Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications
Abstract
:1. Introduction
2. General Principles of P-C Bond Formation
- (1)
- Expensive synthesis approaches,
- (2)
- Toxicity of the starting materials,
- (3)
- Non-desirable wastes or by-product,
- (4)
- Multiple purification steps,
- (5)
- Difficulty handling of the chemical materials.
3. Flame Retardants with P-Caliphatic Bonds
3.1. Substitution Reactions
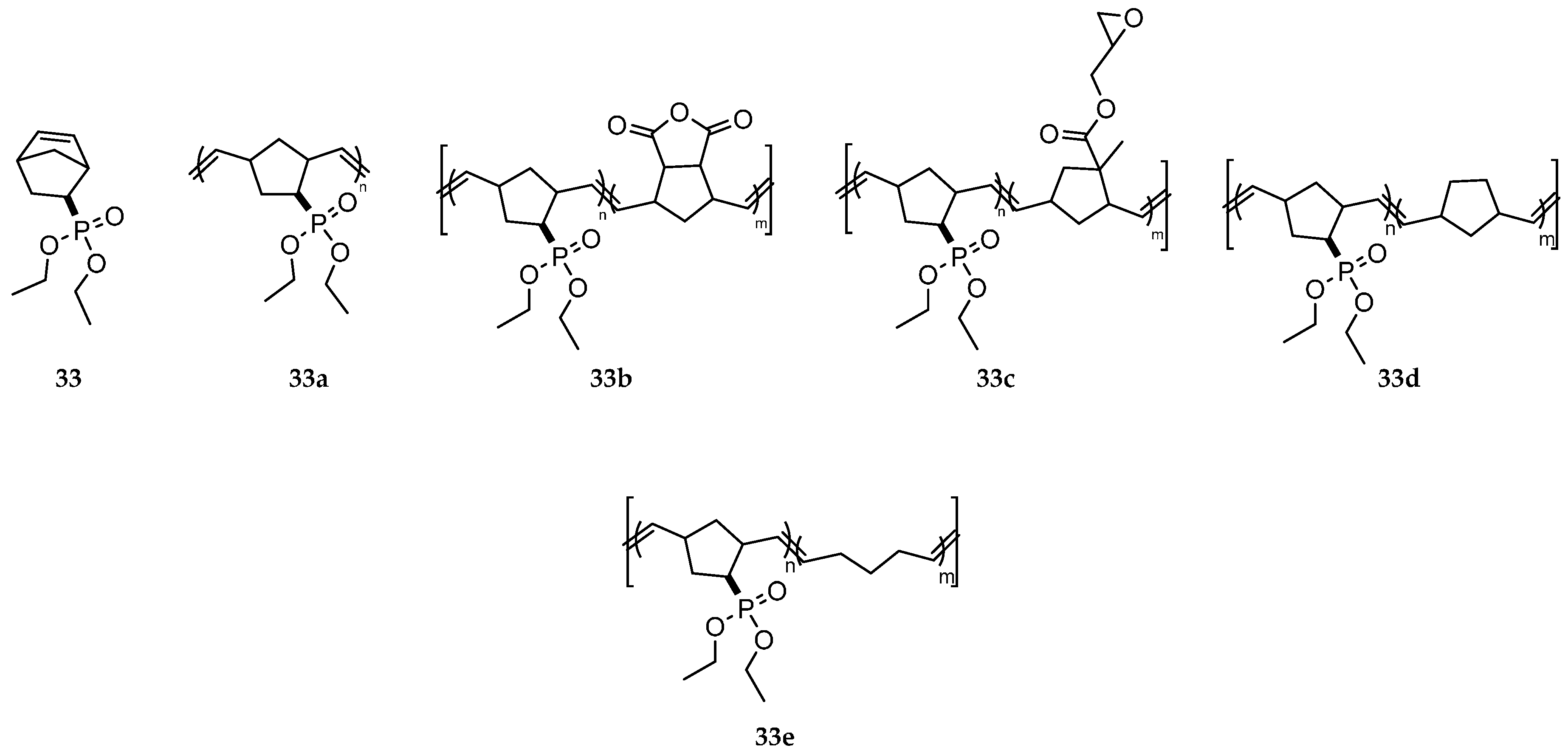
3.2. Addition Reactions
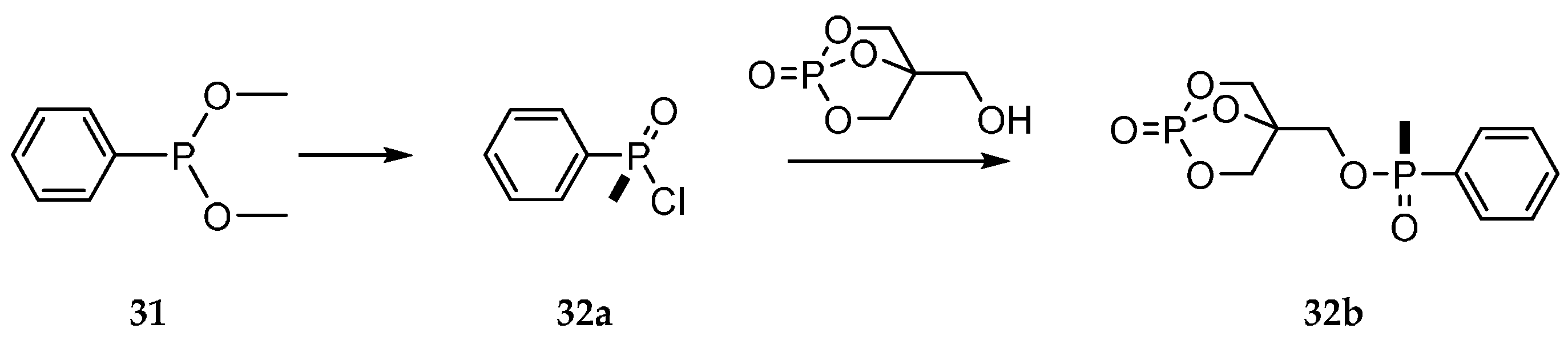
3.3. Michaelis–Arbuzov Type Reactions and Rearrangement
3.4. Grignard Type Reaction
4. Flame Retardants with P-Caromatic Bonds
4.1. Friedel–Crafts Type Reactions
4.2. General Substitution Reactions
4.3. Addition Reactions
4.4. Michaelis–Arbuzov Rearrangement
4.5. [1,3]-Sigmatropic Rearrangement
4.6. Phospho-Fries Rearrangement
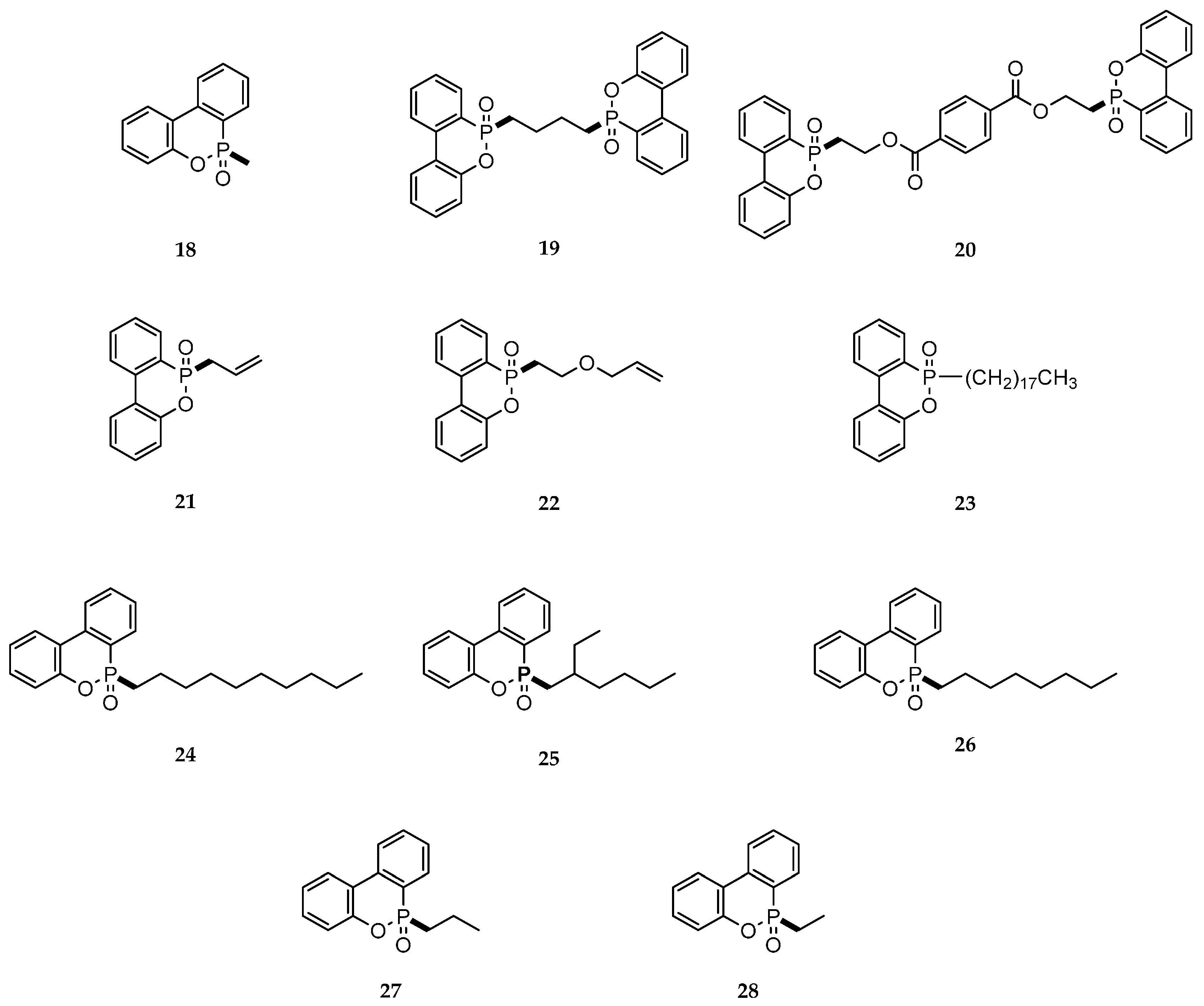
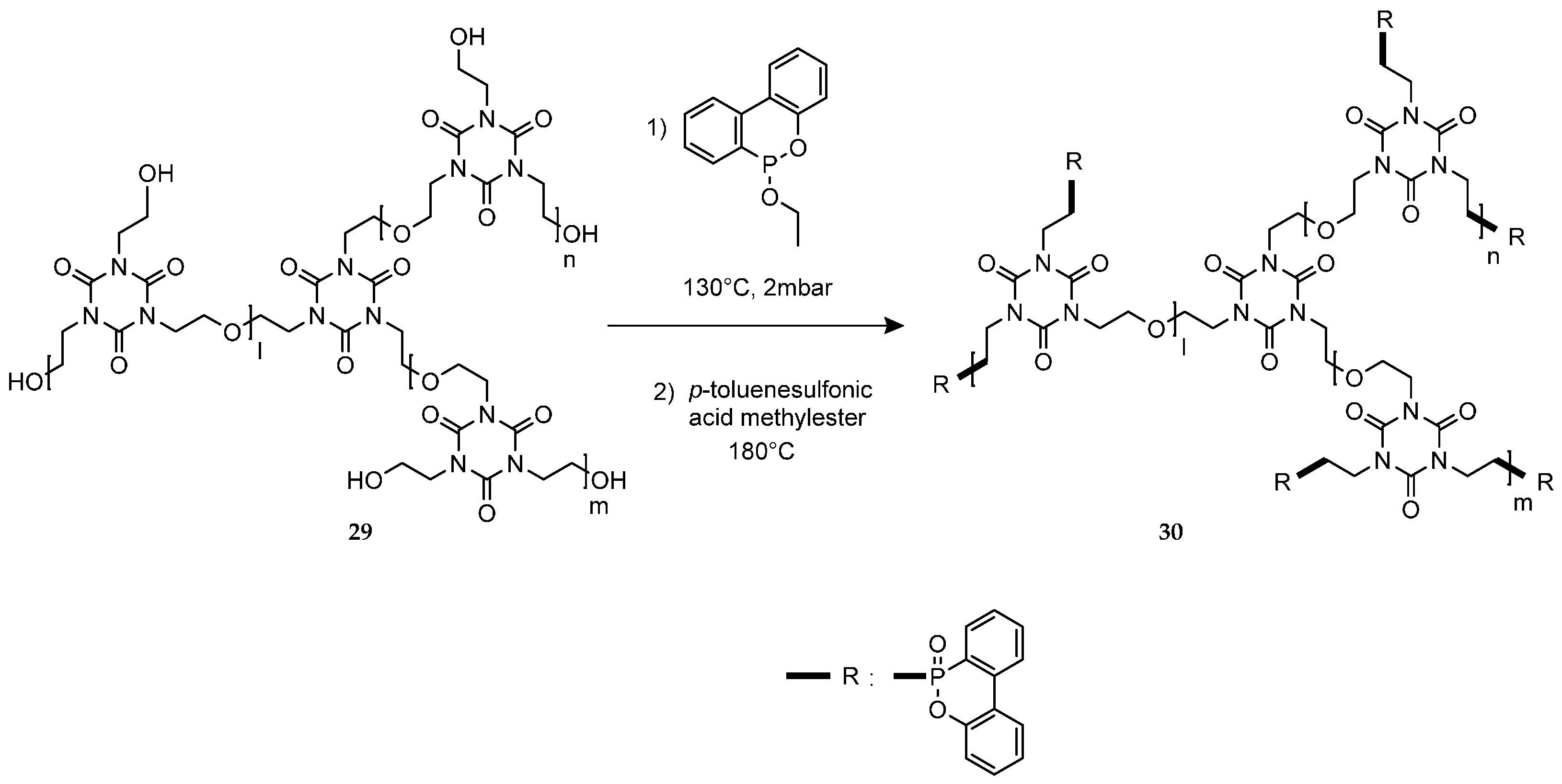
4.7. Grignard Type Reactions
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ABS | Acrylonitril butadiene styrene |
| AOPH | Aluminium–organophosphorus hybrids |
| BKZ-VB | Swiss standart fire test (vertical specimen) |
| CPPPA | 4-Carboxyphenylphenylphosphinic acid |
| DBU | 1,8-Diazabicyclo(5.4.0)undec-7-ene |
| DDM | Diaminodiphenylmethane |
| DETDA | Diethyldiamino toluene |
| DFDPS | 4,4-Difluorodiphenylsulfone |
| DGEBA | Diglycidyl ether of bisphenol-A epoxy resin |
| DIP-MS | Direct insertion probe mass spectroscopy |
| DOPO | 10-Dihydro-9-oxa-10-phosphaphenanthrene 10-oxide |
| DPPO | 2,8-Dimethyl-phenoxaphosphine-10-oxide |
| DSC | Differential scanning calorimetry |
| EDX | Energy-dispersive X-ray spectroscopy |
| EVA | Ethylene-vinyl acetate |
| FPUF | Flexible polyurethane foams |
| FR | Flame retardant |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HAPP | Hexaallyltriphosphazene |
| KMnO4 | Potassium permanganate |
| LOI | Limiting oxygen index |
| MCC | Microscale Combustion Calorimetry |
| m-CPBA | meta-Chloroperoxybenzoic acid |
| MMPP | Methyl methylphenylphosphinate |
| m.p. | Melting point |
| N2 | Nitrogen |
| PA6 | Polyamide 6 |
| PC | Polycarbonate |
| PCFC | Pyrolysis-combustion flow calorimetry |
| PEEK | Polyether ether ketone |
| PEK | Polyether ketones |
| PES | Poly(ethersulfone) |
| PHRR | Lower peak heat release rate |
| PMMD | Poly(ethylene glycol) methacrylate/methyl methacrylate/diethyl allylphosphonate |
| PN-TLCP | Thermotropic liquid crystal polymer |
| POSS | Polyhedral oligomeric silsesquioxane |
| PSU | Polysulfones |
| PU | Polyurethane |
| Py-GC-MS | Pyrolysis-gas chromatography mass spectroscopy |
| ROMP | Ring opening metathesis polymerisation |
| RPUF | Rigid polyurethane foams |
| SEM | Scanning electron microscope |
| SET | Self-extinguishing time |
| TCPP | Tris(1-chloro-2-propyl) phosphate |
| Tdx% | Temperature for x% weight loss |
| TEOS | Tetraethyl orthosilicate |
| TGA | Thermogravimetric analysis |
| THR | Total heat release |
| TPA | Terephthalic acid |
| TSR | Total smoke release |
| UL94 HB | Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances (horizontal specimen) |
| UL94 VA or UL94 VB | Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances (vertical specimen) |
| UV | Ultraviolet |
| WAXD | Wide-angle X-ray scattering |
| XPS | X-ray photoelectron spectroscopy |
References
- Demchuk, O.M.; Jasinski, R. Organophosphorus ligands: Recent developments in design, synthesis, and application in environmentally benign catalysis. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 245–253. [Google Scholar] [CrossRef]
- Majoral, J.-P. Topics in Current Chemistry; Springer: Berlin, Germany, 2002; p. 244. [Google Scholar]
- Majoral, J.-P. Topics in Current Chemistry; Springer: Berlin, Germany, 2003; p. 263. [Google Scholar]
- Demkowicz, S.; Rachon, J.; Dasko, M.; Kozak, W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in dopo-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Michal Sobkowski, A.K.; Stawinski, J. Recent advances in h-phosphonate chemistry. Part 2. Synthesis of c-phosphonate derivatives. In Topics in Current Chemistry; Montchamp, J.-L., Ed.; Springer: Berlin, Germany, 2015; Volume 361, pp. 179–216. [Google Scholar]
- Ma, J.; Yang, J.; Huang, Y.; Ke, C. Aluminum-organophosphorus hybrid nanorods for simultaneously enhancing the flame retardancy and mechanical properties of epoxy resin. J. Mater. Chem. 2012, 22, 2007–2017. [Google Scholar]
- Montchamp, J.-L. Carbon-hydrogen to carbon-phosphorus transformations. In Topics in Current Chemistry; Montchamp, J.-L., Ed.; Springer: Berlin, Germany, 2015; Volume 361, pp. 217–252. [Google Scholar]
- Menigaux, D.; Belmont, P.; Brachet, E. Light on unsaturated hydrocarbons—“Gotta heterofunctionalize them all”. Eur. J. Org. Chem. 2017, 2017, 2008–2055. [Google Scholar] [CrossRef]
- Pan, X.-Q.; Zou, J.-P.; Yi, W.-B.; Zhang, W. Recent advances in sulfur- and phosphorous-centered radical reactions for the formation of s–c and p–c bonds. Tetrahedron 2015, 71, 7481–7529. [Google Scholar] [CrossRef]
- Wauters, I.; Debrouwer, W.; Stevens, C.V. Preparation of phosphines through c–p bond formation. Beilstein J. Org. Chem. 2014, 10, 1064–1096. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, J.-S.; Han, L.-B. Dehydrogenative coupling involving p(o)-h bonds: A powerful way for the preparation of phosphoryl compounds. Dalton Trans. 2016, 45, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Pullarkat, S.A. Recent progress in palladium-catalyzed asymmetric hydrophosphination. Synthesis 2016, 48, 493–503. [Google Scholar] [CrossRef]
- Liang, S.; Neisius, M.; Mispreuve, H.; Naescher, R.; Gaan, S. Flame retardancy and thermal decomposition of flexible polyurethane foams: Structural influence of organophosphorus compounds. Polym. Degrad. Stab. 2012, 97, 2428–2440. [Google Scholar] [CrossRef]
- Butnaru, I.; Fernandez-Ronco, M.P.; Czech-Polak, J.; Heneczkowski, M.; Bruma, M.; Gaan, S. Effect of meltable triazine-dopo additive on rheological, mechanical, and flammability properties of pa6. Polymer 2015, 7, 1541–1563. [Google Scholar] [CrossRef]
- Agarwal, V.; Borisova, S.A.; Metcalf, W.W.; van der Donk, W.A.; Nair, S.K. Structural and mechanistic insights into c-p bond hydrolysis by phosphonoacetate hydrolase. Chem. Biol. 2011, 18, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.S.; Williams, H.J.; Raushel, F.M. Intermediates in the transformation of phosphonates to phosphate by bacteria. Nature 2011, 480, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Ternan, N.G.; Hamilton, J.T.; Quinn, J.P. Initial in vitro characterisation of phosphonopyruvate hydrolase, a novel phosphate starvation-independent, carbon-phosphorus bond cleavage enzyme in burkholderia cepacia pal6. Arch. Microbiol. 2000, 173, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hemberger, P.; Levalois-Grützmacher, J.; Grützmacher, H.; Gaan, S. Probing phosphorus nitride (p≡n) and other elusive species formed upon pyrolysis of dimethyl phosphoramidate. Chemistry 2017, 23, 5595–5601. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hemberger, P.; Neisius, N.M.; Bodi, A.; Grützmacher, H.; Levalois-Grützmacher, J.; Gaan, S. Elucidating the thermal decomposition of dimethyl methylphosphonate by vacuum ultraviolet (vuv) photoionization: Pathways to the po radical, a key species in flame-retardant mechanisms. Chemistry 2015, 21, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Salmeia, K.; Gaan, S.; Malucelli, G. Recent advances for flame retardancy of textiles based on phosphorus chemistry. Polymers 2016, 8, 319. [Google Scholar] [CrossRef]
- Salmeia, K.; Fage, J.; Liang, S.; Gaan, S. An overview of mode of action and analytical methods for evaluation of gas phase activities of flame retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Stawinski, J.; Kraszewski, A. How to get the most out of two phosphorus chemistries. Studies on h-phosphonates. Acc. Chem. Res. 2002, 35, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G.; Grun, A.; Balint, E.; Kiss, N.Z.; Bagi, P.; Toke, L. Green chemical syntheses and applications within organophosphorus chemistry. Struct. Chem. 2017, 28, 431–443. [Google Scholar] [CrossRef]
- Luo, K.; Yang, W.-C.; Wu, L. Photoredox catalysis in organophosphorus chemistry. Asian J. Org. Chem. 2017, 6, 350–367. [Google Scholar] [CrossRef]
- Sharova, E.V.; Artyushin, O.I.; Odinets, I.L. Synthetic routes to carbamoylmethylphosphoryl compounds—Extractants for the processing of spent nuclear fuels. Russ. Chem. Rev. 2014, 83, 95–119. [Google Scholar] [CrossRef]
- Haegele, G. Michaelis-arbuzov reactions of perhalogenated cyclobutenes with trialkyl phosphites. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2094–2109. [Google Scholar] [CrossRef]
- Keglevich, G.; Kiss, N.Z.; Balint, E.; Bagi, P.; Grun, A.; Kovacs, T.; Henyecz, R.; Abranyi-Balogh, P. Milestones in microwave-assisted organophosphorus chemistry. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1416–1420. [Google Scholar] [CrossRef]
- Enders, D.; Saint-Dizier, A.; Lannou, M.-I.; Lenzen, A. The phospha-michael addition in organic synthesis. Eur. J. Org. Chem. 2005, 2006, 29–49. [Google Scholar] [CrossRef]
- Herrera, R.P. Organocatalytic hydrophosphonylation reaction of carbonyl groups. Chem. Rec. 2017. [Google Scholar] [CrossRef] [PubMed]
- Montchamp, J.-L. Phosphinate chemistry in the 21st century: A viable alternative to the use of phosphorus trichloride in organophosphorus synthesis. Acc. Chem. Res. 2014, 47, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Troev, K.D. Structure and spectral characteristics of h-phosphonates. In Chemistry and Application of H-Phosphonates; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2006; pp. 11–22. [Google Scholar]
- Yu, X.; Ge, B.; Zhang, Y.; Wang, X.; Wang, D. Silver-mediated phosphonylation of c(sp2)−h bonds with p−h bonds: Direct c−h functionalization of ferrocenyl anilides and dialkyl phosphites under palladium- and copper-free conditions. Asian J. Org. Chem. 2016, 5, 1253–1259. [Google Scholar] [CrossRef]
- Renaud, J.-L.; Gaillard, S. Iron-catalyzed carbon-nitrogen, carbon-phosphorus, and carbon-sulfur bond formation and cyclization reactions. In Iron Catalysis ii; Bauer, E., Ed.; Springer: Cham, Switzerland, 2015; pp. 83–144. [Google Scholar]
- Guin, J.; Wang, Q.; van Gemmeren, M.; List, B. The catalytic asymmetric abramov reaction. Angew. Chem. Int. Ed. 2015, 54, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Troev, K.D. Methods for preparation and physical properties of h-phosphonates. In Chemistry and Application of H-Phosphonates; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2006; pp. 1–9. [Google Scholar]
- Balint, E.; Tajti, A.; Adam, A.; Csontos, I.; Czugler, M.; Keglevich, G.; Karaghiosoff, K.; Abranyi-Balogh, P. The synthesis of α-aryl-α-aminophosphonates and α-aryl-α-aminophosphine oxides by the microwave-assisted pudovik reaction. Beilstein J. Org. Chem. 2017, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, C.; Thaslim Basha, S.; Madhava, G.; Nayab Rasool, S.; Adam, S.; Durga Srinivasa Murthy, S.; Naga Raju, C. Synthesis, spectral characterization and bioactivity evaluation of novel α-aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 267–270. [Google Scholar] [CrossRef]
- Kamath, P.; Rajan, R.; Deshpande, S.H.; Montgomery, M.; Lal, M. An efficient synthesis of 3-phosphorylated benzoxaboroles via the pudovik reaction. Synthesis 2017. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, J.; Chen, T.; Han, L.-B. Nickel-catalyzed phosphorylation of phenol derivatives via c–o/p–h cross-coupling. J. Org. Chem. 2016, 81, 3911–3916. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, T.; Zhou, Y.; Yin, S.-F.; Han, L.-B. Mechanistic studies on the palladium-catalyzed cross dehydrogenative coupling of p(o)–h compounds with terminal alkynes: Stereochemistry and reactive intermediates. Organometallics 2015, 34, 5095–5098. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Liu, Y.; Yang, H.; Liu, X.; Wang, J.; Li, X.; Jiang, J. Copper-catalyzed p–h insertions of α-imino carbenes for the preparation of 3-phosphinoylindoles. Org. Lett. 2017, 19, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Lu, Q.; Peng, L.; Liu, C.; Wang, G.; Lei, A. Dioxygen-induced oxidative activation of a p-h bond: Radical oxyphosphorylation of alkenes and alkynes toward β-oxy phosphonates. Chem. Commun. 2016, 52, 12338–12341. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yoshimura, A.; Chen, T.; Saga, Y.; Han, L.-B. Air-induced double addition of p(o)-h bonds to alkynes: A clean and practical method for the preparation of 1,2-bisphosphorylethanes. Green Chem. 2017, 19, 1502–1506. [Google Scholar] [CrossRef]
- Rafikov, S.R.; Chelnokova, G.N.; Dzhilkibaeva, G.M. Michaelis-arbuzov reaction kinetics and the use of this reaction for polymer synthesis. Tr. Inst. Khim. Nauk Akad. Nauk Kaz. SSR 1969, 23, 80–97. [Google Scholar]
- Shaikh, R.S.; Düsel, S.J.S.; König, B. Visible-light photo-arbuzov reaction of aryl bromides and trialkyl phosphites yielding aryl phosphonates. ACS Catal. 2016, 6, 8410–8414. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, D.; Mo, F.; Zhang, Y.; Wang, J. Metal-free aromatic carbon–phosphorus bond formation via a sandmeyer-type reaction. J. Org. Chem. 2016, 81, 11603–11611. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Cai, R.; Xu, C.; Chen, H.; Shi, X. Nucleophile promoted gold redox catalysis with diazonium salts: C-br, c-s and c-p bond formation through catalytic sandmeyer coupling. Chem. Sci. 2016, 7, 6190–6196. [Google Scholar] [CrossRef]
- Yu, R.; Chen, X.; Martin, S.F.; Wang, Z. Differentially substituted phosphines via decarbonylation of acylphosphines. Org. Lett. 2017, 19, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kotani, S.; Sugiura, M.; Nakajima, M. First asymmetric abramov-type phosphonylation of aldehydes with trialkyl phosphites catalyzed by chiral lewis bases. Tetrahedron 2008, 64, 6415–6419. [Google Scholar] [CrossRef]
- Zhang, P.; Fan, H.; Tian, S.; Chen, Y.; Yan, J. Synergistic effect of phosphorus-nitrogen and silicon-containing chain extenders on the mechanical properties, flame retardancy and thermal degradation behavior of waterborne polyurethane. RSC Adv. 2016, 6, 72409–72422. [Google Scholar] [CrossRef]
- Bisaro, F.; Gouverneur, V. Desymmetrization by direct cross-metathesis producing hitherto unreachable p-stereogenic phosphine oxides. Tetrahedron 2005, 61, 2395–2400. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Zhang, S. Enhancing polymer flame retardancy by reaction with phosphorylated polyols. Part 2. Cellulose treated with a phosphonium salt urea condensate (proban cc) flame retardant. Fire Mater. 2002, 26, 173–182. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, Y.J.; Jiang, P. Phosphonium sulfonates as flame retardants for polycarbonate. Polym. Degrad. Stab. 2016, 130, 165–172. [Google Scholar] [CrossRef]
- Panmand, D.S.; Tiwari, A.D.; Panda, S.S.; Monbaliu, J.-C.M.; Beagle, L.K.; Asiri, A.M.; Stevens, C.V.; Steel, P.J.; Hall, C.D.; Katritzky, A.R. New benzotriazole-based reagents for the phosphonylation of various n-, o-, and s-nucleophiles. Tetrahedron Lett. 2014, 55, 5898–5901. [Google Scholar] [CrossRef]
- Liu, Z.; De Campo, F. Conjugated Diene Phosphinate Compounds, Preparation Method and Use Thereof. U.S. Patent 20120208975 A1, 2014. [Google Scholar]
- Liu, Z.; De Campo, F. N-Substituted Acrylamides, Preparation Method and Use Thereof. Patent WO2011050534A1, 2011. [Google Scholar]
- Neisius, N.M.; Liang, S.; Gaan, S.; Mispreuve, H.; Nascher, R.; Rentsch, D. Flame retardants for flexible polyurethane foams: Structure-property-relationship studies. In Proceedings of the 243rd ACS National Meeting & Exposition, San Diego, CA, USA, 2012. [Google Scholar]
- Çakmakçı, E.; Mülazim, Y.; Kahraman, M.V.; Apohan, N.K. Flame retardant thiol-ene photocured coatings. React. Funct. Polym. 2011, 71, 36–41. [Google Scholar] [CrossRef]
- Zeytuncu, B.; Çakmakçı, E.; Kahraman, M.V. Allyl phosphonium salt-modified clay for photocured coatings: Influence on the properties of polyester acrylate-based coatings. Polym. Compos. 2015, 36, 946–954. [Google Scholar] [CrossRef]
- Mai, N.L.; Koo, Y.-M. Computer-aided design of ionic liquids for high cellulose dissolution. ACS Sustain. Chem. Eng. 2016, 4, 541–547. [Google Scholar] [CrossRef]
- Yang, L.; Han, X.Y.; Li, L.L.; Bi, C.L.; Tang, X.J.; Zhang, B.G. Synthesis of aluminium diethylphosphinate by gas-liquid free radical addition reaction under atmospheric pressure. Adv. Mater. Res. 2011, 194–196, 2237–2240. [Google Scholar] [CrossRef]
- Lee, M.S.; Ju, B.J.; Lee, B.G.; Hong, S.H. Non-Halogen Flameproof Resin Composition. U.S. Patent 20100152345, 2008. [Google Scholar]
- Liu, X.; Salmeia, K.A.; Rentsch, D.; Hao, J.; Gaan, S. Thermal decomposition and flammability of rigid pu foams containing some dopo derivatives and other phosphorus compounds. J. Anal. Appl. Pyrolysis 2017, 124, 219–229. [Google Scholar] [CrossRef]
- Benin, V.; Cui, X.; Morgan, A.B.; Seiwert, K. Synthesis and flammability testing of epoxy functionalized phosphorous-based flame retardants. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Worku, A.Z.; Mullins, M.J. Phosphorus-Containing Compounds Useful for Making Halogen-Free, Ignition-Resistant Polymers. Patent WO2012161926, 2012. [Google Scholar]
- Li, S.; Wang, J.; Li, Y.; Wu, G.; Wang, Y.; Wang, W.; Guo, J. Preparation and applications of the tertiary copolymer poly(ethylene glycol) methacrylate/methyl methacrylate/diethyl allylphosphonate. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Xie, C.; Gao, A.; Chang, Z.; Kwon Oh, J.; Yang, P.; Li, P. Phosphonated homopolymers and copolymers via ring opening metathesis polymerization: Tg tuning, flame resistance, and photolithography. J. Polym. Sci. Part A 2016, 54, 1396–1408. [Google Scholar] [CrossRef]
- König, A.; Kroke, E. Flame retardancy working mechanism of methyl-dopo and mppp in flexible polyurethane foam. Fire Mater. 2012, 36, 1–15. [Google Scholar] [CrossRef]
- Artner, J.; Ciesielski, M.; Ahlmann, M.; Walter, O.; Döring, M.; Perez, R.M.; Altstädt, V.; Sandler, J.K.W.; Schartel, B. A novel and effective synthetic approach to 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (dopo) derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 2131–2148. [Google Scholar] [CrossRef]
- Dittrich, U.; Just, B.; Doring, M.; Ciesielski, M. Process for the Preparation of 9, 10-Dihydro-9-oxa-10-organylphosphaphenanthrene-10-oxide and Derivatives of the Same Substituted on the Phenyl Groups. U.S. Patent 20050038279, 2005. [Google Scholar]
- Zang, L.; Wagner, S.; Ciesielski, M.; Müller, P.; Döring, M. Novel star-shaped and hyperbranched phosphorus-containing flame retardants in epoxy resins. Polym. Adv. Technol. 2011, 22, 1182–1191. [Google Scholar] [CrossRef]
- Hoang, D.; Kim, J. Flame-retarding behaviors of novel spirocyclic organo-phosphorus compounds based on pentaerythritol. Macromol. Res. 2015, 23, 579–591. [Google Scholar] [CrossRef]
- Nguyen, C.; Lee, M.; Kim, J. Relationship between structures of phosphorus compounds and flame retardancies of the mixtures with acrylonitrile-butadiene-styrene and ethylene-vinyl acetate copolymer. Polym. Adv. Technol. 2011, 22, 512–519. [Google Scholar] [CrossRef]
- Kang, N.; Du, Z.; Li, H.; Zhang, C. Synthesis and characterization of p/si flame retardant and its application in epoxy systems. Polym. Adv. Technol. 2012, 23, 1329–1334. [Google Scholar] [CrossRef]
- Karataş, S.; Hoşgör, Z.; Kayaman-Apohan, N.; Güngör, A. Preparation and characterization of phosphine oxide containing organosilica hybrid coatings by photopolymerization and sol–gel process. Prog. Org. Coat. 2009, 65, 49–55. [Google Scholar] [CrossRef]
- Worku, A.Z.; Mullins, M.J. Phosphorus-Containing Compounds Useful for Making Halogen-Free, Ignition-Resistant Polymers. U.S. Patent 20160319107 A1, 2016. [Google Scholar]
- Su, W.C.; Sheng, C.S. Method for Preparing a Biphenylphosphonate Compound. U.S. Patent 20050101793, 2005. [Google Scholar]
- Tian, C.; Liu, P.; Ye, G.; Xu, J. Soluble polyarylates containing phosphorus in main chains with thermal stability. J. Appl. Polym. Sci. 2013, 130, 3521–3529. [Google Scholar] [CrossRef]
- Campbell, I.G.M.; Way, J.K. Synthesis and stereochemistry of heterocyclic phosphorus compounds. Part I. Preparation of (+)- and (-)-10-p-dimethylaminophenyl-9,10-dihydro-9-aza-10-phosphaphenanthrene. J. Chem. Soc. 1960, 5034–5041. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Kubba, V.P. New heteroaromatic compounds. Part vi. Novel heterocyclic compounds of phosphorus. J. Am. Chem. Soc. 1960, 82, 5685–5688. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Ciesielski, M.; Zevaco, T.; Doering, M. New phosphacyclic molecules and their application as flame retardants for epoxy resins. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 989–996. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Zevaco, T.; Ciesielski, M.; Walter, O.; Doring, M. Synthesis and reactivity of 6h-dibenzo[c,e][1,2]oxaphosphinine 6-sulfide, a novel thiophosphacyclic molecule. Heterocycles 2011, 83, 743–753. [Google Scholar]
- Schäfer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Döring, M. Synthesis and properties of flame-retardant epoxy resins based on dopo and one of its analog dppo. J. Appl. Polym. Sci. 2007, 105, 685–696. [Google Scholar] [CrossRef]
- Schäfer, A.; Seibold, S.; Walter, O.; Döring, M. Novel high tg flame retardancy approach for epoxy resins. Polym. Degrad. Stab. 2008, 93, 557–560. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Sun, Q.-X.; Wu, J.-S.; Wang, L.-S. Solubilities of 2-carboxyethylphenylphosphinic acid and 4-carboxyphenylphenylphosphinic acid in water. J. Chem. Eng. Data 2003, 48, 1073–1075. [Google Scholar] [CrossRef]
- Sacristán, M.; Ronda, J.C.; Galià, M.; Cádiz, V. Phosphorus-containing soybean-oil copolymers: Cross-metathesis of fatty acid derivatives as an alternative to phosphorus-containing reactive flame retardants. J. Appl. Polym. Sci. 2011, 122, 1649–1658. [Google Scholar] [CrossRef]
- Mandal, B.; Filler, R. Thermal Runaway Inhibitors. U.S. Patent 20030003358 A1, 2003. [Google Scholar]
- Li, X.; Chen, H.; Wang, W.; Liu, Y.; Zhao, P. Synthesis of a formaldehyde-free phosphorus-nitrogen flame retardant with multiple reactive groups and its application in cotton fabrics. Polym. Degrad. Stab. 2015, 120, 193–202. [Google Scholar] [CrossRef]
- Zuo, J.-D.; Liu, S.-M.; Sheng, Q. Synthesis and application in polypropylene of a novel of phosphorus-containing intumescent flame retardant. Molecules 2010, 15, 7593–7602. [Google Scholar] [CrossRef] [PubMed]
- Chun Shan, W.; Ching Hsuan, L. Synthesis and properties of phosphorus containing polyarylates derived from 2-(6-oxido-6h-dibenzoxaphosphorin-6-yl)-1,4-dihydroxy phenylene. Polymer 1999, 40, 4387–4398. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, Y.; Zhang, L.; Zhen, X.; Zhao, Y. Synthesis of flame-retardant phosphaphenanthrene derivatives with high phosphorus contents. Aust. J. Chem. 2014, 67, 1688–1692. [Google Scholar] [CrossRef]
- Bai, Z.; Song, L.; Hu, Y.; Yuen, R.K.K. Preparation, flame retardancy, and thermal degradation of unsaturated polyester resin modified with a novel phosphorus containing acrylate. Ind. Eng. Chem. Res. 2013, 52, 12855–12864. [Google Scholar] [CrossRef]
- Xia, Y.; Ge, X.; Hou, C.; Wang, C.; Ma, W. Synthesis and characterization of a new kind of flame-retardant thermotropic liquid crystal containing 1,8-octanediamine softsegment. Polym.-Plast. Technol. Eng. 2013, 52, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H. Thermal degradation behaviors of epoxy resin/poss hybrids and phosphorus-silicon synergism of flame retardancy. J. Polym. Sci. Part B 2010, 48, 693–705. [Google Scholar] [CrossRef]
- Ho, T.-H.; Hwang, H.-J.; Shieh, J.-Y.; Chung, M.-C. Thermal, physical and flame-retardant properties of phosphorus-containing epoxy cured with cyanate ester. React. Funct. Polym. 2009, 69, 176–182. [Google Scholar] [CrossRef]
- Seibold, S.; Schäfer, A.; Lohstroh, W.; Walter, O.; Döring, M. Phosphorus-containing terephthaldialdehyde adducts—Structure determination and their application as flame retardants in epoxy resins. J. Appl. Polym. Sci. 2008, 108, 264–271. [Google Scholar] [CrossRef]
- Hoffmann, T.; Pospiech, D.; Häußler, L.; Sahre, K.; Komber, H.; Harnisch, C.; Auf Der Landwehr, M.; Schäfer, A.; Döring, M. Phosphorus-containing polysulfones—A comparative study. High Perform. Polym. 2010, 22, 715–741. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Wang, C.-S. Synthesis and luminescent characteristics of novel phosphorus containing light-emitting polymers. Polymer 2001, 42, 1035–1045. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Vlad-Bubulac, T.; Hamciuc, C.; Aflori, M. Synthesis and properties of novel phosphorus-containing thermotropic liquid crystalline copoly(ester imide)s. J. Polym. Sci. Part A 2010, 48, 5391–5403. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Vlad-Bubulac, T.; Hamciuc, C.; Doroftei, F. Synthesis and characterization of new phosphorus-containing poly(ester imide)s. High Perform. Polym. 2010, 22, 916–929. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Yu, Q.; Wu, Q.; Gao, J. Synthesis and property of flame retardant epoxy resins modified with 2-(diphenylphosphinyl)-1,4-benzenediol. Chem. Res. Chin. Univ. 2014, 30, 868–873. [Google Scholar] [CrossRef]
- Chen, G.-C.H.; Huang, C.J.; Tu, A.P.; Hwang, K.Y. Phosphorus-Containing Compound and Method for Preparing the Same. US20100105939, 2009. [Google Scholar]
- Benin, V.; Gardelle, B.; Morgan, A.B. Heat release of polyurethanes containing potential flame retardants based on boron and phosphorus chemistries. Polym. Degrad. Stab. 2014, 106, 108–121. [Google Scholar] [CrossRef]
- Ivan, N.; Benin, V.; Morgan, A.B. Preparation of phosphonoterephthalic acids via palladium-catalyzed coupling of aromatic iodoesters. Synth. Commun. 2013, 43, 1831–1836. [Google Scholar] [CrossRef]
- Finocchiaro, P.; Consiglio, G.A.; Imbrogiano, A.; Failla, S. Synthesis and characterization of new organic phosphonates monomers as flame retardant additives for polymers. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1689–1701. [Google Scholar] [CrossRef]
- Dumitrascu, A.; Howell, B.A. Flame-retarding vinyl polymers using phosphorus-functionalized styrene monomers. Polym. Degrad. Stab. 2011, 96, 342–349. [Google Scholar] [CrossRef]
- Rectenwald, M.F.; Gaffen, J.R.; Rheingold, A.L.; Morgan, A.B.; Protasiewicz, J.D. Phosphoryl-rich flame-retardant ions (frions): Towards safer lithium-ion batteries. Angew. Chem. Int. Ed. 2014, 53, 4173–4176. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, B.; Redmore, D. O-hydroxyaryl diphosphonic acids. J. Org. Chem. 1984, 49, 4018–4021. [Google Scholar] [CrossRef]
- Ghosh, A.; Bera, D.; Wang, D.-Y.; Komber, H.; Mohanty, A.K.; Banerjee, S.; Voit, B. Synthesis, characterization and properties of new semifluorinated poly(arylene ether phosphine oxide)s. Macromol. Mater. Eng. 2012, 297, 145–154. [Google Scholar] [CrossRef]
- Yoon, T.H.; Jeong, K.; Jo, Y.J. Triarylphosphine Oxide Derivatives Containing Fluorine Substituent and Preparing Method Thereof. U.S. Patent 20020095057 A1, 2002. [Google Scholar]

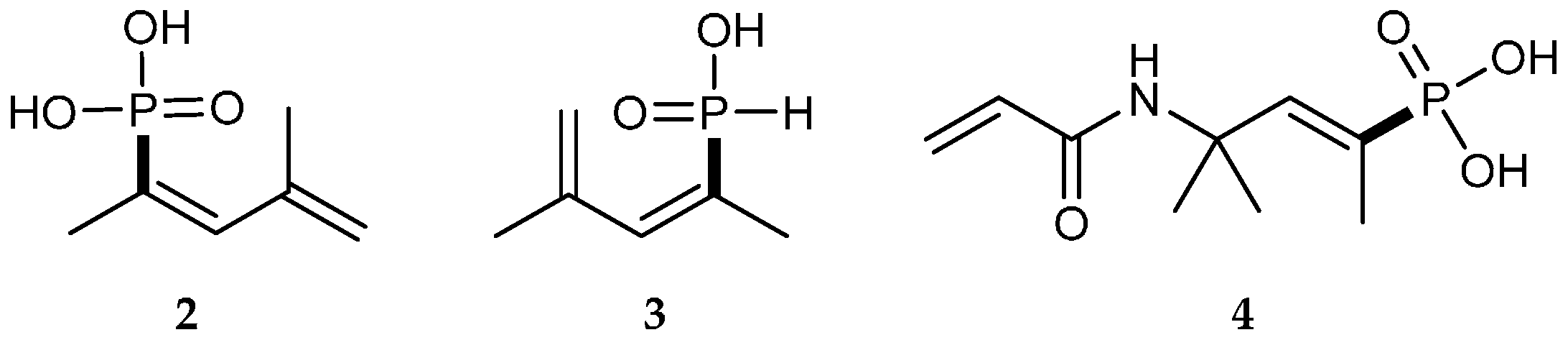

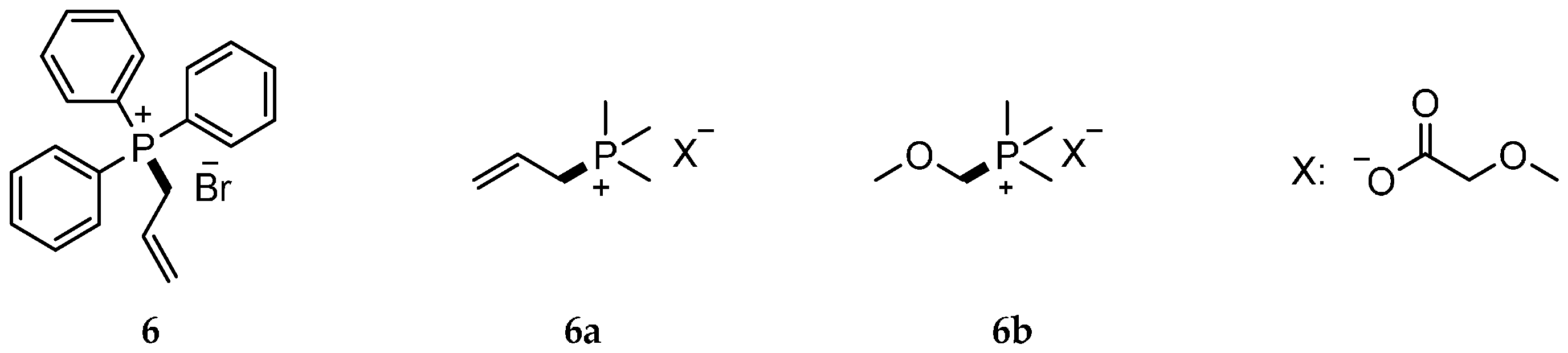
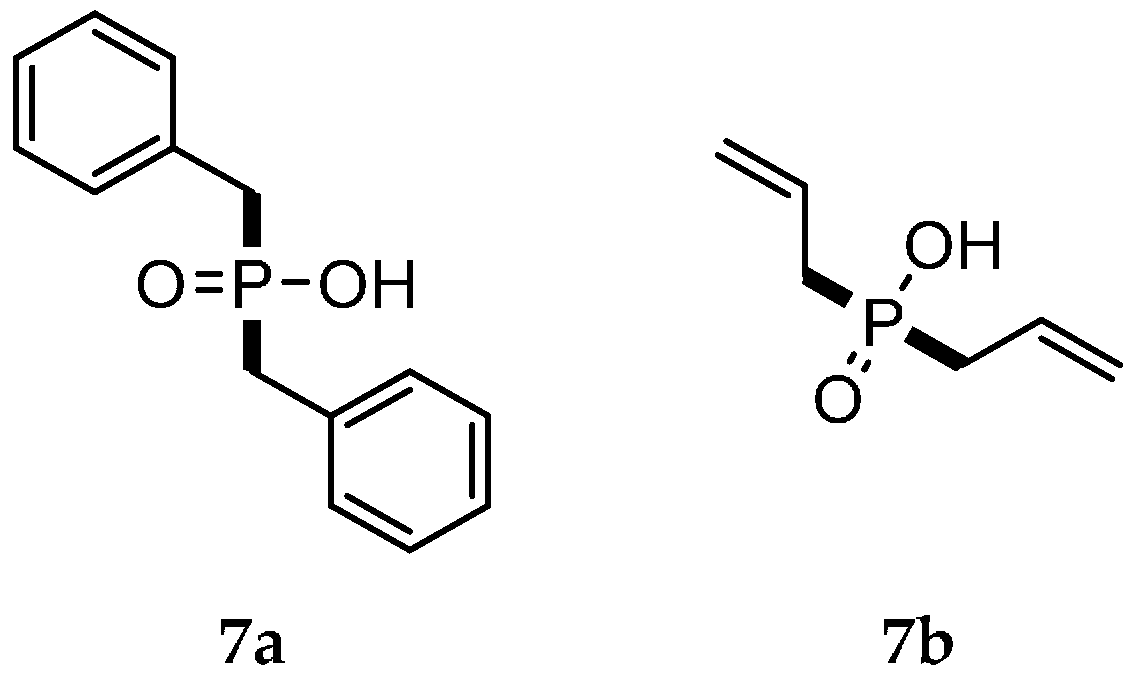
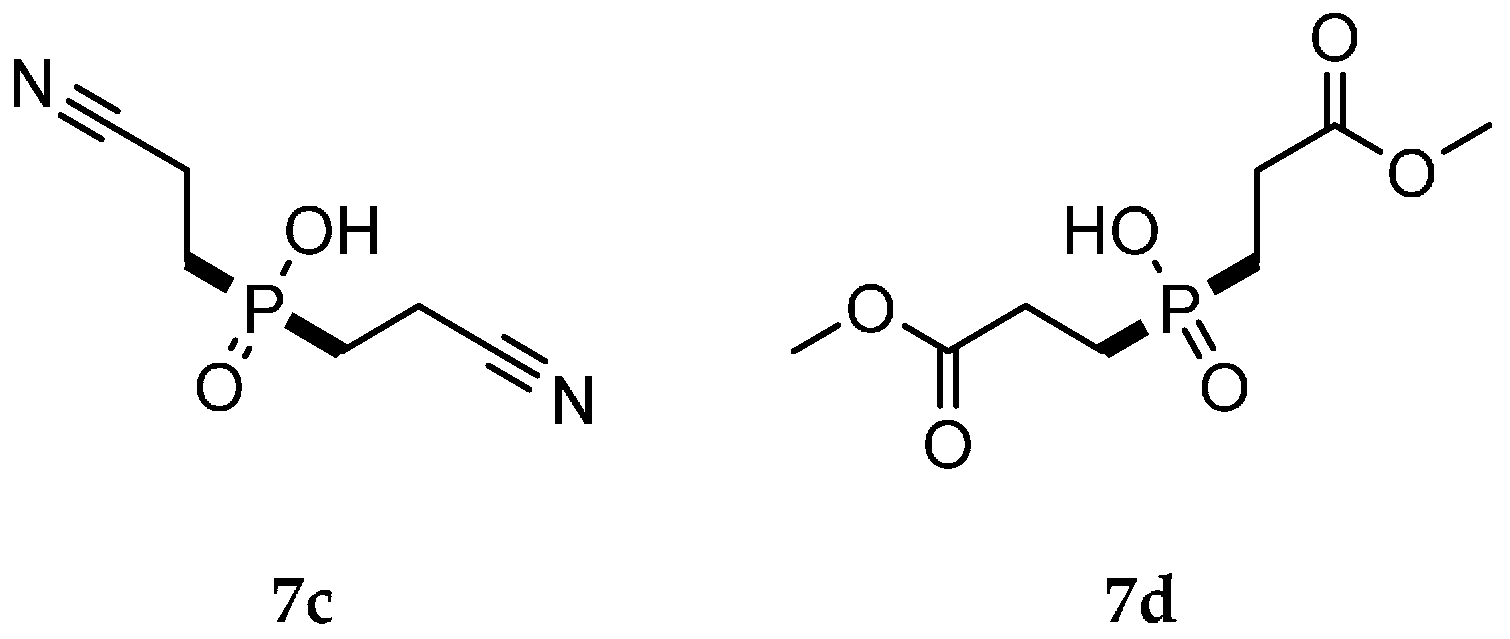
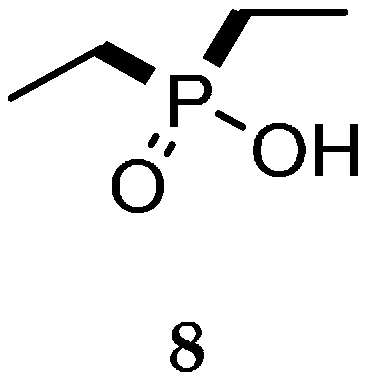
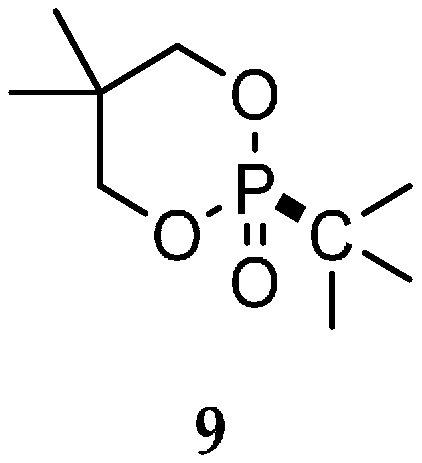
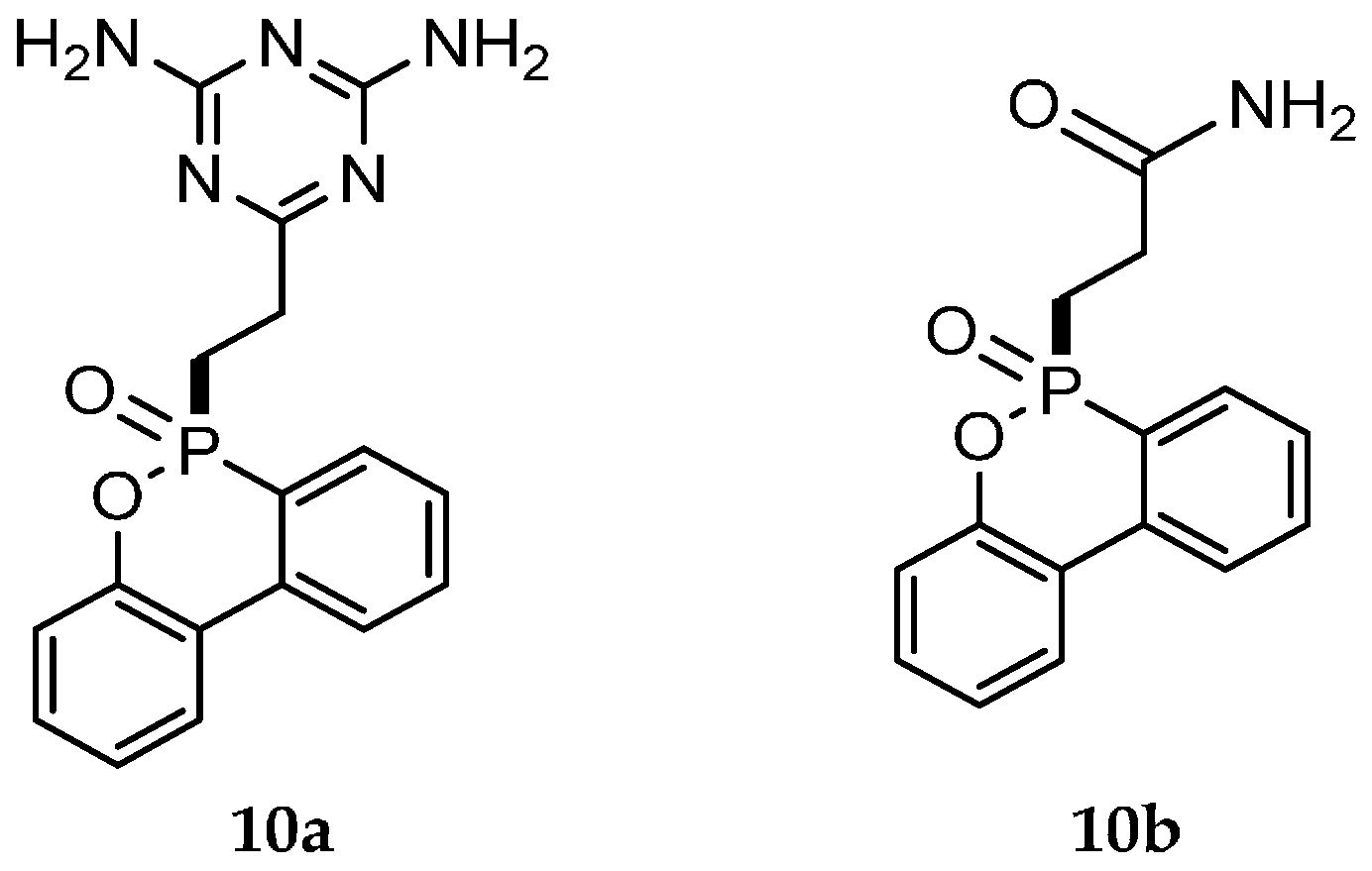


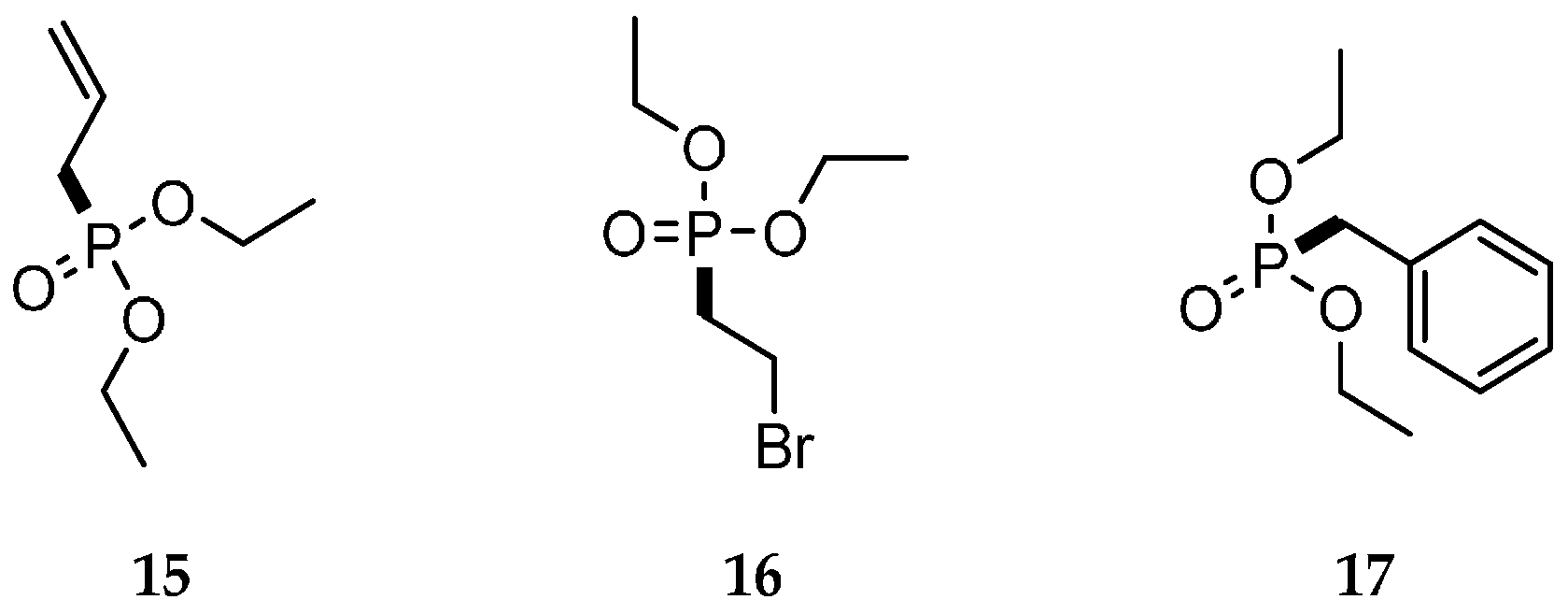
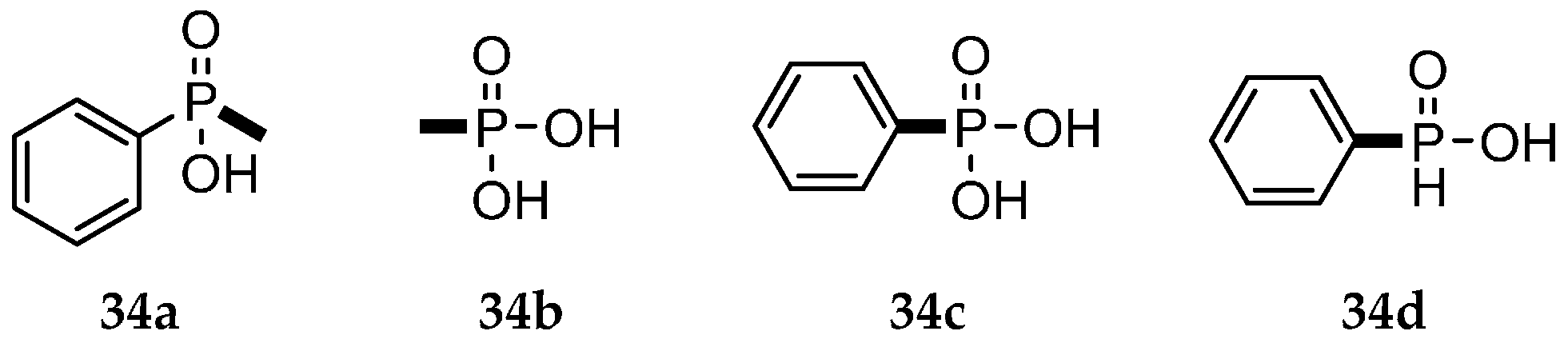
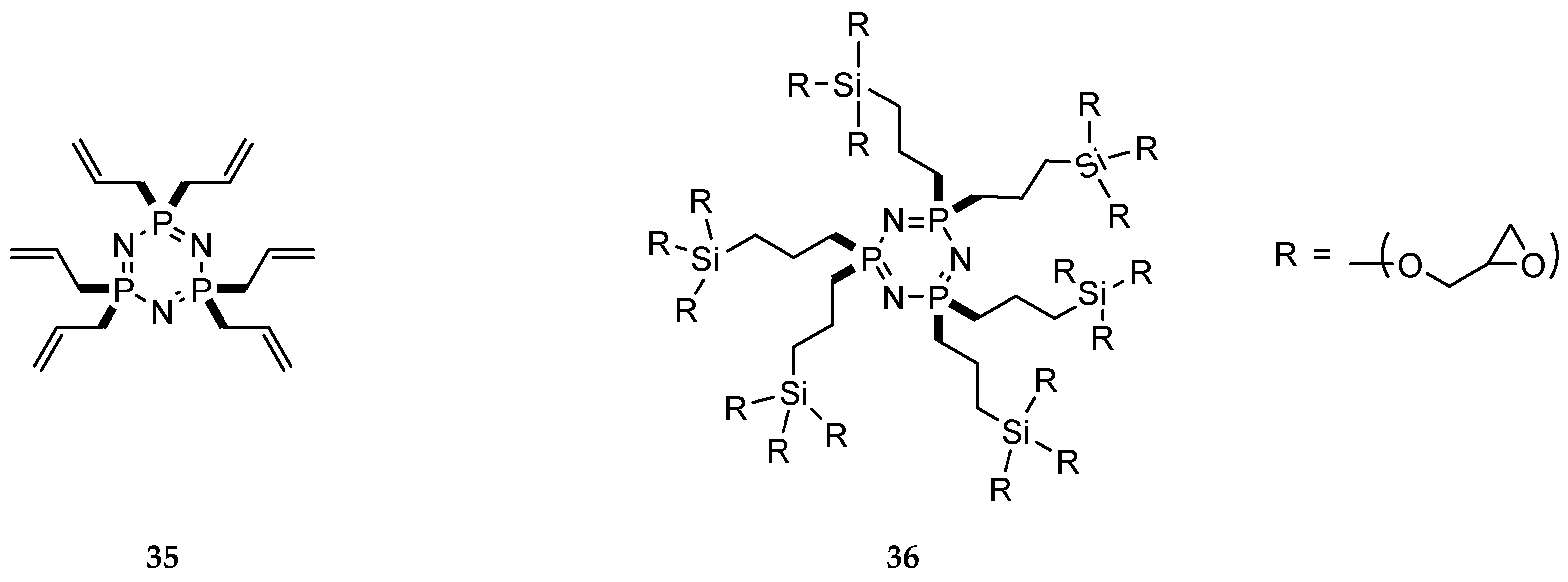
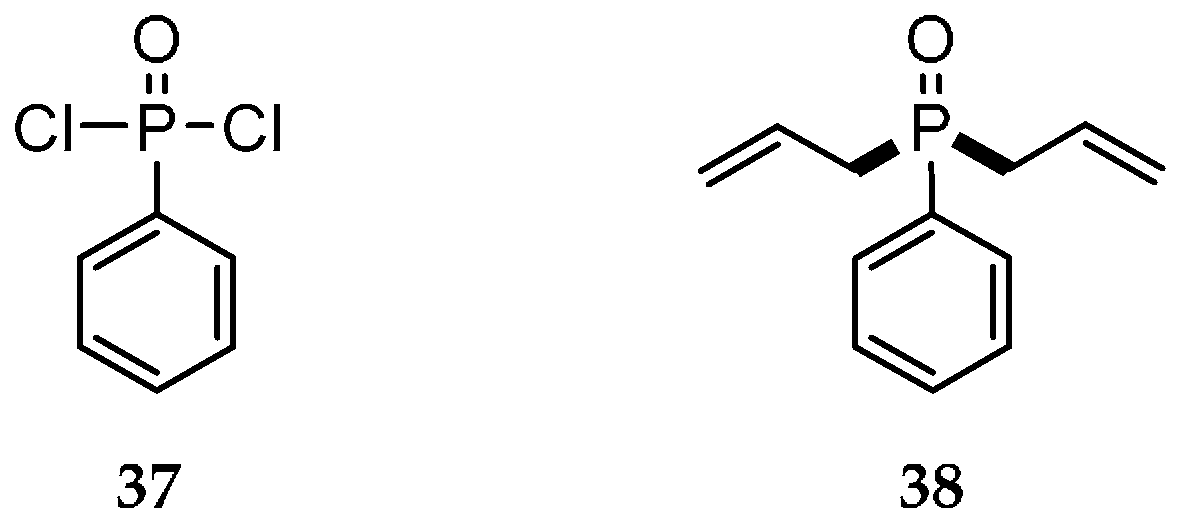
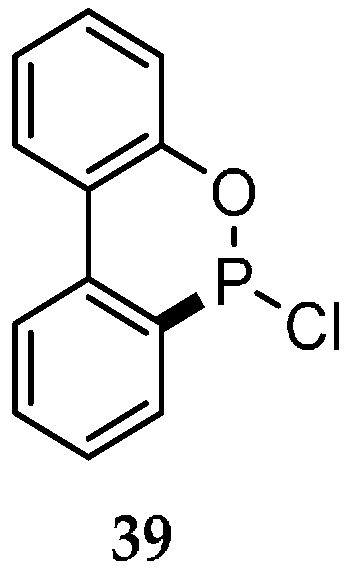
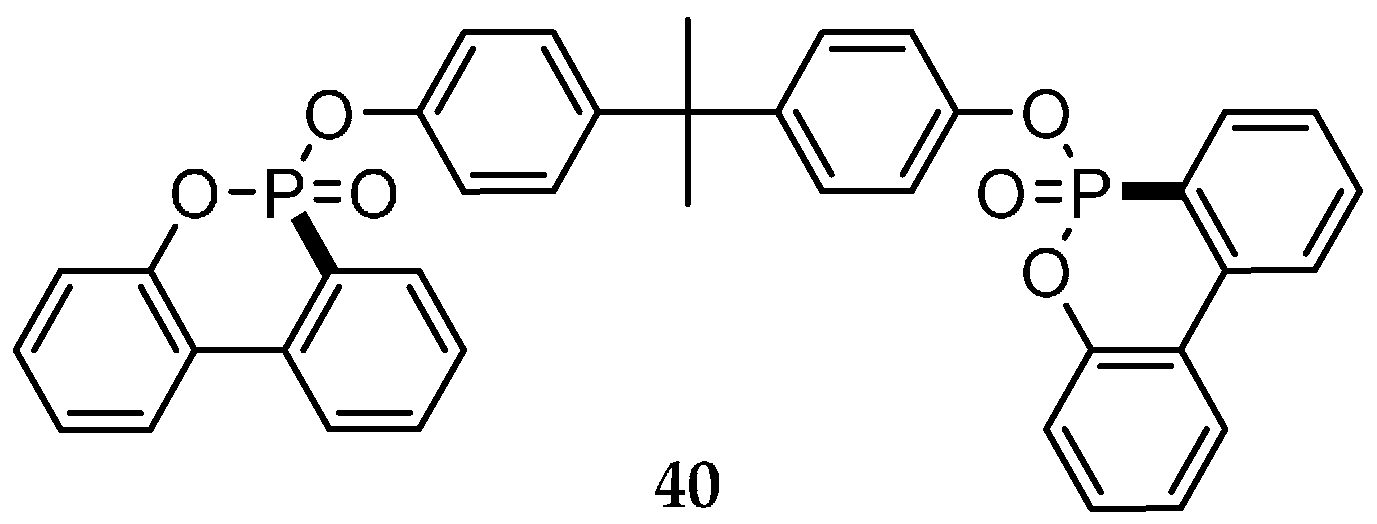
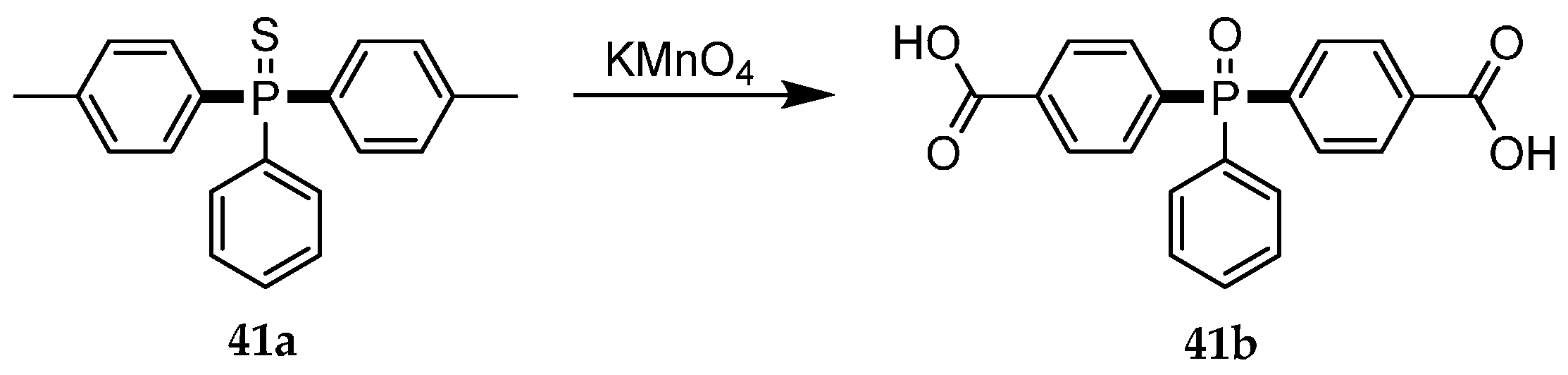
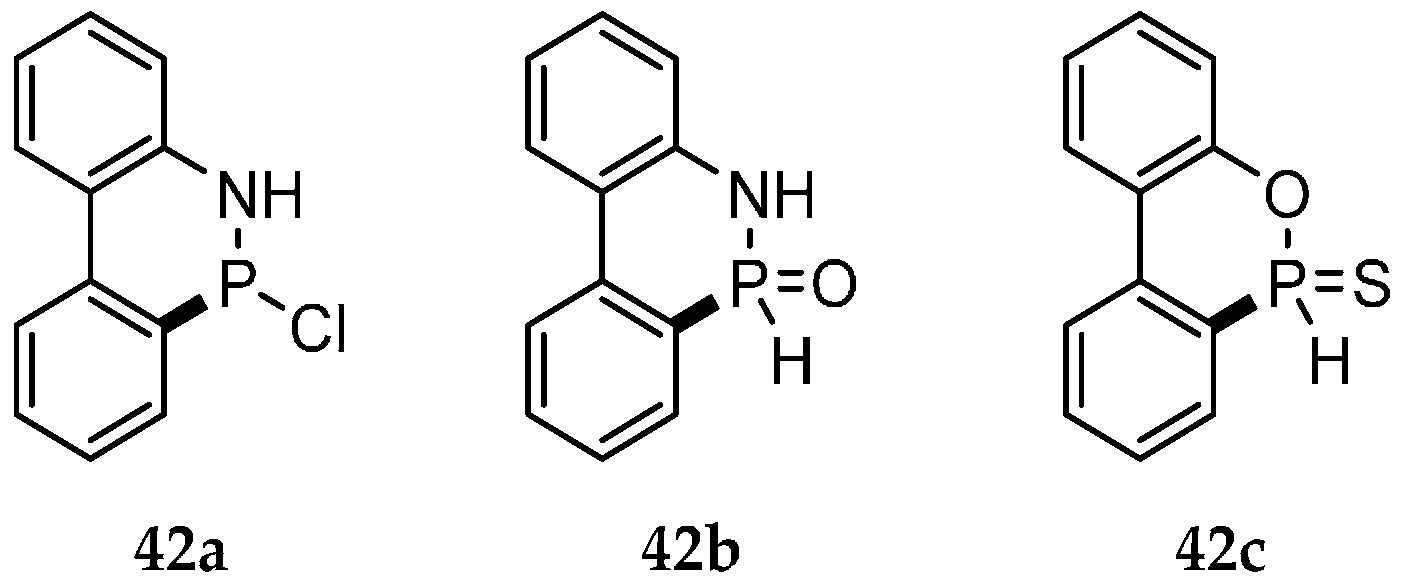
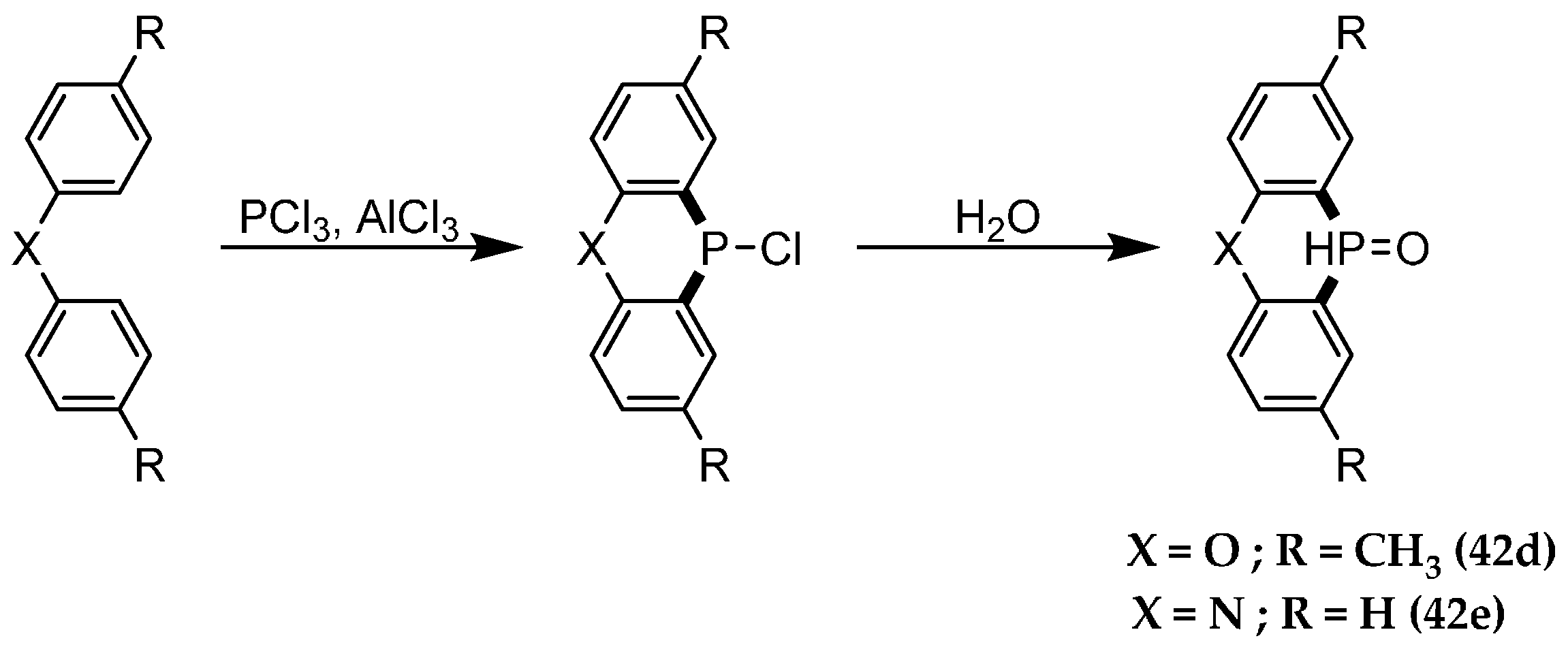
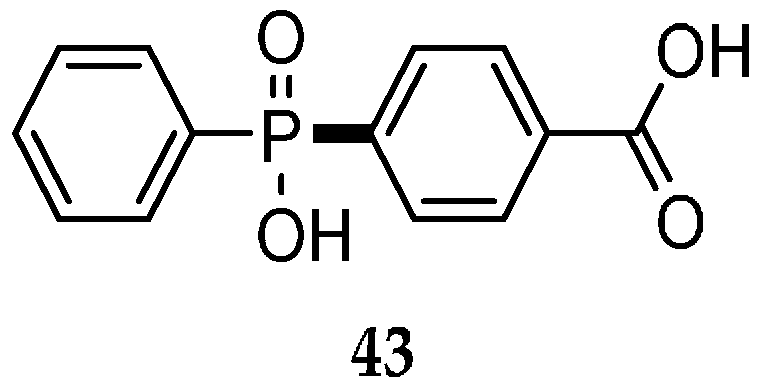
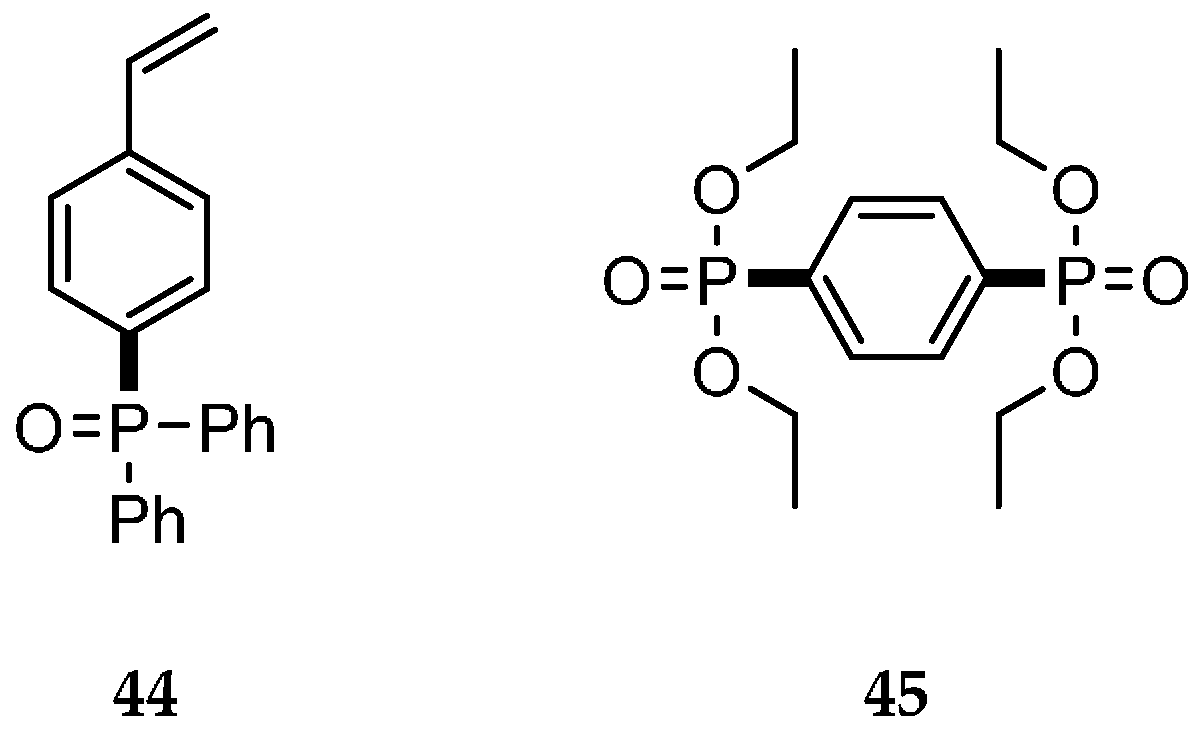
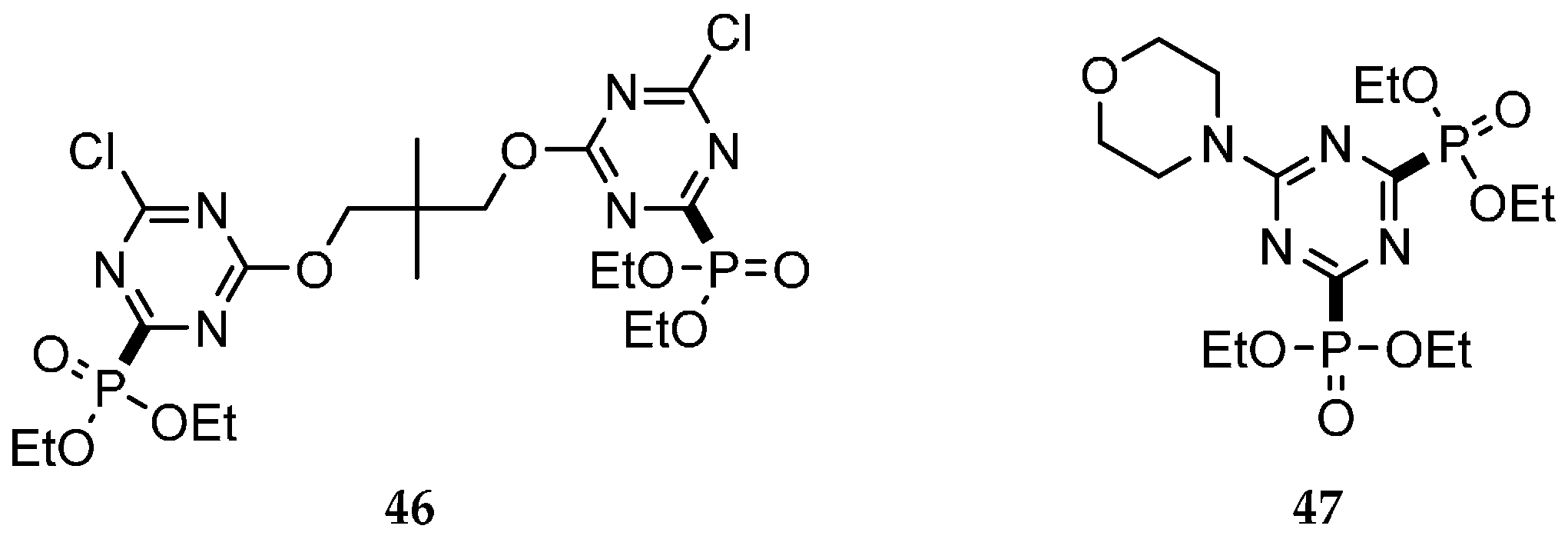
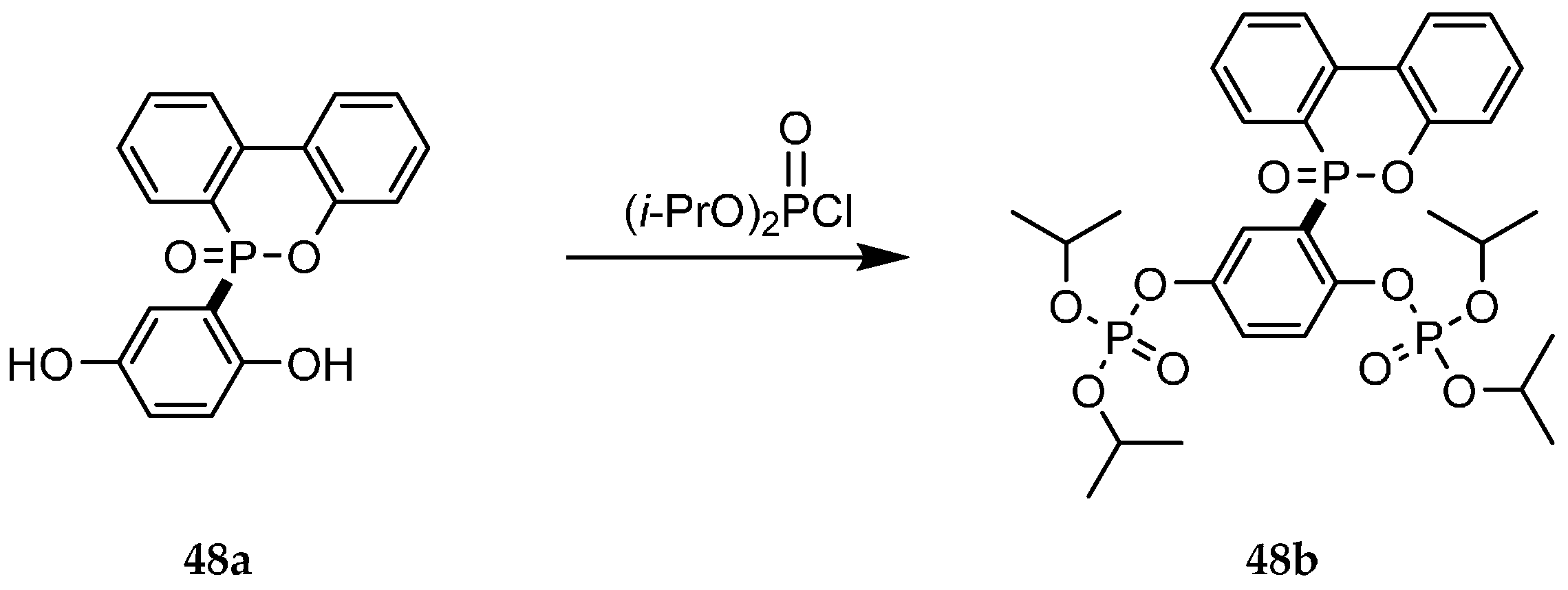
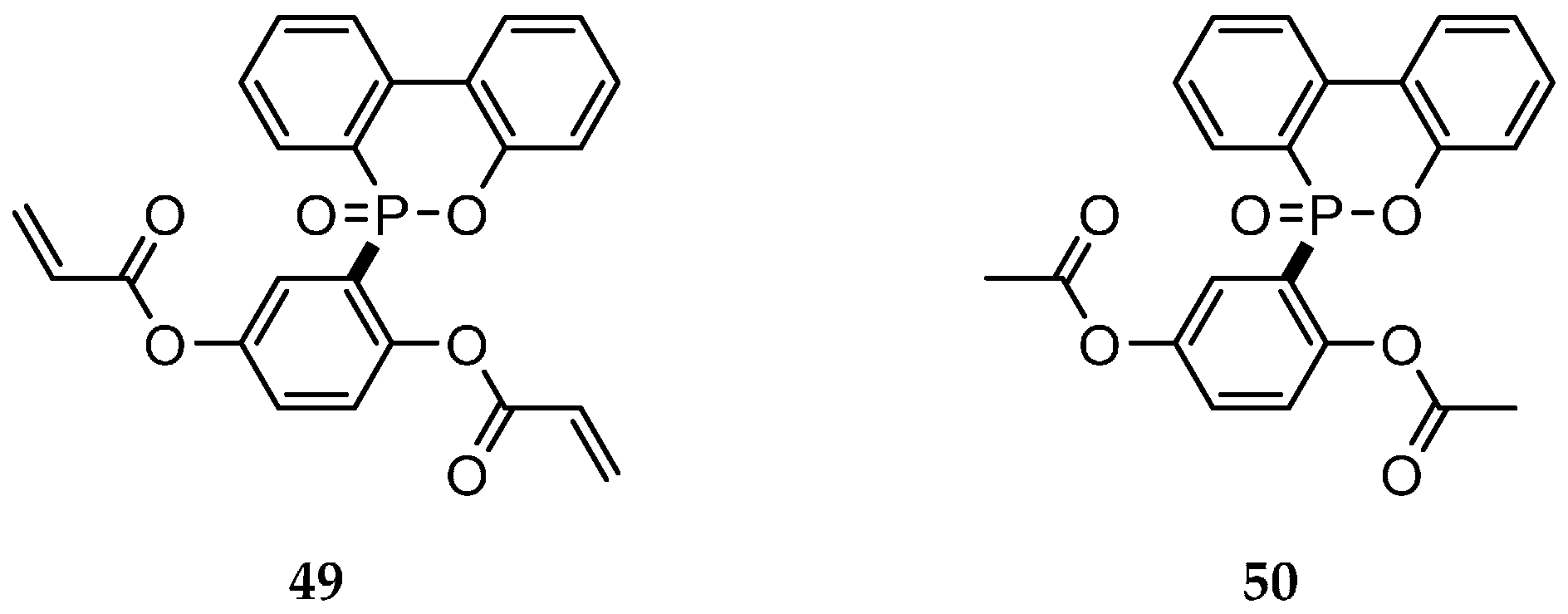
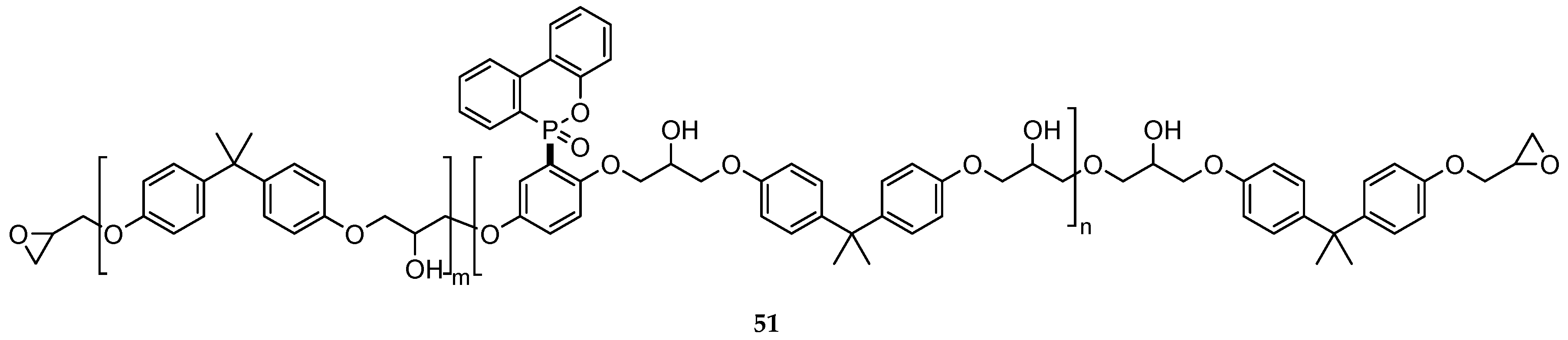
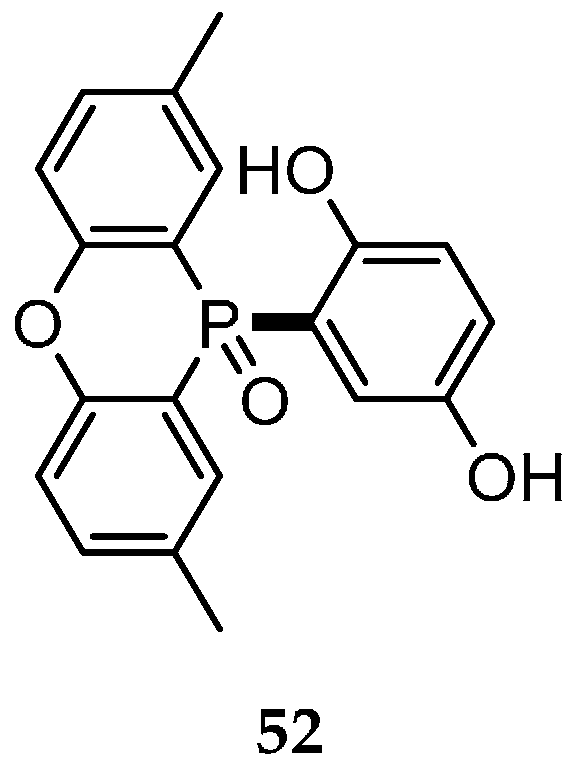

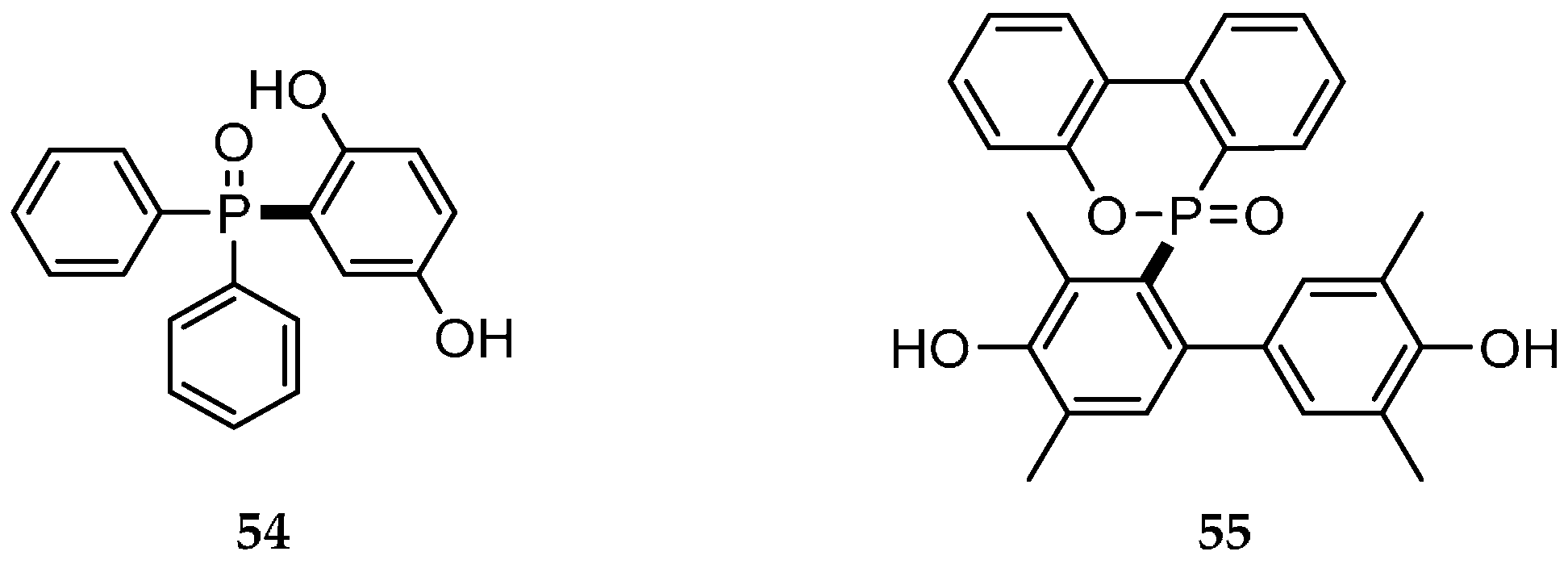





| Reaction Type | General Examples | Selected Ref. |
|---|---|---|
| Pudovik reaction |  | [6,30,37,38,39] |
| Kabachnik–Fields reaction |  | [6,30] |
| Michaelis–Becker reaction |  | [26] |
| Nucleophilic ring opening of epoxides |  | [6] |
| C-O/P-H cross coupling reaction |  | [40] |
| Dehydrogenative coupling with terminal alkynes |  | [6,12,41] |
| P-H insertion of α-Imino-carbenes |  | [42] |
| O2-induced radical oxyphosphorylation reaction |  | [43] |
 | [44] | |
| Phospha–Michael addition reaction |  | [5,15,29] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications. Materials 2017, 10, 784. https://doi.org/10.3390/ma10070784
Wendels S, Chavez T, Bonnet M, Salmeia KA, Gaan S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications. Materials. 2017; 10(7):784. https://doi.org/10.3390/ma10070784
Chicago/Turabian StyleWendels, Sophie, Thiebault Chavez, Martin Bonnet, Khalifah A. Salmeia, and Sabyasachi Gaan. 2017. "Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications" Materials 10, no. 7: 784. https://doi.org/10.3390/ma10070784













