Improvement of Cr-Co-Mo Membrane Surface Used as Barrier for Bone Regeneration through UV Photofunctionalization: An In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Contact Angle
2.2. In Vitro Culture
3. Discussion
4. Materials and Methods
4.1. Cr-Co-Mo Samples and Surface Characterization
4.2. Ultraviolet-Light Irradiation
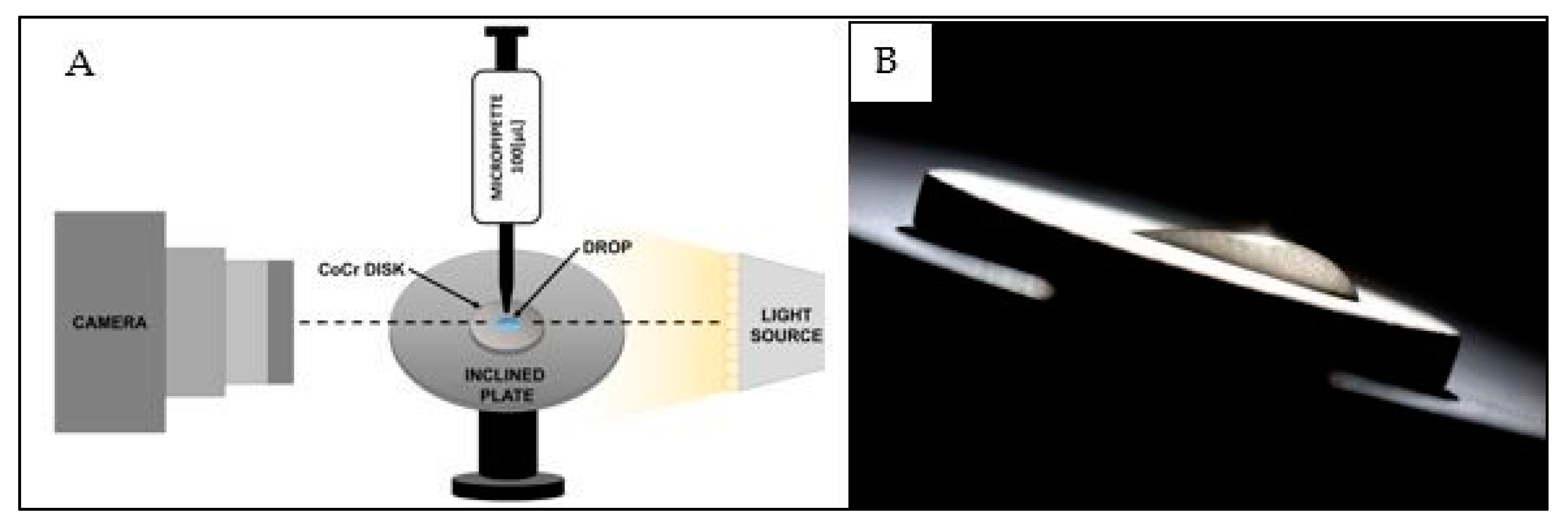
4.3. Contact Angle Measurement
4.4. Immersion in R-SBF
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Current concepts review-corrosion of metal orthopaedic implants. J. Bone Jt. Surg. Am. 1998, 80, 268–282. [Google Scholar] [CrossRef]
- Larsson Wexell, C.; Thomsen, P.; Aronsson, B.O.; Tengvall, P.; Rodahl, M.; Lausmaa, J.; Kasemo, B.; Ericson, L.E. Bone response to surface-modified titanium implants: Studies on the early tissue response to implants with different surface characteristics. Int. J. Biomater. 2013. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part I: Review of titanium alloys, surface characteristics and treatments. Med. Oral Patol. Oral Cir. Bucal 2016, 21, 514. [Google Scholar] [CrossRef]
- Mengucci, P.; Barucca, G.; Gatto, A.; Bassoli, E.; Denti, L.; Fiori, F.; Girardinc, E.; Bastianonia, P.; Rutkowski, B.; Czyrska-Filemonowicz, A. Effects of thermal treatments on microstructure and mechanical properties of a Co–Cr–Mo–W biomedical alloy produced by laser sintering. J. Mech. Behav. Biomed. Mater. 2016, 60, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Chenakin, S.P.; Filatova, V.S.; Makeeva, I.N.; Vasylyev, M.A. Ultrasonic impact treatment of CoCrMo alloy: Surface composition and properties. Appl. Surf. Sci. 2017, 408, 11–20. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, W.; Chu, K. Fabrication, tribological and corrosion behaviors of ultra-fine grained Co–28Cr–6Mo alloy for biomedical applications. J. Mech. Behav. Biomed. Mater. 2016, 60, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.C.; Goswami, T. Knee implants-Review of models and biomechanics. Mater. Des. 2009, 30, 398–413. [Google Scholar] [CrossRef]
- Sánchez-De Jesús, F.; Bolarín-Miró, A.M.; Torres-Villaseñor, C.A.; Cortés-Escobedo, C.A.; Betancourt-Cantera, J.A. Mechanical alloying of biocompatible Co-28Cr-6Mo alloy. J. Mater. Sci. Mater. Med. 2010, 21, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Grgązka-Dahlke, M.; Dąbrowski, J.R.; Dąbrowski, B. Modification of mechanical properties of sintered implant materials on the base of Co-Cr-Mo alloy. J. Mater. Process. Technol. 2008, 204, 199–205. [Google Scholar] [CrossRef]
- Shahgaldi, B.F.; Heatley, F.W.; Dewar, A.; Corrin, B. In vivo corrosion of cobalt-chromium and titanium wear particles. J. Bone Jt. Surg. 1995, 77, 962–966. [Google Scholar]
- Allen, M.J.; Myer, B.J. The ffects of particulate cobalt, chromium and cobalt-chromium alloy on human osteoblast-like cells in vitro. J. Bone Jt. Surg. 1997, 79, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Decco, O.; Cura, A.; Beltrán, V.; Lezcano, F.; Engelke, W. Bone augmentation in rabbit tibia using microfixed cobalt-chromium membranes with whole blood, tricalcium phosphate and bone marrow cells. Int. J. Clin. Exp. Med. 2015, 8, 135–144. [Google Scholar] [PubMed]
- Decco, O.A.; Beltrán, V.; Zuchuat, J.I.; Cura, A.C.; Lezcano, M.F.; Engelke, W. Bone Augmentation in Rabbit Tibia Using Microfixed Cobalt-Chromium Membranes with Whole Blood and Platelet-Rich Plasma. Materials 2015, 8, 4843–4856. [Google Scholar] [CrossRef]
- Mustafa, K.; Wroblewski, J.; Lopez, B.S.; Wennerberg, A.; Hultenby, K.; Arvidson, K. Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin. Oral Implant. Res. 2001, 12, 515–525. [Google Scholar] [CrossRef]
- Jayaraman, M.; Meyer, U.; Bühner, M.; Joos, U.; Wiesmann, H.P. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials 2004, 25, 625–631. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Tanaka, M.; Ishijima, M.; Iwasaki, C.; Park, W.; Ogawa, T. Effect of photofunctionalization on Ti6Al4V screw stability placed in segmental bone defects in rat femurs. J. Oral Maxillofac. Surg. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- Al Qahtani, M.S.; Wu, Y.; Spintzyk, S.; Krieg, P.; Killinger, A.; Schweizer, E.; Stephana, I.; Scheidelera, L.; Geis-Gerstorfera, J.; Rupp, F. UV-A and UV-C light induced hydrophilization of dental implants. Dent. Mater. 2015, 31, e157–e167. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.W.; Chen, Y.; Yang, G.L.; Wang, X.X.; He, F.M.; Wang, H.M. Effects of storage medium and UV photofunctionalization on time-related changes of titanium surface characteristics and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 485–726. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T. Ultraviolet photofunctionalization of titanium implants. Int. J. Oral Maxillofac. Implants 2014, 29, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Ueno, T.; Suzuki, T.; Iwasa, F.; Yamada, M.; Att, W.; Okada, S.; Ohno, A.; Aita, H.; Kimoto, K.; et al. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int. J. Oral Maxillofac. Implants 2010, 25, 49–62. [Google Scholar] [PubMed]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone–titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium–cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- Tuna, T.; Wein, M.; Swain, M.; Fischer, J.; Att, W. Influence of ultraviolet photofunctionalization on the surface characteristics of zirconia-based dental implant materials. Dent. Mater. 2015, 31, e14–e24. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves, 1st ed.; Springer Science & Business Media: Verlag, NY, USA, 2013. [Google Scholar]
- Tadmor, R. Line energy and the relation between advancing, receding, and young contact angles. Langmuir 2004, 20, 7659–7664. [Google Scholar] [CrossRef] [PubMed]
- Alfarsi, M.A.; Hamlet, S.M.; Ivanovski, S. Titanium surface hydrophilicity enhances platelet activation. Dent. Mater. J. 2014, 33, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. A 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon deposition attenuates osteoblast activity on titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Toma, S.; Lasserre, J.; Brecx, M.C.; Nyssen-Behets, C. In vitro evaluation of peri-implantitis treatment modalities on Saos-2osteoblasts. Clin. Oral Implants Res. 2016, 27, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Cassinelli, C.; Bruzzone, G.; Carpi, A.; Di Santi, G.; Giardino, R.; Fini, M. Surface chemistry effects of topographic modification of titanium dental implant surfaces: 1. Surface analysis. Int. J. Oral Maxillofac. Implants 2003, 18, 40–45. [Google Scholar] [PubMed]
- Kasemo, B.; Lausmaa, J. Biomaterial and implant surfaces: A surface science approach. Int. J. Oral Maxillofac. Implants 1988, 3. [Google Scholar] [PubMed]
- Lamour, G.; Hamraoui, A.; Buvailo, A.; Xing, Y.; Keuleyan, S.; Prakash, V.; Eftekhari-Bafrooei, A.; Borguet, E. Contact angle measurements using a simplified experimental setup. J. Chem. Educ. 2010, 87, 1403–1407. [Google Scholar] [CrossRef]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Madureira, P.; Barbosa, M.A.; Aguas, A.P. The influence of functional groups of self-assembled monolayers on fibrous capsule formation and cell recruitment. J. Biomed. Mater. Res. A 2006, 76, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Denzer, A.; Simpson, J. U.S. Patent Application No. 10/492,131. 2002. Available online: https://www.google.com/patents/US20040210309 (accessed on 4 April 2017).
- Zubkov, T.; Stahl, D.; Thompson, T.L.; Panayotov, D.; Diwald, O.; Yates, J.T. Ultraviolet light-induced hydrophilicity effect on TiO2 (110)(1 × 1). Dominant role of the photooxidation of adsorbed hydrocarbons causing wetting by water droplets. J. Phys. Chem. B 2005, 109, 15454–15462. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Miyaji, F.; Kim, H.M.; Nakamura, T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyazaki, T.; Kokubo, T.; Nakamura, T. Revised simulated body fluid. Key Eng. Mater. 2001, 192, 47–50. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
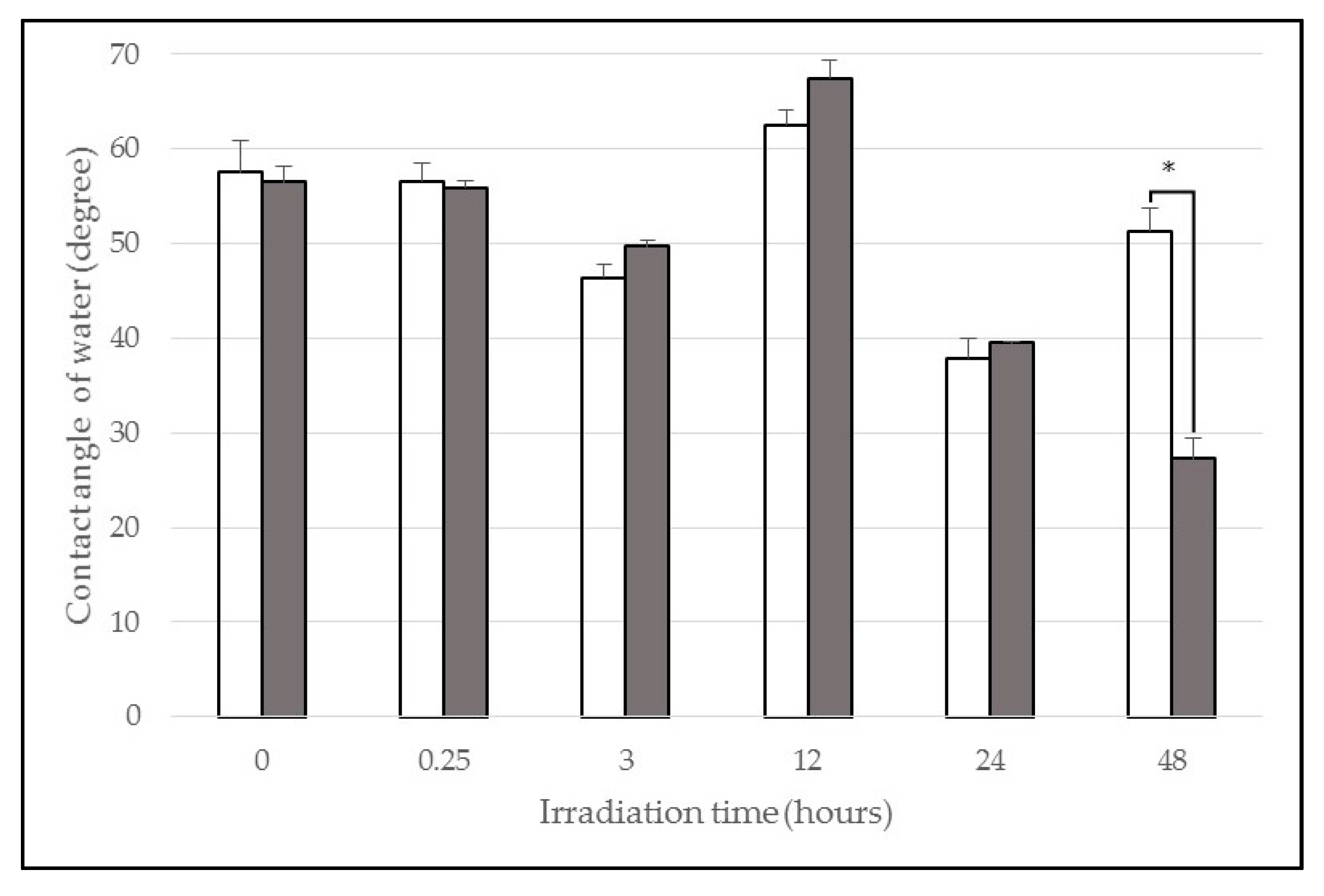
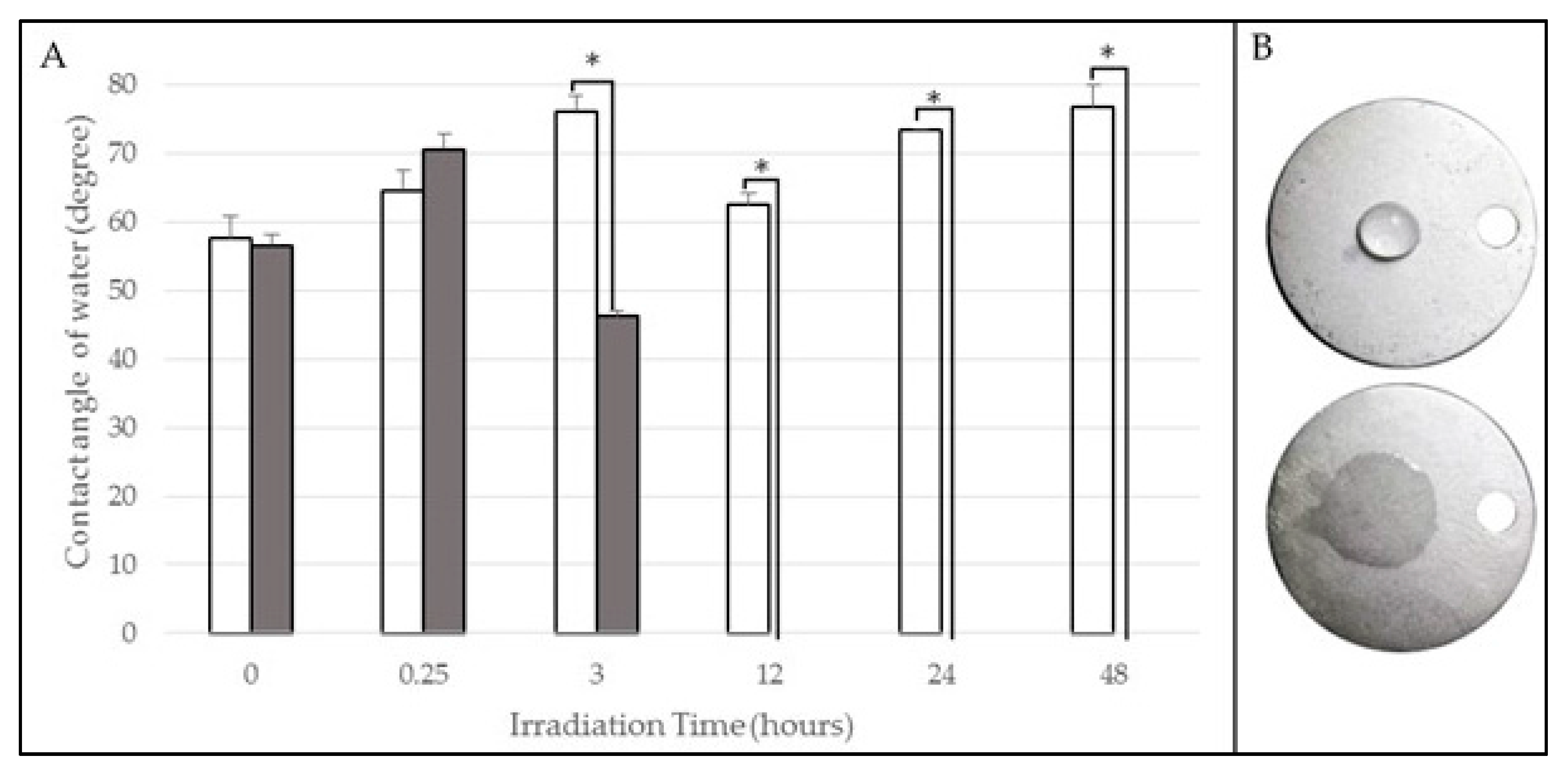
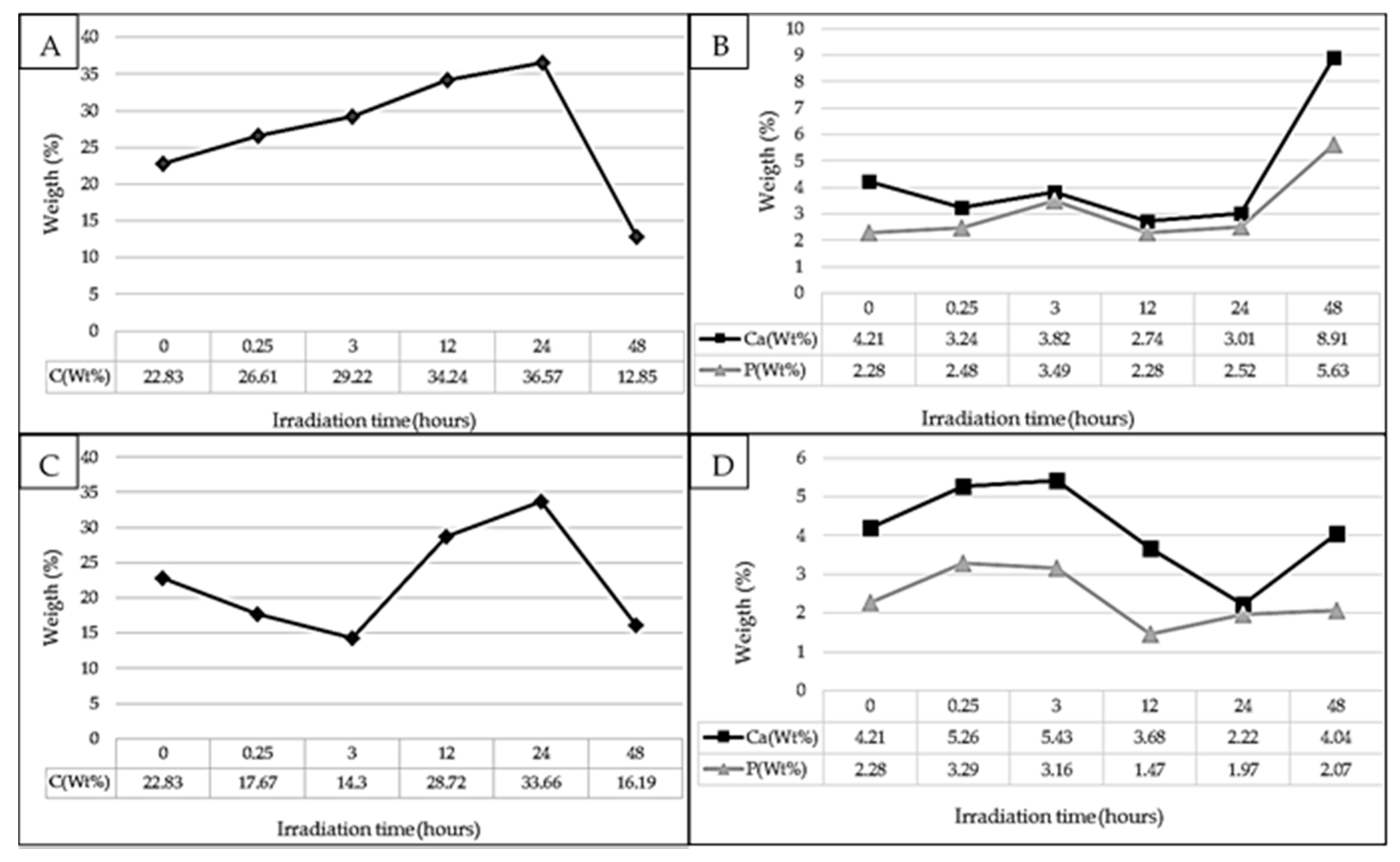
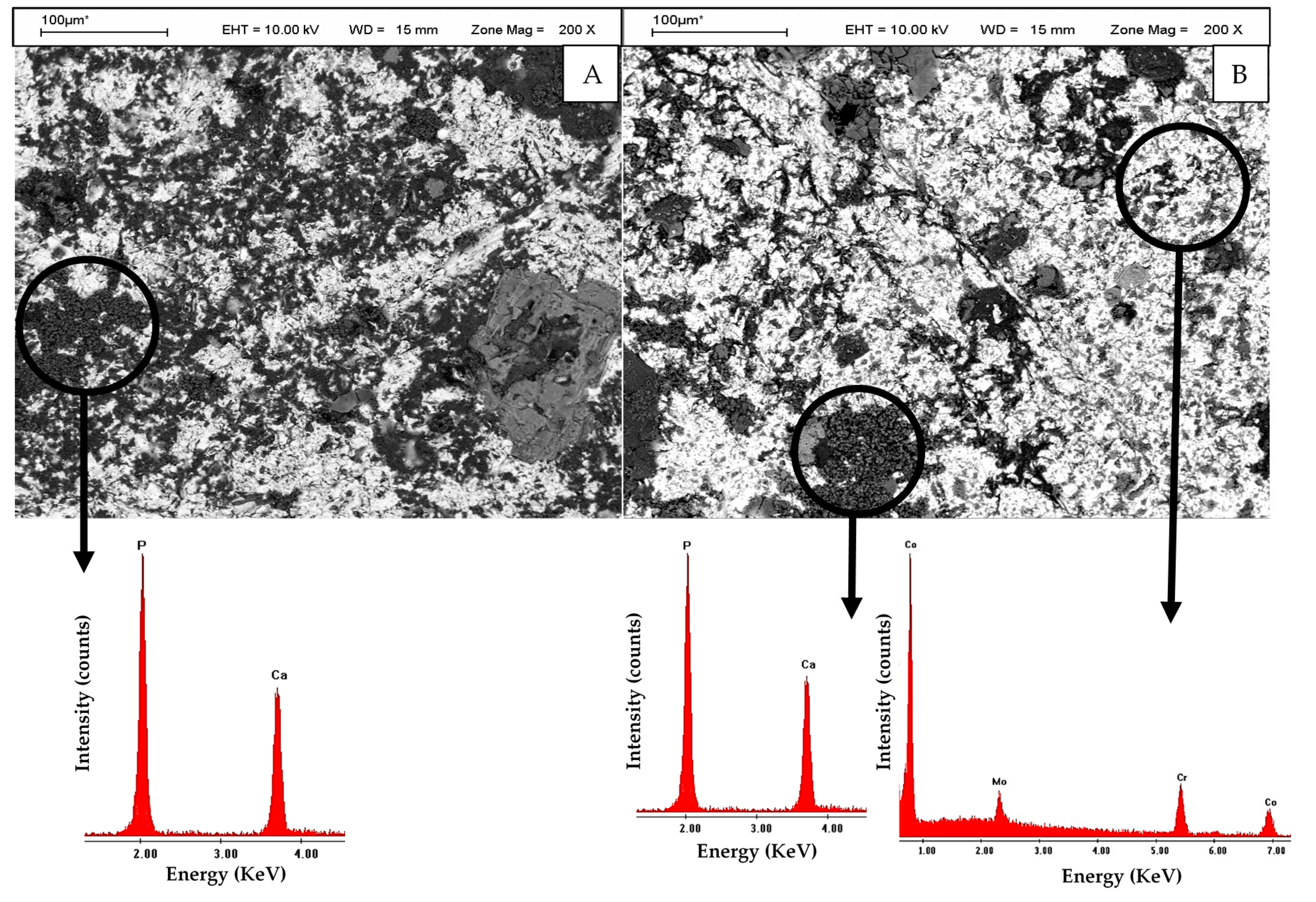


| Wavelength (nm) | Irradiation Time (h) | Contact Angle Pre-Irradiation (°) | Contact Angle Post-Irradiation (°) |
|---|---|---|---|
| 254 (UVC) | 0.25 | 65 ± 3 | 71 ± 2 |
| 3 | 76 ± 2 | 46 ± 1 | |
| 12 | 62 ± 2 | 0 ± 0 | |
| 24 | 73 ± 0 | 0 ± 0 | |
| 48 | 77 ± 3 | 0 ± 0 | |
| 365 (UVA) | 0.25 | 55 ± 2 | 56 ± 1 |
| 3 | 46 ± 1 | 50 ± 1 | |
| 12 | 63 ± 2 | 67 ± 2 | |
| 24 | 38 ± 2 | 39 ± 0 | |
| 48 | 51 ± 2 | 27 ± 2 | |
| Control | Non-irradiated | 58 ± 3 | 57 ± 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decco, O.; Zuchuat, J.; Farkas, N. Improvement of Cr-Co-Mo Membrane Surface Used as Barrier for Bone Regeneration through UV Photofunctionalization: An In Vitro Study. Materials 2017, 10, 825. https://doi.org/10.3390/ma10070825
Decco O, Zuchuat J, Farkas N. Improvement of Cr-Co-Mo Membrane Surface Used as Barrier for Bone Regeneration through UV Photofunctionalization: An In Vitro Study. Materials. 2017; 10(7):825. https://doi.org/10.3390/ma10070825
Chicago/Turabian StyleDecco, Oscar, Jésica Zuchuat, and Nicolás Farkas. 2017. "Improvement of Cr-Co-Mo Membrane Surface Used as Barrier for Bone Regeneration through UV Photofunctionalization: An In Vitro Study" Materials 10, no. 7: 825. https://doi.org/10.3390/ma10070825




