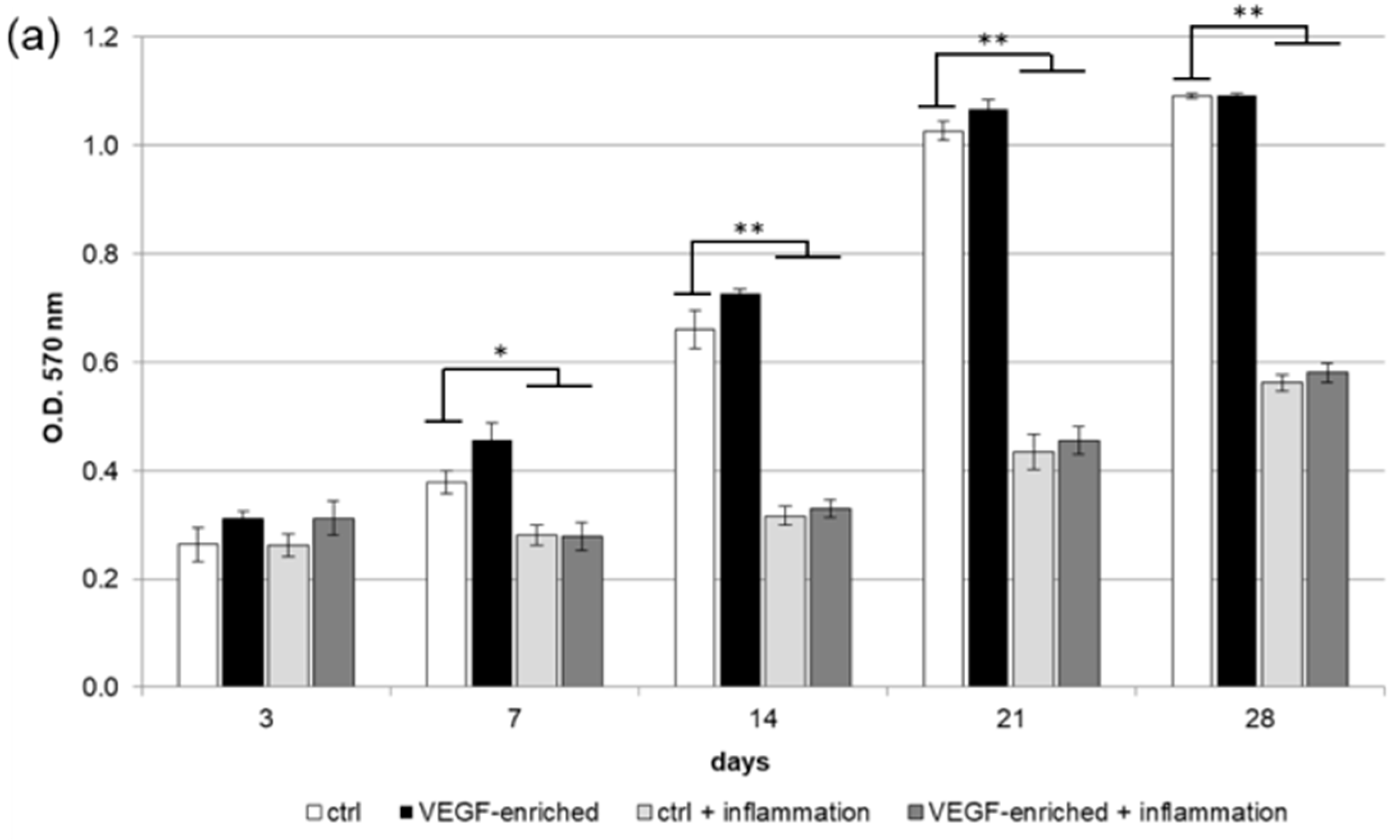
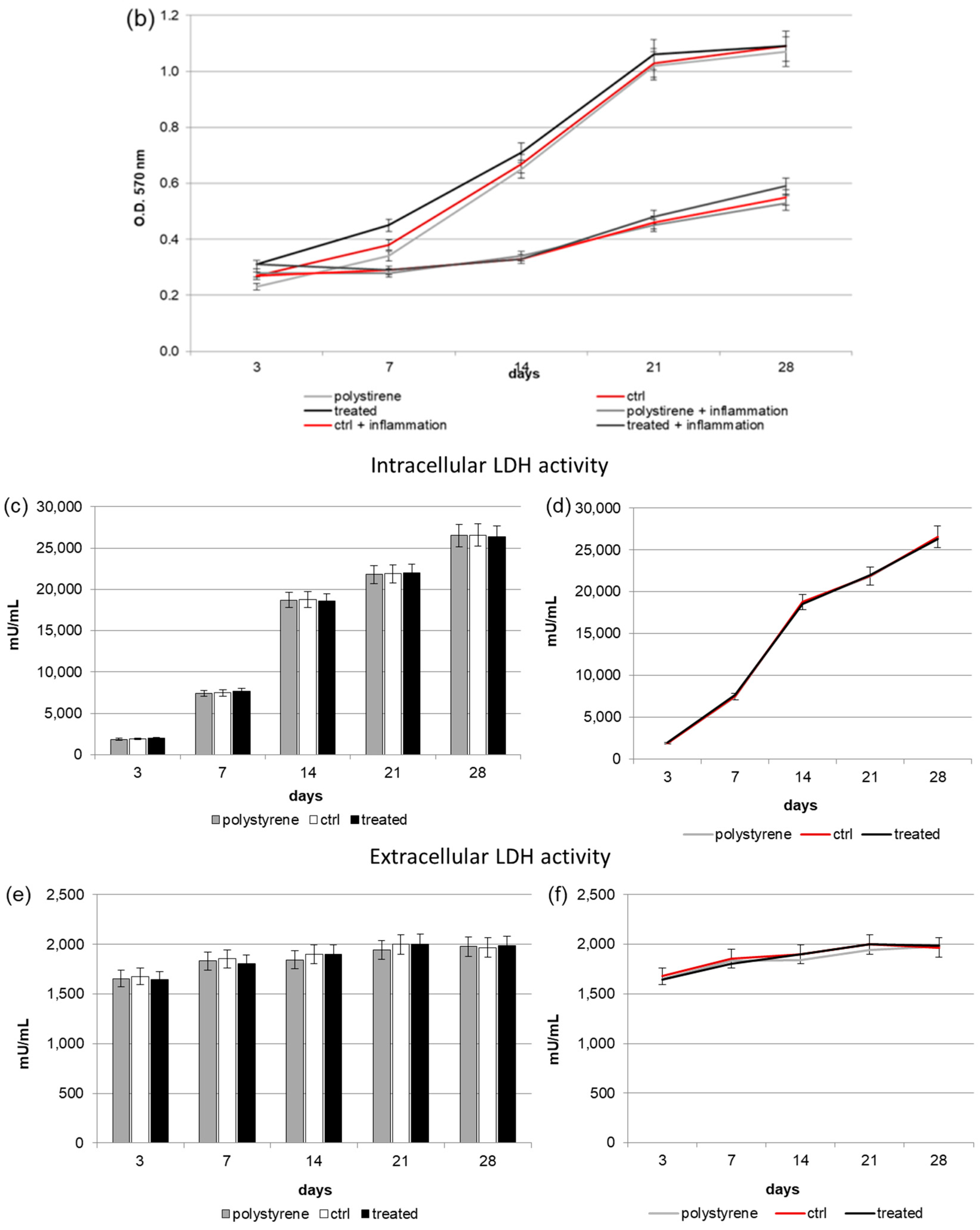
2.2. Biocompatibility and Cell Proliferation
2.3. Intracellular and Extracellular Lactate Dehydrogenase (LDH) Activity
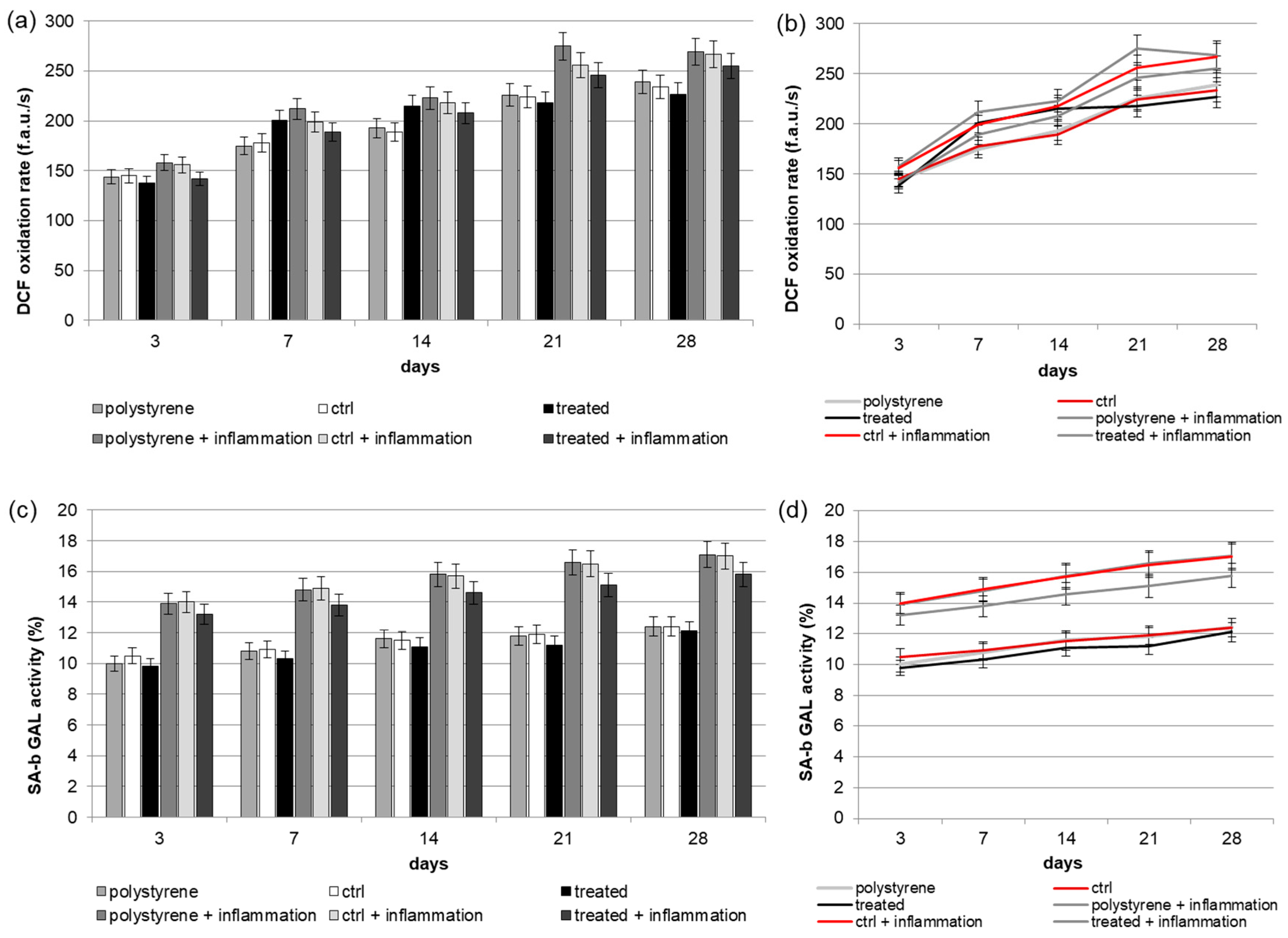
2.4. Reactive Oxygen Species (ROS) Production
2.5. Senescence
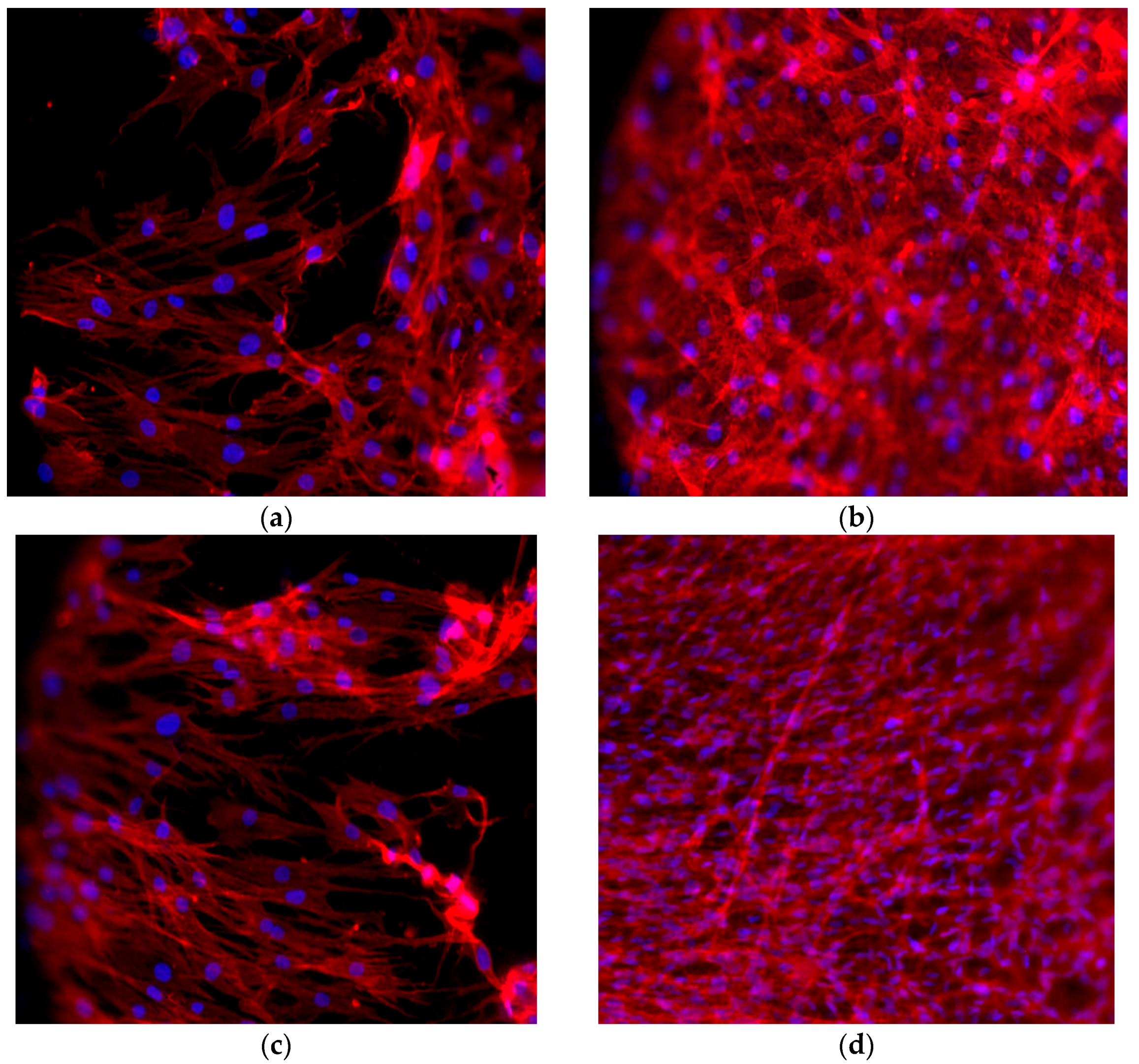
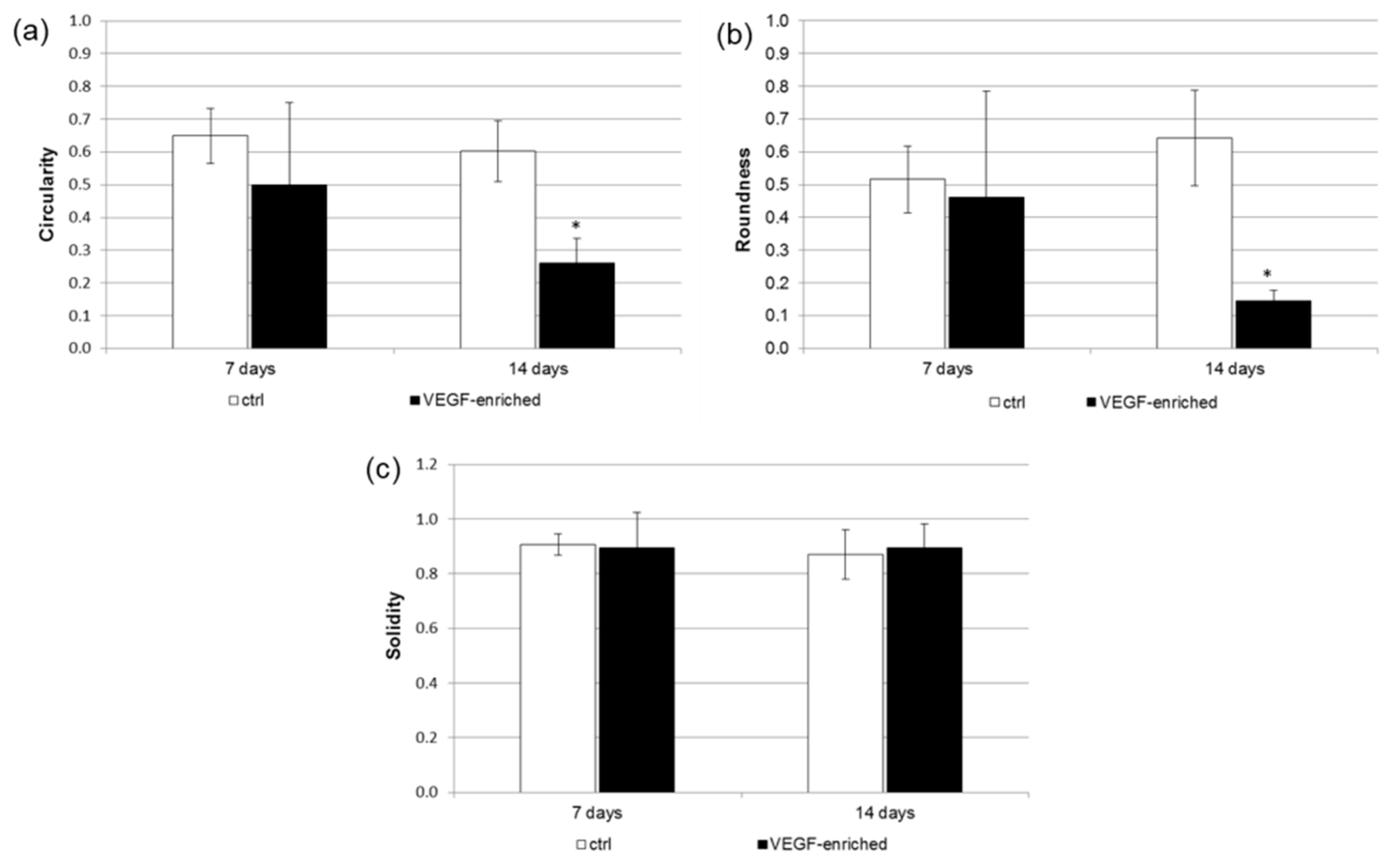
2.6. Cell Morphology
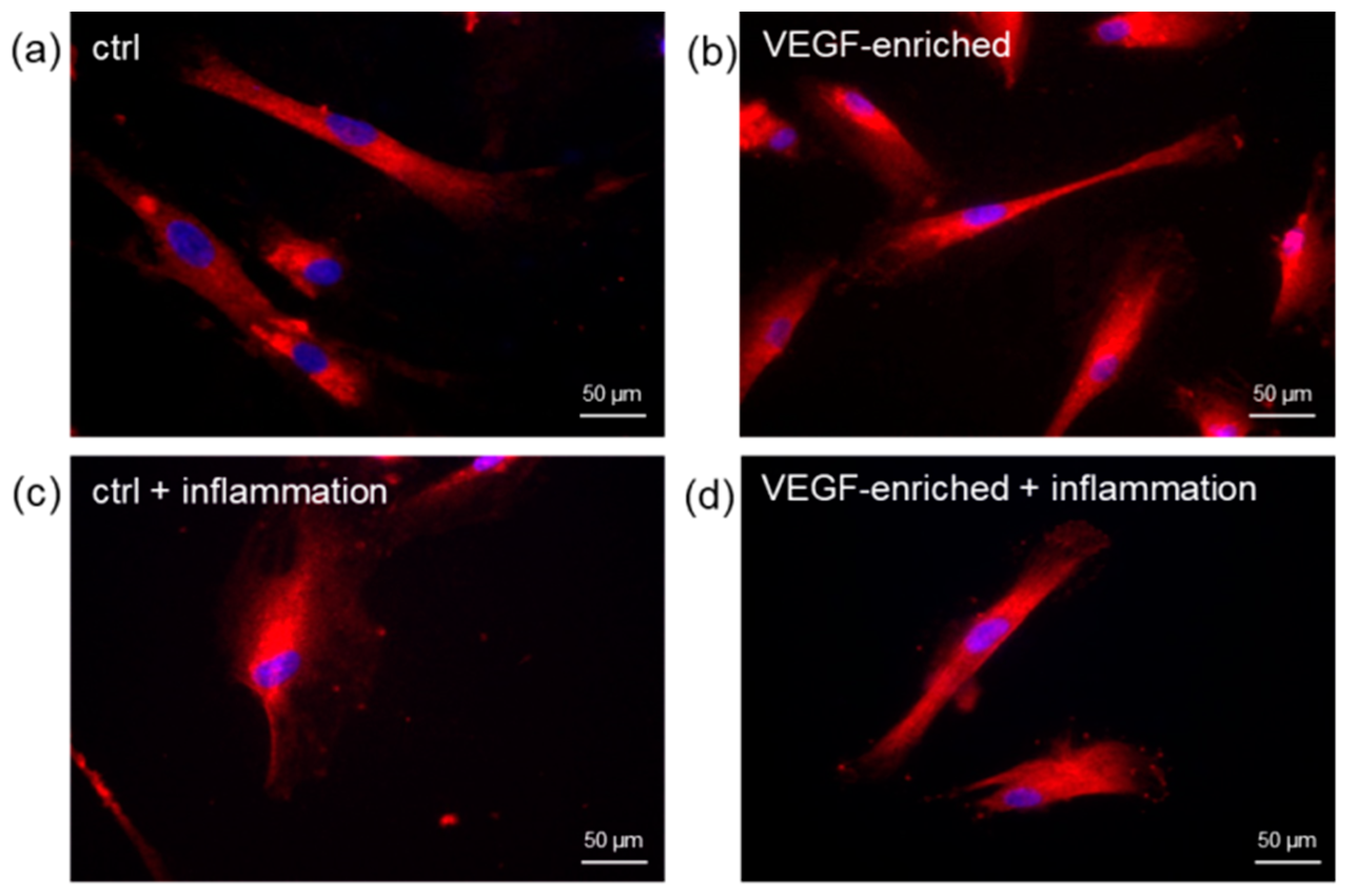
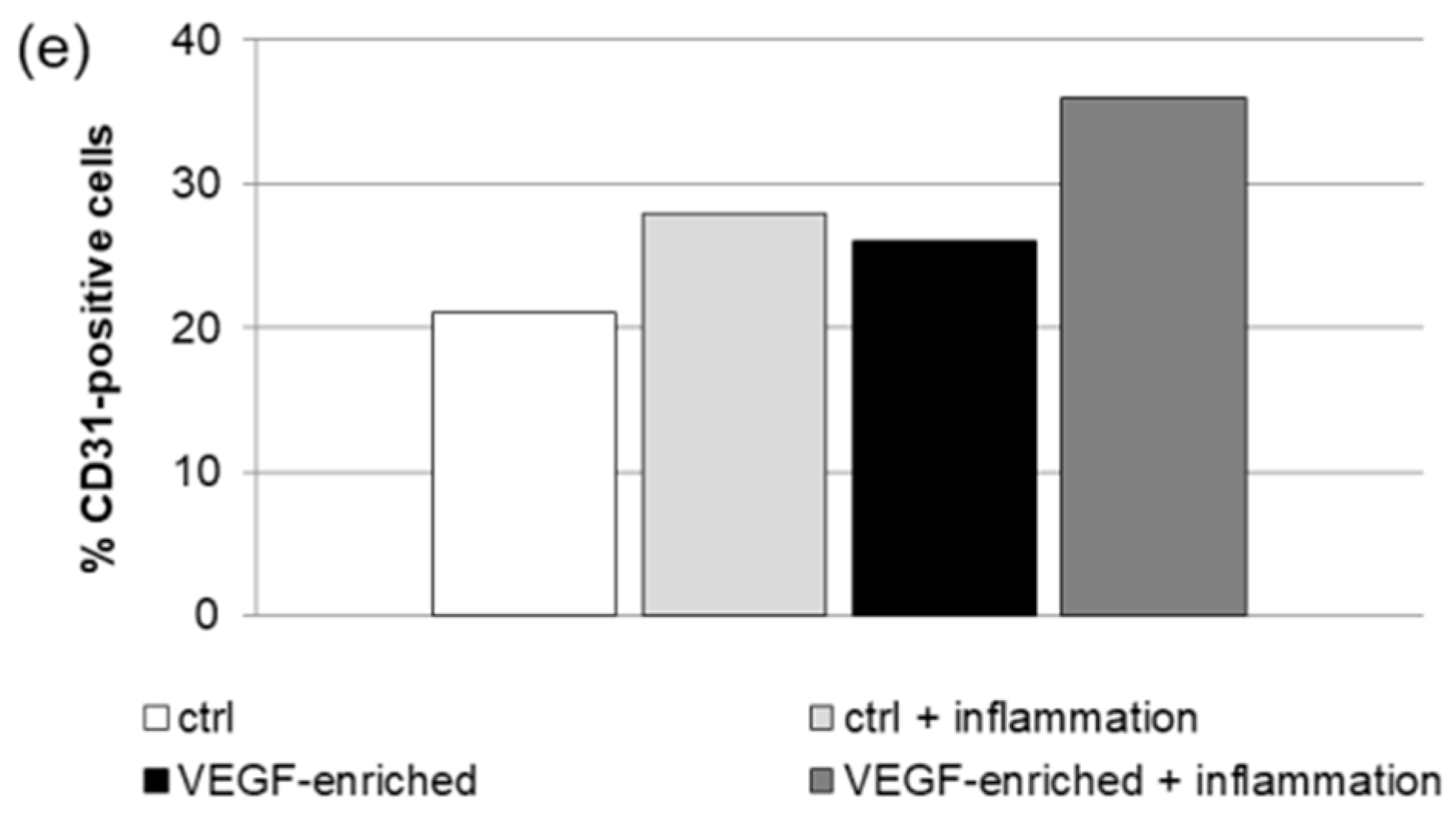
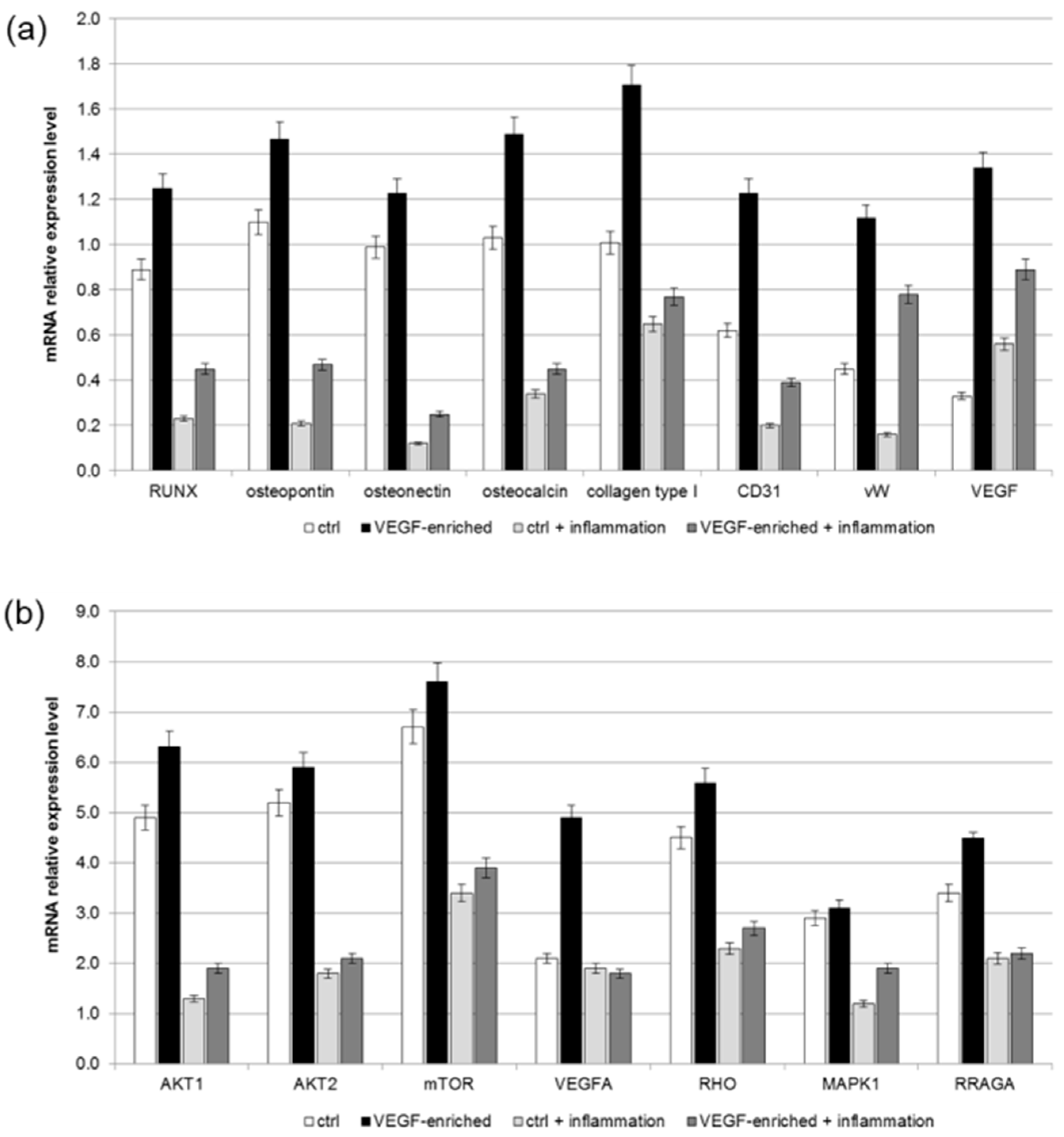
2.7. Effect of VEGF on Endothelial Cell Differentiation
2.8. Osteogenic Commitment
3. Discussion
4. Materials and Methods
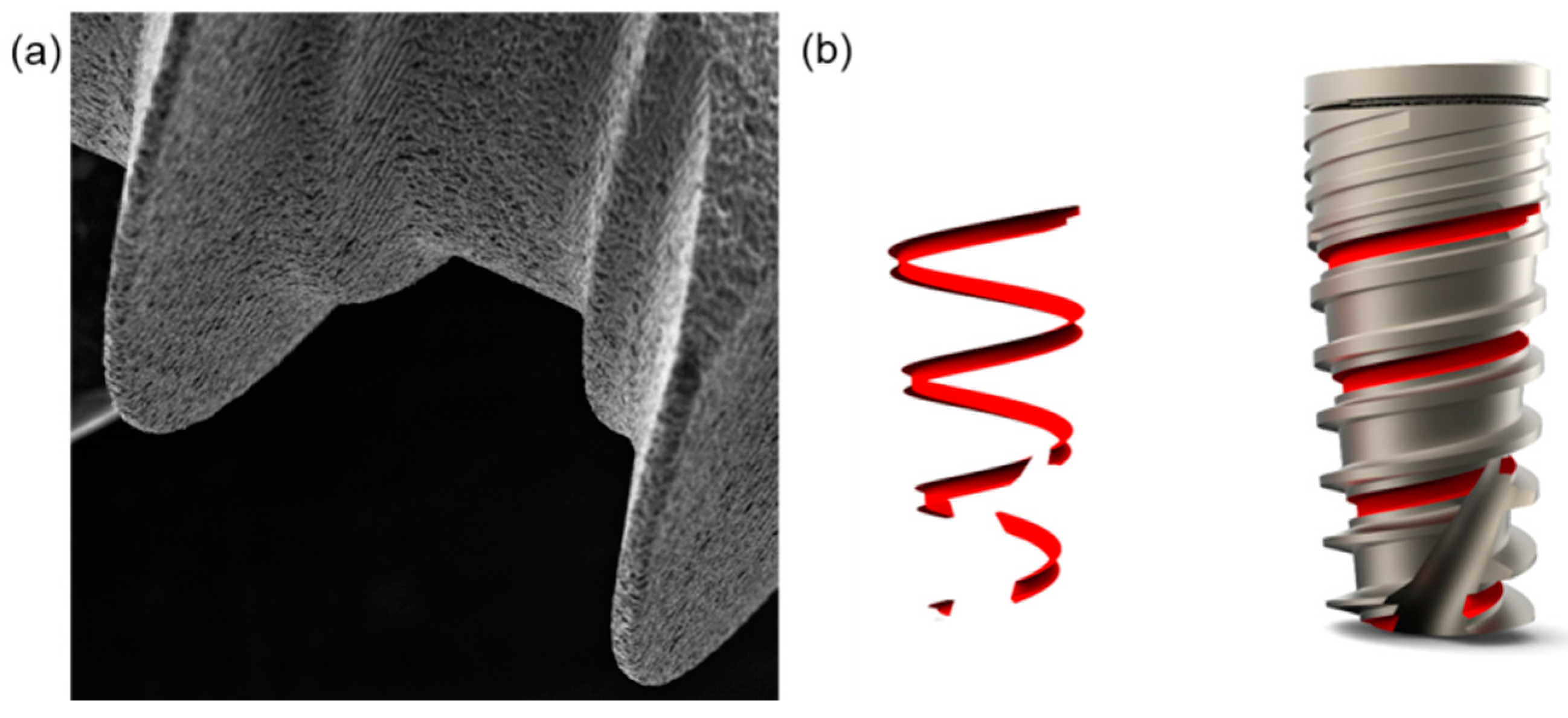
4.1. Dental Implants
4.2. Quantitative Analysis of VEGF Release
4.3. DPSCs Isolation and Seeding onto Dental Implant
4.4. MTT Assay
4.5. LDH Activity
4.6. ROS Measurements
4.7. SA-b GAL Staining
4.8. Cell Shape Analysis
4.9. Immunofluorescence
4.10. Real-Time RT-PCR
4.11. Statistical Analyses
4.12. ALP Activity Measurements
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.W.; Chien, E.Y.; Chien, H.H. Dental implant bioactive surface modifications and their effects on osseointegration: A review. Biomark. Res. 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; Van Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar]
- Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Manero, J.M.; Gil, J.; Kessler, H.; Mas-Moruno, C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf. B Biointerfaces 2015, 128, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Silva-Correia, J.; Zavan, B.; Vindigni, V.; Silva, T.H.; Oliveira, J.M.; Abatangelo, G.; Reis, R.L. Biocompatibility evaluation of ionic- and photo-crosslinked methacrylated gellan gum hydrogels: In vitro and in vivo study. Adv. Healthc. Mater. 2013, 4, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Magdolen, U.; Auernheimer, J.; Dahmen, C.; Schauwecker, J.; Gollwitzer, H.; Tübel, J.; Gradinger, R.; Kesler, H.; Schmitt, M.; Diehl, P. Growth promoting in vitro effect of synthetic cyclic RGD-peptides on human osteoblast-like cells attached to cancellous bone. Int. J. Mol. Med. 2006, 17, 1017–1021. [Google Scholar] [PubMed]
- Figallo, E.; Flaibani, M.; Zavan, B.; Abatangelo, G.; Elvassore, N. Micropatterned biopolymer 3D scaffold for static and dynamic culture of human fibroblasts. Biotechnol. Prog. 2007, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H. Clinical efficacy of growth factors to enhance tissue repair in oral and maxillofacial reconstruction: A systematic review. Clin. Implant Dent. Relat. Res. 2015, 17, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Tocco, I.; Epis, R.; Casadei, A.; Vindigni, V.; Mucci, G.; Zavan, B. Potential for neural differentiation of mesenchymal stem cells. Adv. Biochem. Eng. Biotechnol. 2013, 129, 89–115. [Google Scholar] [PubMed]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yu, J.; Liu, Y.; Lu, S.; Liu, H.; Shi, J.; Jin, Y. Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol. Int. 2008, 32, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Farges, J.C.; Romeas, A.; Melin, M.; Pin, J.J.; Lebecque, S.; Lucchini, M.; Bleicher, F.; Magloire, H.J. TGF-beta1 induces accumulation of dendritic cells in the odontoblast layer. J. Dent. Res. 2003, 82, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Scheufler, C.; Sebald, W.; Hülsmeyer, M. Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J. Mol. Biol. 1999, 287, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Açil, Y.; Springer, I.N.; Broek, V.; Terheyden, V.; Jepsen, S. Effects of bone morphogenetic protein-7 stimulation on osteoblasts cultured on different biomaterials. J. Cell. Biochem. 2002, 86, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ogawa, M.; Hata, Y.; Bessho, K.J. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J. Endod. 2004, 30, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gluhak-Heinrich, J.; Martinez, M.; Li, T.; Wu, Y.; Chuang, H.H.; Chen, L.; Dong, J.; Gay, I.; MacDougall, M. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J. Biol. Chem. 2008, 283, 19359–19370. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.; Porter, J.D.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 2005, 203, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.K.; Savage, N.W.; Young, W.G.; Gupta, G.S.; Breier, B.H.; Waters, M.J. Expression and regulation of insulin-like growth factor-I in the rat incisor. Growth Factors 1993, 8, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Huang, D.; Lu, X.; Feng, G.; Xing, J.; Lu, J.; Xu, K.; Xia, W.; Meng, Y.; Tao, T.; et al. Insulin-like growth factor 1 can promote proliferation and osteogenic differentiation of human dental pulp stem cells via mTOR pathway. Dev. Growth Differ. 2014, 56, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- D’ Alimonte, I.; Nargi, E.; Mastrangelo, F.; Falco, G.; Lanuti, P.; Marchisio, M.; Miscia, S.; Robuffo, I.; Capogreco, M.; Buccella, S.; et al. Vascular endothelial growth factor enhances in vitro proliferation and osteogenic differentiation of human dental pulp stem cells. J. Biol. Regul. Homeost. Agents 2011, 25, 57–69. [Google Scholar]
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardin, C.; Bressan, E.; Ferroni, L.; Nalesso, E.; Vindigni, V.; Stellini, E.; Pinton, P.; Sivolella, S.; Zavan, B. In vitro concurrent endothelial and osteogenic commitment of adipose-derived stem cells and their genomical analyses through comparative genomic hybridization array: Novel strategies to increase the successful engraftment of tissue-engineered bone grafts. Stem Cells Dev. 2012, 21, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Murata, Y.; Liu, Y.; Nicolae, C.; Olsen, B.R.; Berendsen, A.D. Vegfa regulates perichondrial vascularity and osteoblast differentiation in bone development. Development 2015, 142, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.D.; Olsen, B.R. How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells. J. Histochem. Cytochem. 2014, 62, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part I. review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Novaes, A.; Papalexiou, V.; Grisi, M.; Souza, S.; Taba, M.; Kajiwara, J. Influence of implant microstructure on the osseointegration of immediate implants placed in periodontally infected sites. A histomorphometric strudy in dogs. Clin. Oral Implants Res. 2004, 15, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Burgos, P.; Rasmusson, L.; Meirelles, L.; Senerby, L. Early bone tissue response to turned and oxidized implants in a rabbit tibia. Clin. Implant Dent. Relat. Res. 2008, 10, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiropaidis, A.; Sorensen, R.; Albandar, J.; Hall, J.; Wikesjo, U. Bone formation of titanium porous oxide (TiUnite) oral implants in type IV bone. Clin. Oral Implants Res. 2005, 16, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.; Moineddin, R.; Davies, J. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 2007, 28, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.; Moineddin, R.; Davies, J. Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium based implant surfaces. J. Biomed. Mater. Res. A 2009, 90, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Wang, Z.; Horger, K.; Mooney, D.J.; Krebsbach, P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Miner. Res. 2006, 21, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.K.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Pinton, P.; Stellini, E.; Botticelli, D.; Sivolella, S.; Zavan, B. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS ONE 2012, 7, e49146. [Google Scholar] [CrossRef] [PubMed]
- Zulato, E.; Favaretto, F.; Veronese, C.; Campanaro, S.; Marshall, J.D.; Romano, S.; Cabrelle, A.; Collin, G.B.; Zavan, B.; Belloni, A.S.; et al. ALMS1-deficient fibroblasts over-express extra-cellular matrix components, display cell cycle delay and are resistant to apoptosis. PLoS ONE 2011, 6, e19081. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Giorgi, C.; Bagnara, G.P.; Vindigni, V.; Abatangelo, G.; Cortivo, R. Osteogenic and chondrogenic differentiation: Comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur. J. Histochem. 2007, 51, 1–8. [Google Scholar] [PubMed]
- Rocca, A.; Marino, A.; Rocca, V.; Moscato, S.; de Vito, G.; Piazza, V.; Mazzolai, B.; Mattoli, V.; Ngo-Anh, T.J.; Ciofani, G. Barium titanate nanoparticles and hypergravity stimulation improve differentiation of mesenchymal stem cells into osteoblasts. Int. J. Nanomed. 2015, 10, 433–445. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavan, B.; Ferroni, L.; Gardin, C.; Sivolella, S.; Piattelli, A.; Mijiritsky, E. Release of VEGF from Dental Implant Improves Osteogenetic Process: Preliminary In Vitro Tests. Materials 2017, 10, 1052. https://doi.org/10.3390/ma10091052
Zavan B, Ferroni L, Gardin C, Sivolella S, Piattelli A, Mijiritsky E. Release of VEGF from Dental Implant Improves Osteogenetic Process: Preliminary In Vitro Tests. Materials. 2017; 10(9):1052. https://doi.org/10.3390/ma10091052
Chicago/Turabian StyleZavan, Barbara, Letizia Ferroni, Chiara Gardin, Stefano Sivolella, Adriano Piattelli, and Eitan Mijiritsky. 2017. "Release of VEGF from Dental Implant Improves Osteogenetic Process: Preliminary In Vitro Tests" Materials 10, no. 9: 1052. https://doi.org/10.3390/ma10091052
APA StyleZavan, B., Ferroni, L., Gardin, C., Sivolella, S., Piattelli, A., & Mijiritsky, E. (2017). Release of VEGF from Dental Implant Improves Osteogenetic Process: Preliminary In Vitro Tests. Materials, 10(9), 1052. https://doi.org/10.3390/ma10091052






