Zinc Sorption Studies on Pectin-Based Biosorbents
Abstract
:1. Introduction
2. Results and Discussion
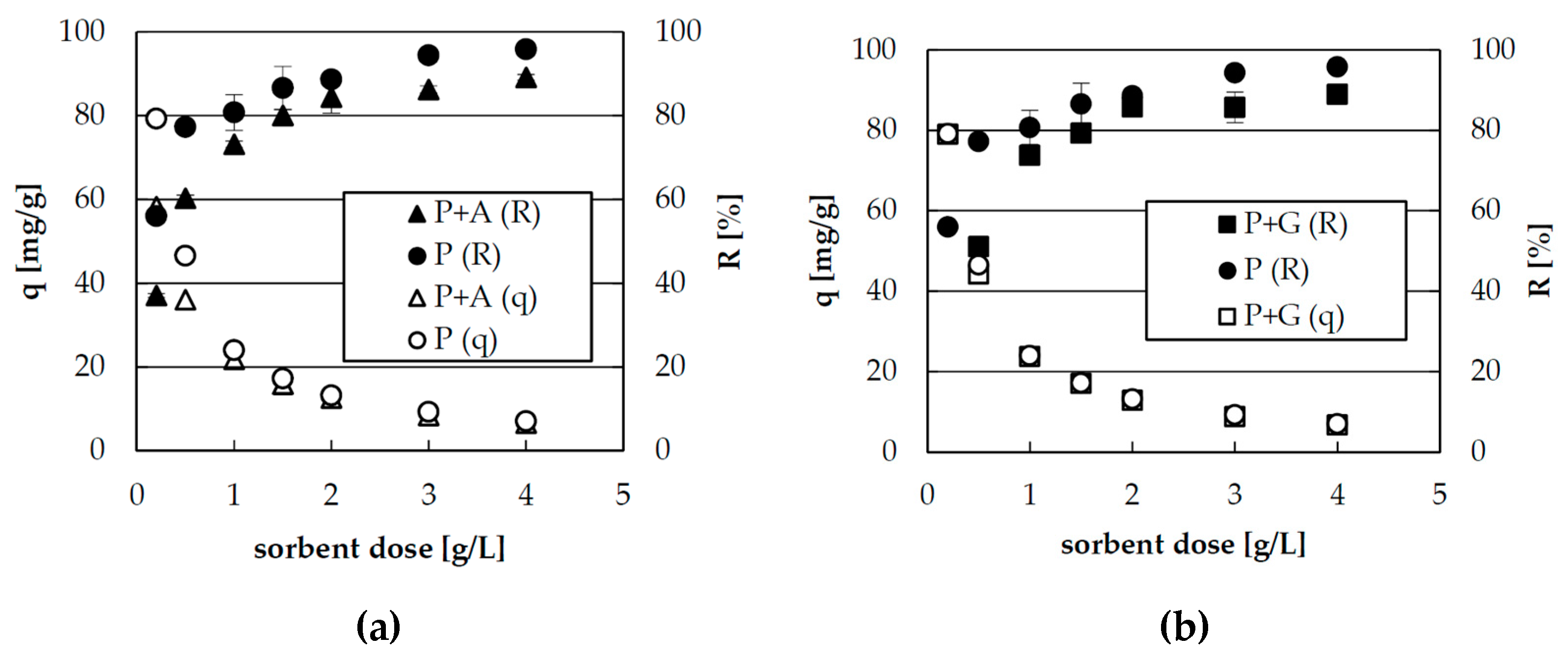
2.1. Effect of Sorbent Dose on Zinc(II) Ions Sorption
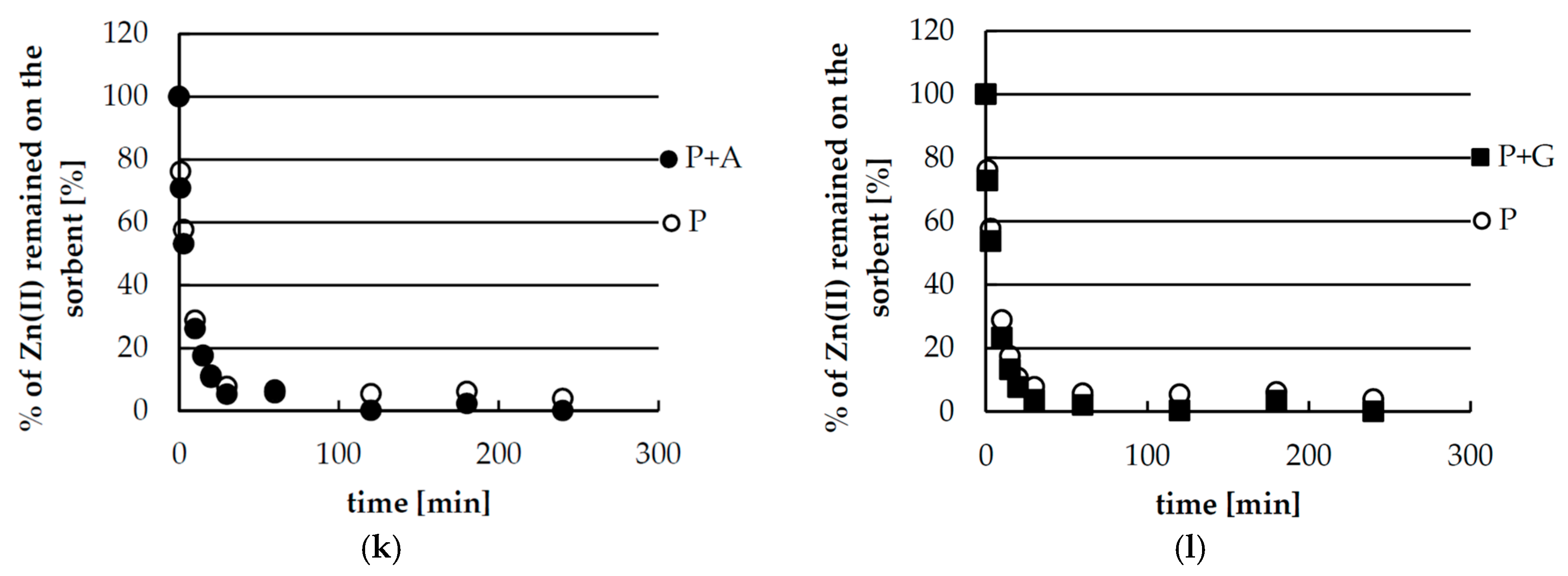
2.2. Kinetics Studies of Zinc(II) Ions Sorption
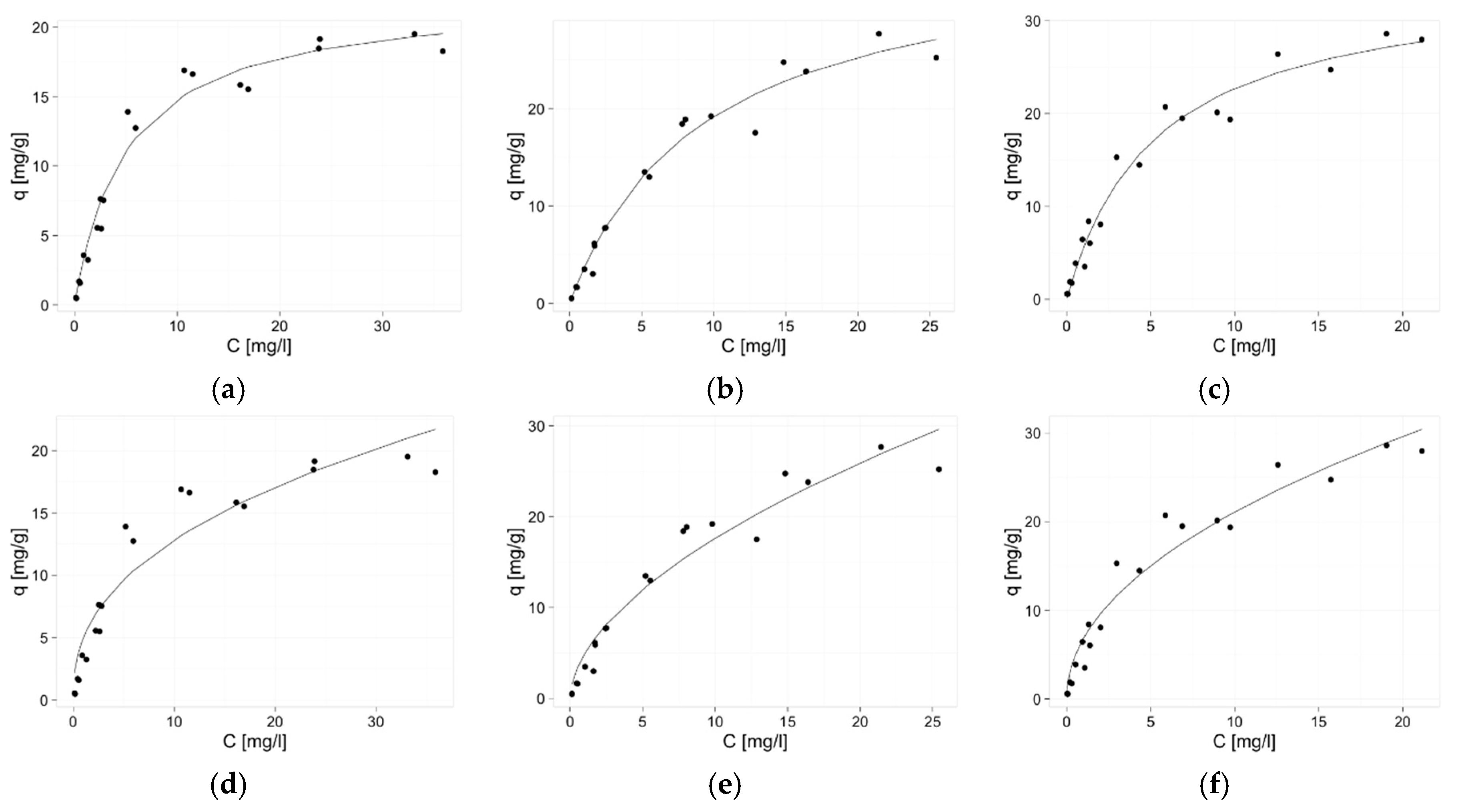
2.3. Isotherms of Zinc(II) Ions Sorption
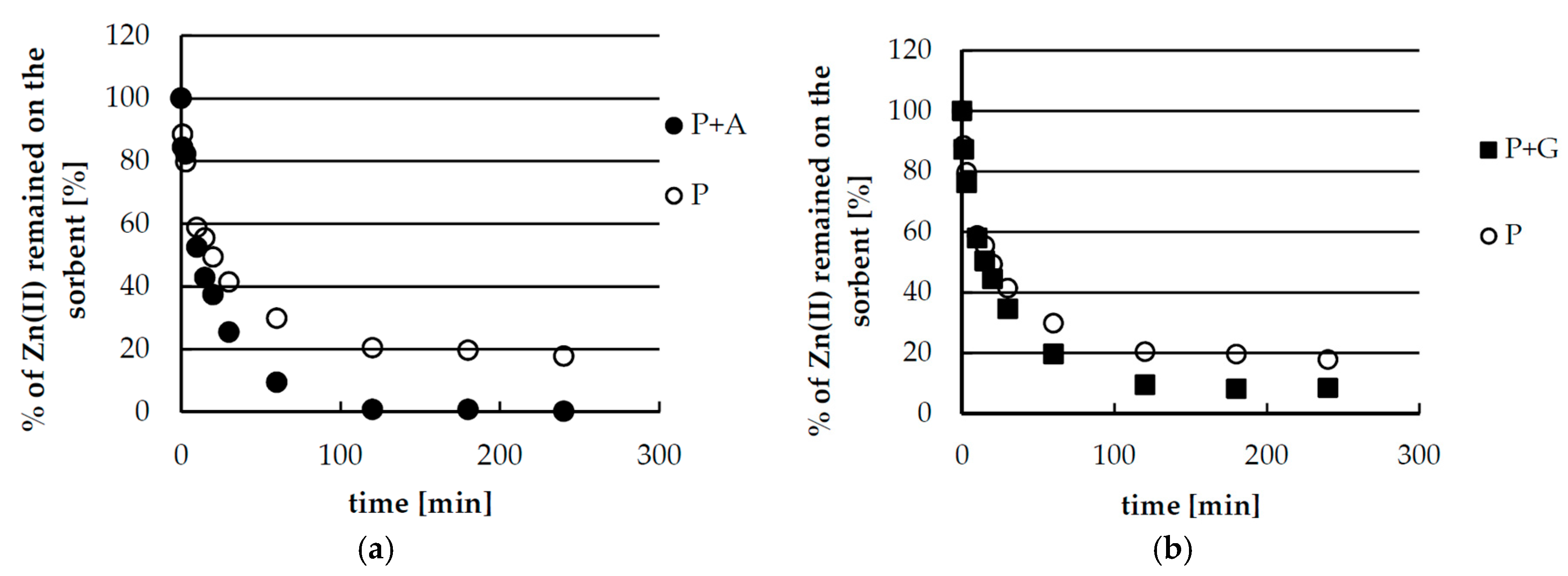
2.4. Desorption of Zinc(II) from Biosorbents—Equlibrium
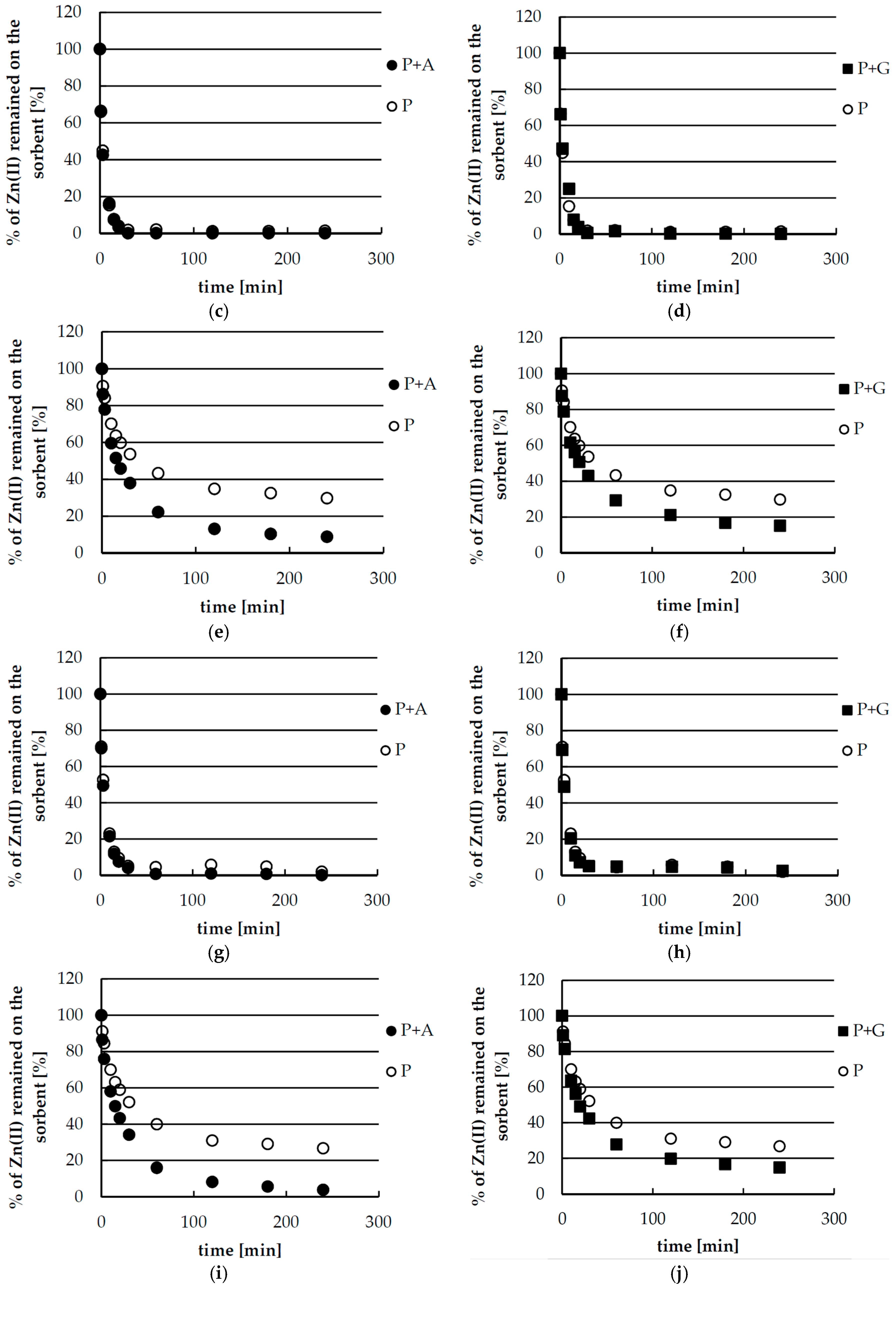
2.5. Desorption of Zinc(II) from Biosorbents—Kinetics
3. Materials and Methods
3.1. Materials
3.2. Analytical Method
3.3. General Procedure for Zinc(II) Ion Sorption Studies
- —the initial concentration of zinc(II) ions in the solution (mg/L),
- c—the final concentration of zinc(II) ions in the solution (mg/L),
- V—the volume of the solution (L),
- m—the mass of the sorbent (g).
3.4. Effect of Sorbent Dose on Zinc(II) Ions Sorption
3.5. Kinetics Studies of Zinc(II) Ions Sorption
- qm—the zinc(II) ions sorbed on one gram of biosorbent at equilibrium (adsorption capacity) (mg/g),
- qt—the zinc(II) ions sorbed on one gram of biosorbent at time “t” (mg/g),
- k1—the rate constant of pseudo-first order sorption model (1/min),
- k2—the rate constant of pseudo-second order sorption model (g/mg·min),
- t—the time (min).
3.6. Isotherms of Zinc(II) Ions Sorption
- q—the zinc(II) ions sorbed on one gram of biosorbent at equilibrium (sorption capacity) (mg/g),
- qm—the maximum sorption capacity (mg/g),
- B—the equilibrium constant that corresponds to the sorption energy (L/mg),
- c—the equilibrium concentration of zinc(II) ions in the solution (mg/L),
- K—((mg/g)(L/mg)1/n) corresponds to the relative sorption capacity,
- n—corresponds to the sorption intensity of the sorbent.
3.7. Desorption of Zinc(II) from Biosorbents—Equilibrium
3.8. Desorption of Zinc(II) from Biosorbents—Kinetics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn(II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017, 598, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Dermentzis, K.K.; Christoforidis, A.; Valsamidou, E. Removal of nickel, copper, zinc and chromium from synthetic and industrial wastewater by electrocoagulation. Int. J. Environ. Sci. 2011, 1, 697–710. [Google Scholar] [CrossRef]
- Kočanová, V.; Cuhorka, J.; Dušek, L.; Mikulášek, P. Application of nanofiltration for removal of zinc from industrial wastewater. Desalin. Water Treat. 2017, 75, 342–347. [Google Scholar] [CrossRef]
- Foroutan, R.; Esmaeili, H.; Rishehri, S.D.; Sadeghzadeh, F.; Mirahmadi, S.; Kosarifard, M.; Ramavandi, B. Zinc, nickel, and cobalt ions removal from aqueous solution and plating plant wastewater by modified Aspergillus flavus biomass: A dataset. Data Br. 2017, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Girczys, J.; Kupich, I. Chemical composition of mine water discharged from Zn-Pb ore mines in view of its management. J. Polish Miner. Eng. Soc. 2010, 11, 1–8. [Google Scholar]
- Wulan, D.R.; Cahyaningsih, S. Djaenudin Influence of voltage input to heavy metal removal from electroplating wastewater using electrocoagulation process. In 1st International Symposium on Green Technology for Value Chains; IOP Science: Philadelphia, PA, USA, 2016; pp. 1–7. [Google Scholar]
- European Community (EC). Directive 2010/75/EU of the European Parliament and of the Council on industrial emissions (integrated pollution prevention and control). Off. J. Eur. Union 2010, 334, 17–119. [Google Scholar]
- Polish Ministry of Environment (MŚ). Rozporządzenie Ministra Środowiska w sprawie warunków, jakie należy spełnić przy wprowadzaniu ścieków do wód lub do ziemi, oraz w sprawie substancji szczególnie szkodliwych dla środowiska wodnego. Dz. U. 2014, POZ., 1800. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), Regulations and Advisories. Regulations and Guidelines Applicable to Zinc and Zinc Compounds; ATSDR: Atlanta, US, 2003.
- Malkoc, S.; Kaynak, E.; Guven, K. Biosorption of zinc(II) on dead and living biomass of Variovorax paradoxus and Arthrobacter viscosus. Desalin. Water Treat. 2016, 57, 15445–15454. [Google Scholar] [CrossRef]
- Zhou, C.; Gong, X.; Han, J.; Guo, R. Removal of Pb(II) and Zn(II) from Aqueous Solutions by Raw Crab Shell: A Comparative Study. Water Environ. Res. 2016, 88, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Bulgariu, L.; Hlihor, R.M.; Bulgariu, D.; Gavrilescu, M. Sorptive removal of cadmium(II) ions from aqueous solution by mustard biomass. Environ. Eng. Manag. J. 2012, 11, 1969–1976. [Google Scholar]
- Ng, Z.-G.; Lim, J.-W.; Isa, M.H.; Pasupuleti, V.R.; Yunus, N.M.; Lee, K.-C. Adsorptive removal of hexavalent chromium using sawdust: Enhancement of biosorption and bioreduction. Sep. Sci. Technol. 2017, 52, 1707–1716. [Google Scholar] [CrossRef]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Petrović, J.; Mihajlović, M.; Ćosović, A.; Stanković, S. Mechanism of adsorption of Cu2+ and Zn2+ on the corn silk (Zea mays L). Ecol. Eng. 2017, 99, 83–90. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process. Saf. Environ. Prot. 2016, 102, 336–352. [Google Scholar] [CrossRef]
- Rodrigues, A.C.D.; do Amaral Sobrinho, N.M.B.; dos Santos, F.S.; dos Santos, A.M.; Pereira, A.C.C.; Lima, E.S.A. Biosorption of Toxic Metals by Water Lettuce (Pistia stratiotes) Biomass. Water. Air Soil Pollut. 2017, 228, 156. [Google Scholar] [CrossRef]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Amer, M.W.; Ahmad, R.A.; Awwad, A.M. Biosorption of Cu(II), Ni(II), Zn(II) and Pb(II) ions from aqueous solution by Sophora japonica pods powder. Int. J. Ind. Chem. 2015, 6, 67–75. [Google Scholar] [CrossRef]
- Ebrahimzadeh Rajaei, G.; Aghaie, H.; Zare, K.; Aghaie, M. Adsorption of Cu(II) and Zn(II) ions from aqueous solutions onto fine powder of Typha latifolia L. root: Kinetics and isotherm studies. Res. Chem. Intermed. 2013, 39, 3579–3594. [Google Scholar] [CrossRef]
- Chaidir, Z.; Zein, R.; Munaf, E. Research Article Biosorption of zinc (II) ions from aqueous solution by Androgaphis paniculata leaves powder on batch method. J. Chem. Pharm. Res. 2015, 7, 28–38. [Google Scholar]
- Cutillas-Barreiro, L.; Paradelo, R.; Igrexas-Soto, A.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodriguez, E.; Garrote, G.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Valorization of biosorbent obtained from a forestry waste: Competitive adsorption, desorption and transport of Cd, Cu, Ni, Pb and Zn. Ecotoxicol. Environ. Saf. 2016, 131, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Studies on sorption, desorption, regeneration and reuse of sugar-beet pectin gels for heavy metal removal. J. Hazard. Mater. 2010, 178, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Sugar-beet pulp pectin gels as biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics. Chem. Eng. J. 2009, 150, 289–301. [Google Scholar] [CrossRef]
- Mata, Y.N.; Torres, E.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Lead and Gold Removal Using Sugar-Beet Pectin Gels with and without Immobilized Fucus Vesiculosus. Adv. Mater. Res. 2007, 20–21, 599–602. [Google Scholar] [CrossRef]
- Harel, P.; Mignot, L.; Sauvage, J.P.; Junter, G.A. Cadmium Removal from Dilute Aqueous Solution by Gel Beads of Sugar Beet Pectin. Ind. Crop. Prod. 1998, 7, 239–247. [Google Scholar] [CrossRef]
- Fares, M.M.; Tahboub, Y.R.; Khatatbeh, S.T.; Abul-Haija, Y.M. Eco-Friendly, Vascular Shape and Interpenetrating Poly (Acrylic Acid) Grafted Pectin Hydrogels; Biosorption and Desorption Investigations. J. Polym. Environ. 2011, 19, 431–439. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Makarova, K.; Khozhaenko, E.; Kovalev, V. Lead-binding capacity of calcium pectates with different molecular weight. Int. J. Biol. Macromol. 2017, 97, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Moia, T.A.; Mattoso, L.H.C.; Tambourgi, E.B. Pectin-based polymer hydrogel as a carrier for release of agricultural nutrients and removal of heavy metals from wastewater. J. Appl. Polym. Sci. 2010, 117, 3146–3154. [Google Scholar] [CrossRef]
- Jakóbik-Kolon, A.; Milewski, A.K.; Mitko, K.; Lis, A. Preparation of Pectin-Based Biosorbents for Cadmium and Lead Ions Removal. Sep. Sci. Technol. 2014, 49, 1679–1688. [Google Scholar] [CrossRef]
- Jakóbik-Kolon, A.; Milewski, A.K.; Karoń, K.; Bok-Badura, J. New, hybrid pectin-based biosorbents. Sep. Sci. Technol. 2016, 51, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- Jakóbik-Kolon, A.; Bok-Badura, J.; Milewski, A.K.; Mitko, K. Sorption studies of cadmium and lead ions on hybrid polysaccharide biosorbents. Sep. Sci. Technol. in press. [CrossRef]
- Yamada, M.; Shiiba, S. Preparation of pectin-inorganic composite material as accumulative material of metal ions. J. Appl. Polym. Sci. 2015, 132, 42056. [Google Scholar] [CrossRef]
- Jakóbik-Kolon, A.; Bok-Badura, J.; Karoń, K.; Mitko, K.; Milewski, A. Hybrid pectin-based biosorbents for zinc ions removal. Carbohydr. Polym. 2017, 169, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kluczka, J.; Trojanowska, J.; Zołotajkin, M. Utilization of fly ash zeolite for boron removal from aqueous solution. Desalin. Water Treat. 2015, 54, 1839–1849. [Google Scholar] [CrossRef]
- Lutts, S.; Qin, P.; Han, R.-M. Salinity influences biosorption of heavy metals by the roots of the halophyte plant species Kosteletzkya pentacarpos. Ecol. Eng. 2016, 95, 682–689. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T. Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res. 2003, 37, 4486–4496. [Google Scholar] [CrossRef]
- Nasernejad, B.; Zadeh, T.E.; Pour, B.B.; Bygi, M.E.; Zamani, A. Camparison for biosorption modeling of heavy metals (Cr(III), Cu(II), Zn(II)) adsorption from wastewater by carrot residues. Process. Biochem. 2005, 40, 1319–1322. [Google Scholar] [CrossRef]
- Lau, T.C.; Ang, P.O.; Wong, P.K. Development of Seaweed Biomass as Biosorbent for Metal Ions; NCBI: Bethesda, MD, USA, 2003; Volume 47, pp. 49–54.
- Rahman, M.S.; Sathasivam, K.V. Heavy metal biosorption potential of a Malaysian Rhodophyte (Eucheuma denticulatum) from aqueous solutions. Int. J. Environ. Sci. Technol. 2016, 13, 1973–1988. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S. Citation Review of Lagergren Kinetic Rate Equation on Adsorption Reactions. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Duong, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; Volume 2, ISBN 1860941303. [Google Scholar]


| Pseudo-First-Order Kinetics | |||
|---|---|---|---|
| sorbent | P + A | P + G | P |
| R2 | 0.944 | 0.991 | 0.997 |
| qm [mg/g] | 14.2 ± 0.4 | 15.7 ± 0.4 | 16.7 ± 0.02 |
| k1·102 [1/min] | 1.02 ± 0.07 | 1.19 ± 0.01 | 1.26 ± 0.05 |
| Pseudo-Second-Order Kinetics | |||
| sorbent | P + A | P + G | P |
| R2 | 0.989 | 0.982 | 0.992 |
| qm [mg/g] | 18.0 ± 0.8 | 19.4 ± 1.0 | 20.4 ± 0.7 |
| k2·104 [g/mg·min] | 5.41 ± 0.89 | 6.07 ± 1.19 | 6.21 ± 0.82 |
| Calculated Parameters of Langmuir Isotherm | |||
|---|---|---|---|
| sorbent | P + A | P + G | P |
| R2 | 0.973 | 0.976 | 0.973 |
| qm [mg/g] | 22.3 ± 1.0 | 37.0 ± 2.6 | 34.7 ± 2.0 |
| B [L/mg] | 0.196 ± 0.027 | 0.108 ± 0.017 | 0.190 ± 0.029 |
| Calculated Parameters of Freundlich Isotherm | |||
| sorbent | P + A | P + G | P |
| R2 | 0.913 | 0.952 | 0.957 |
| K [(mg/g)(L/mg)1/n] | 4.97 ± 0.63 | 4.90 ± 0.57 | 6.85 ± 0.63 |
| n | 2.43 ± 0.26 | 1.80 ± 0.15 | 2.05 ± 0.16 |
| Sorbent | Sorption Capacity (mg/g) | Reference |
|---|---|---|
| Corn silk | 13.98 | [15] |
| Roots of the halophyte | ~0.5 | [36] |
| Live biomass fungus Mucor rouxii | 7.75 | [37] |
| Carrot residues | 29.61 | [38] |
| Sophora jap. pods | 25.71 | [19] |
| Typha latifolia root | 28.8 | [20] |
| Seaweed biomass | 49.54 | [39] |
| Malaysian Rhodophyte | 43.48 | [40] |
| Crab shell * | 117 | [12] |
| Androgaphis paniculata leaves powder * | 86.96 | [21] |
| Modified EUCALYPTUS SHEATHIANA bark * | 250 | [16] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakóbik-Kolon, A.; Mitko, K.; Bok-Badura, J. Zinc Sorption Studies on Pectin-Based Biosorbents. Materials 2017, 10, 844. https://doi.org/10.3390/ma10070844
Jakóbik-Kolon A, Mitko K, Bok-Badura J. Zinc Sorption Studies on Pectin-Based Biosorbents. Materials. 2017; 10(7):844. https://doi.org/10.3390/ma10070844
Chicago/Turabian StyleJakóbik-Kolon, Agata, Krzysztof Mitko, and Joanna Bok-Badura. 2017. "Zinc Sorption Studies on Pectin-Based Biosorbents" Materials 10, no. 7: 844. https://doi.org/10.3390/ma10070844







