Mechanical Behavior of Stainless Steel Fiber-Reinforced Composites Exposed to Accelerated Corrosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Materials
2.2.1. Stainless Steel Samples
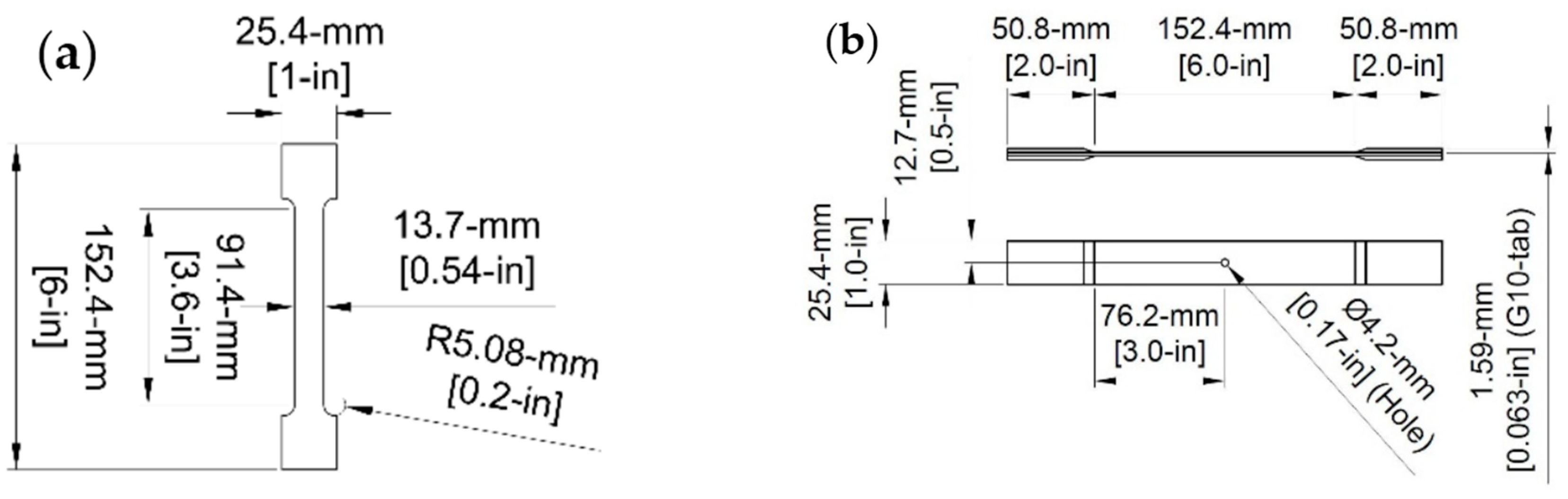
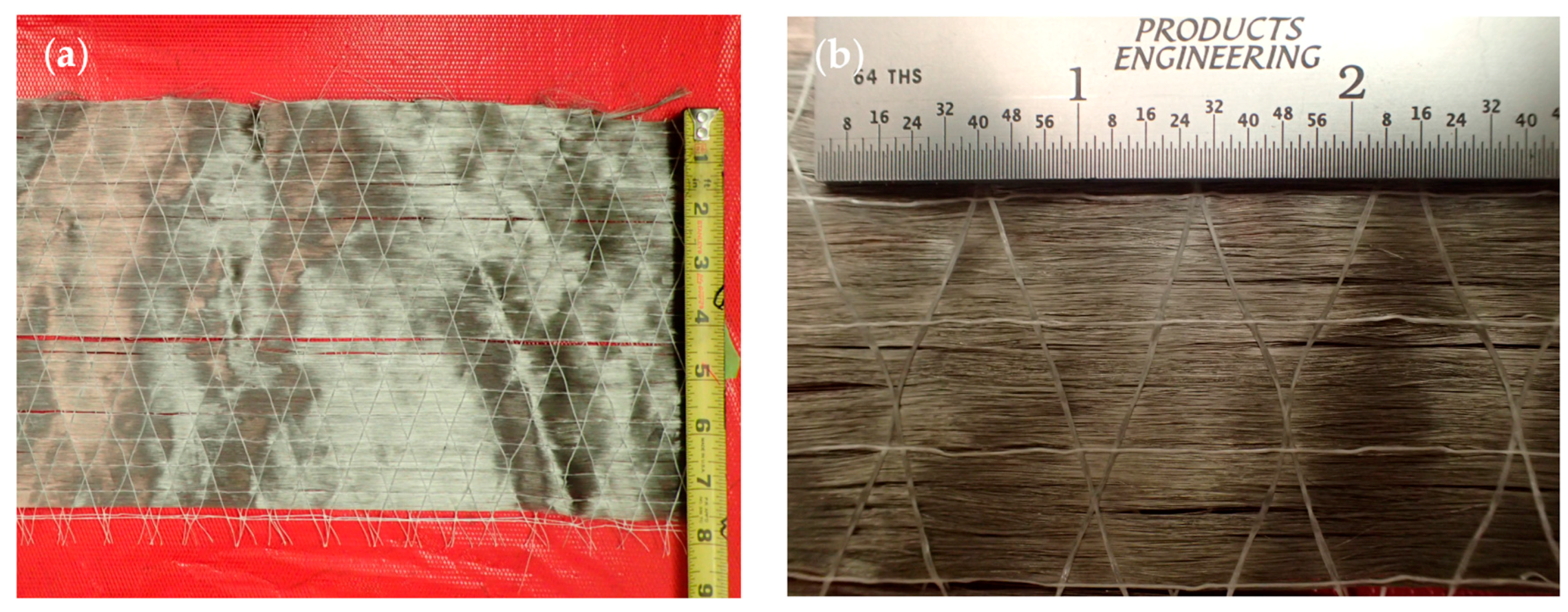
2.2.2. Composite Samples
Steel Reinforcement
Resin
Manufacturing of Composite Specimens
2.3. Experimental Methodology
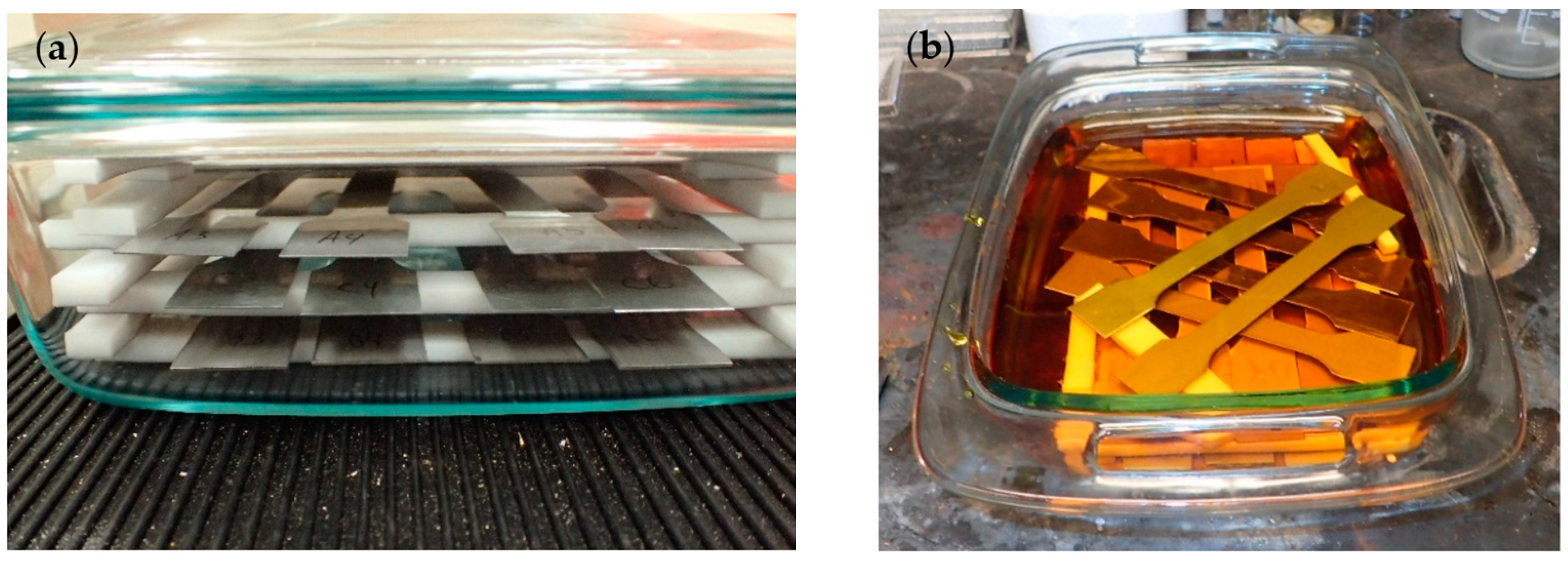
2.3.1. Application of Corrosion Damage
2.3.2. Tensile Testing
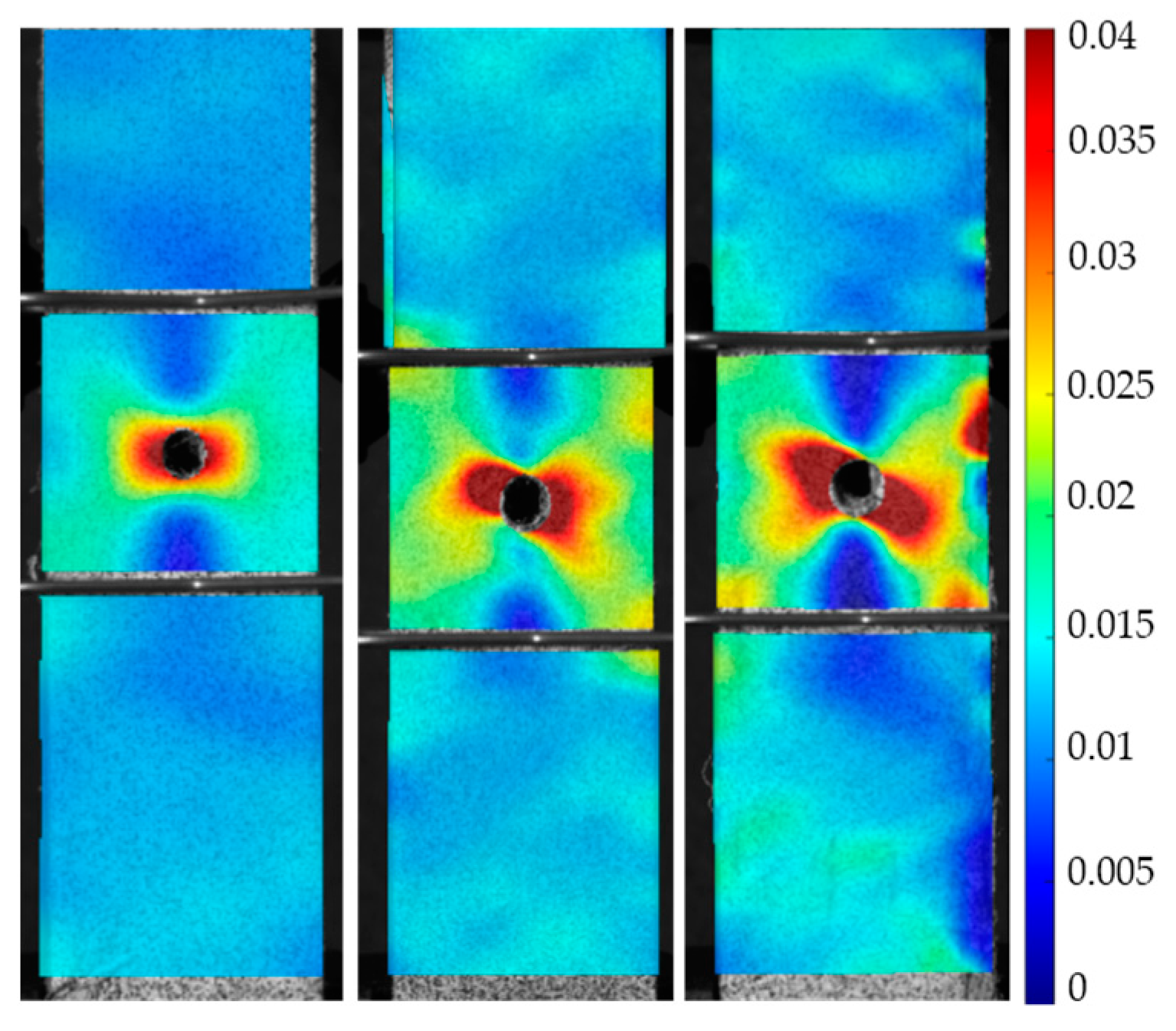
3. Results
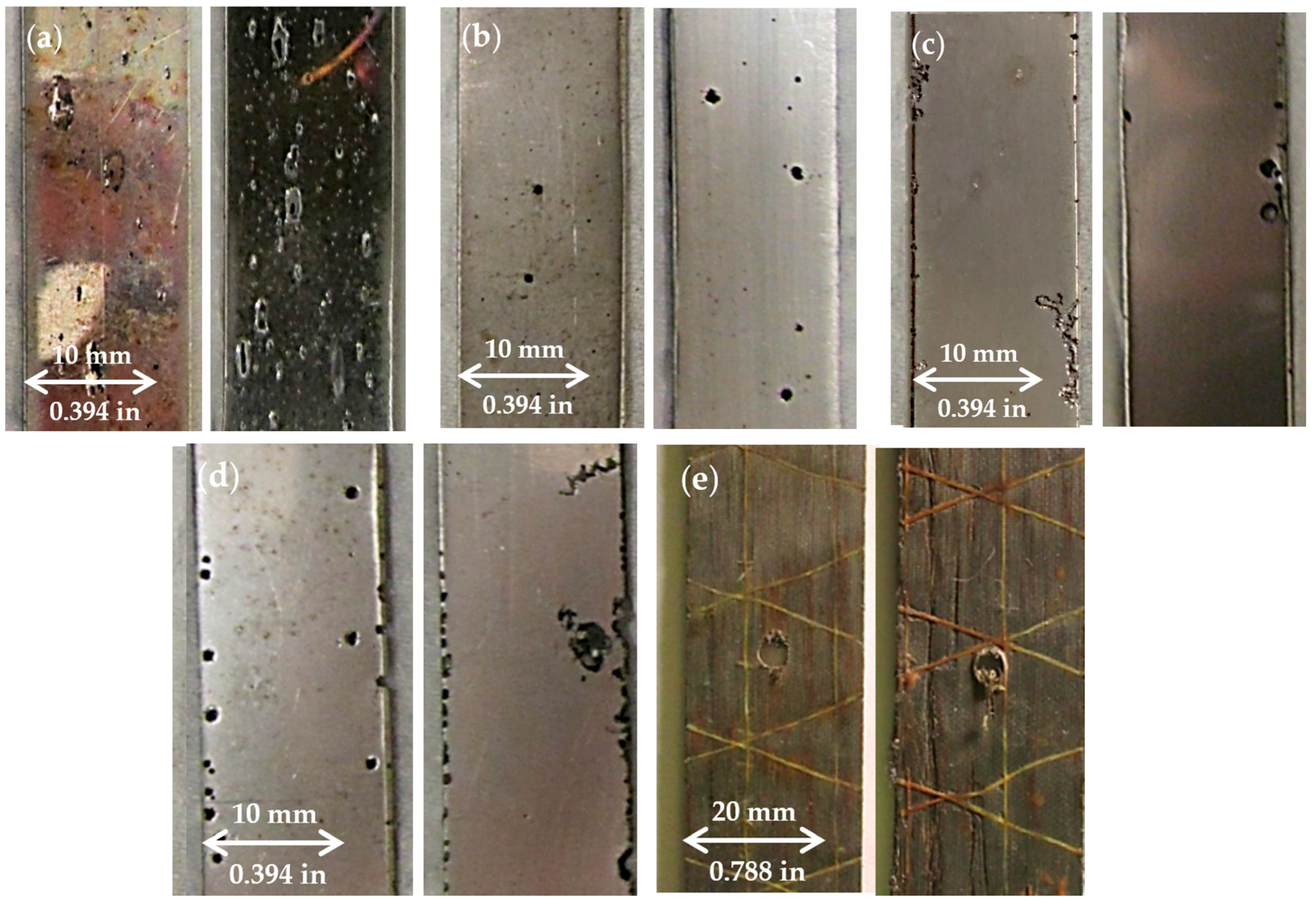
3.1. Corrosion Damage Measurement
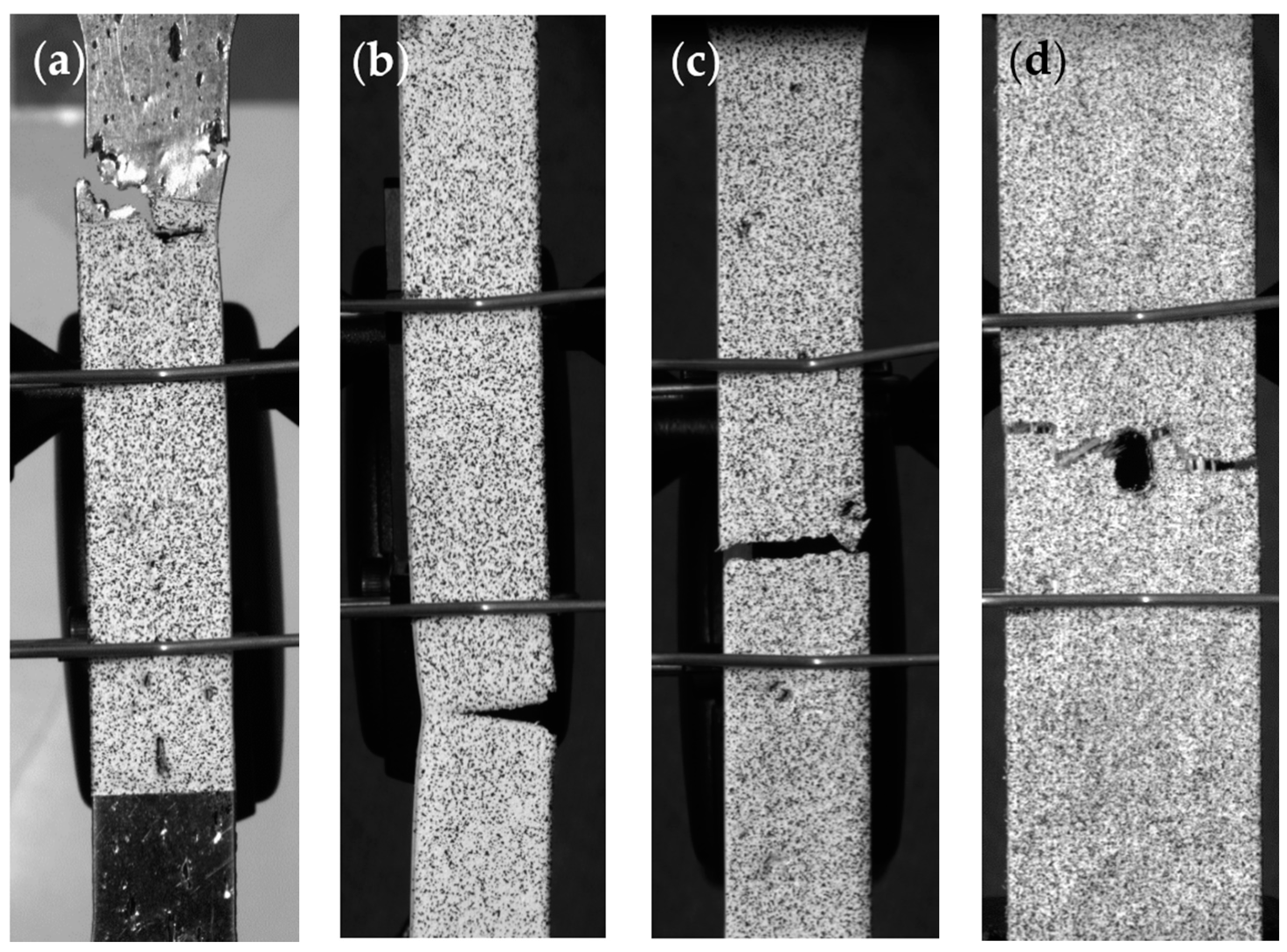
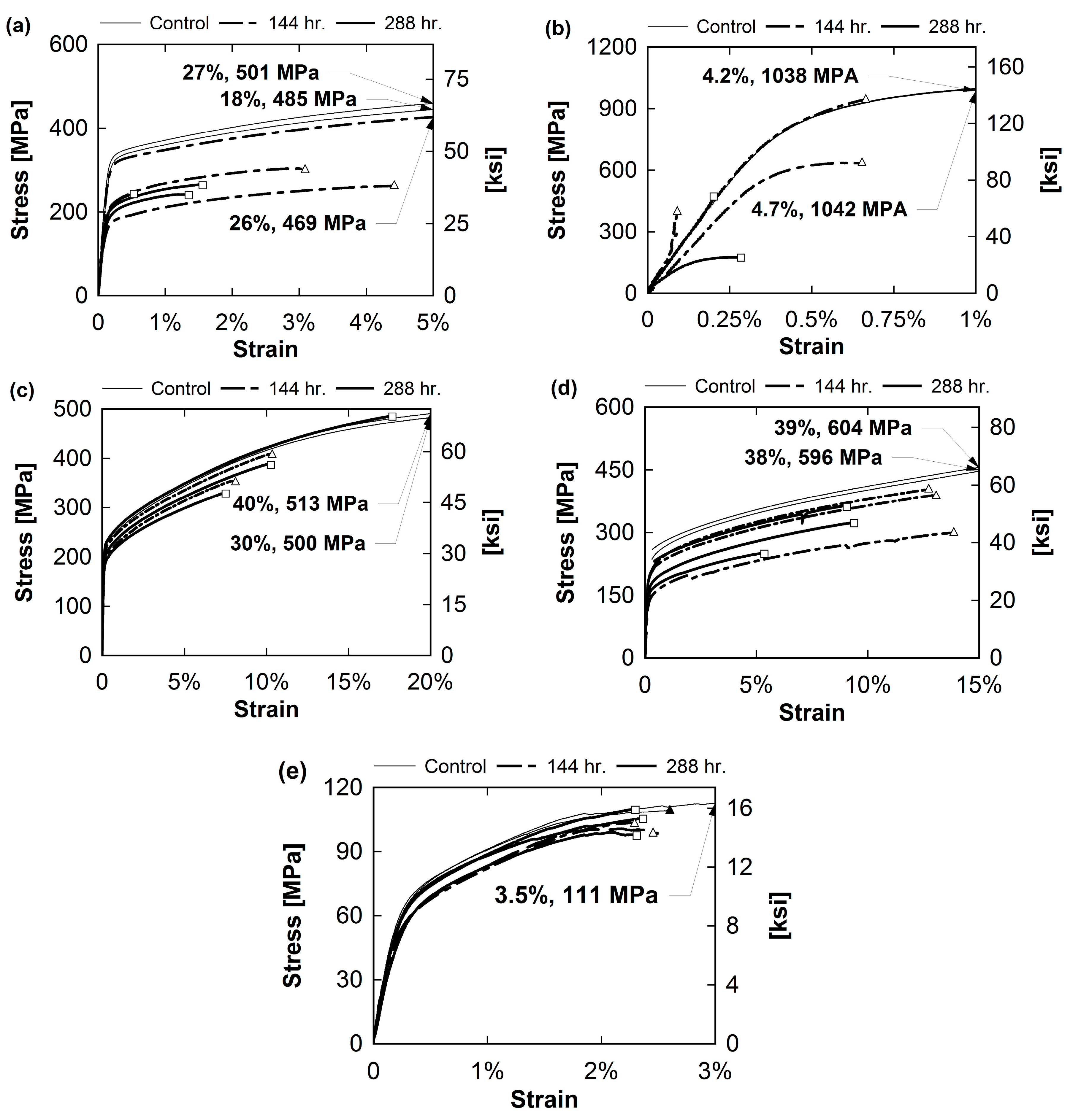
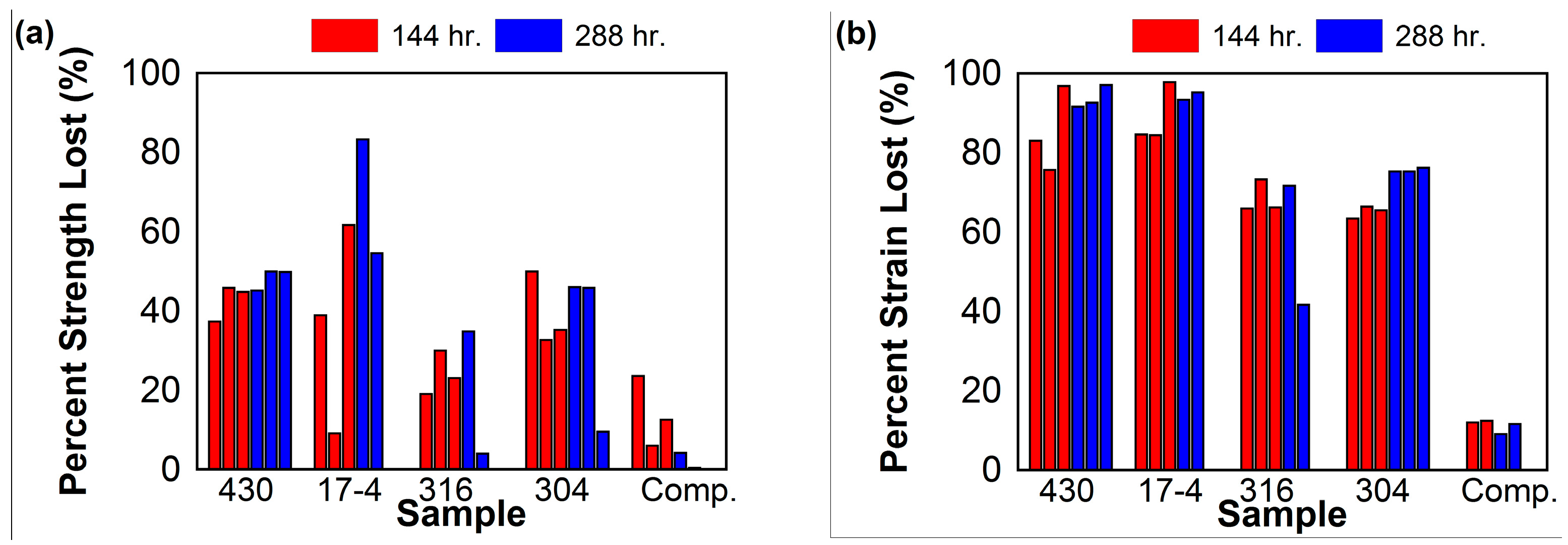
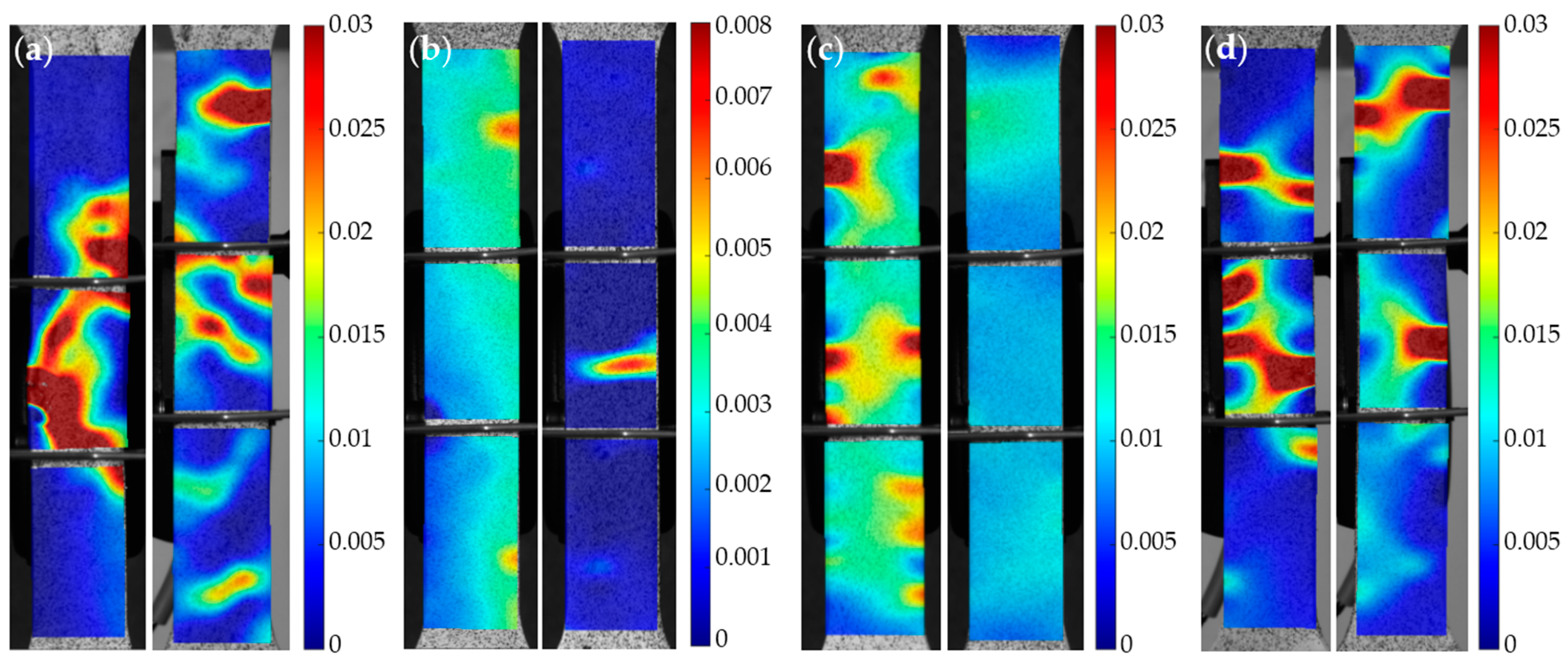
3.2. Tensile Experiment
4. Discussion
5. Conclusions
6. Future Work
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McBride, A.K.; Turek, S.L.; Zaghi, A.E.; Burke, K.A. Mechanical behavior of hybrid glass/steel fiber reinforced epoxy composites. Polymers 2017, 9, 151. [Google Scholar] [CrossRef]
- McBride, A. Mechanical Behavior of Hybrid Glass/Steel Reinforced Epoxy Composites; University of Connecticut: Storrs, CT, USA, 2016. [Google Scholar]
- Myers, T.; Kytömaa, H.; Smith, T. Environmental stress-corrosion cracking of fiberglass: Lessons learned from failures in the chemical industry. J. Hazard. Mater. 2007, 142, 695–704. [Google Scholar] [CrossRef] [PubMed]
- El Maaddawy, T.; Soudki, K. Carbon-fiber-reinforced polymer repair to extend service life of corroded reinforced concrete beams. J. Compos. Constr. 2005, 9, 187–194. [Google Scholar] [CrossRef]
- Triantafyllou, G.G.; Rousakis, T.C.; Karabinis, A.I. Corroded rc beams patch repaired and strengthened in flexure with fiber-reinforced polymer laminates. Compos. B: Eng. 2017, 112, 125–136. [Google Scholar] [CrossRef]
- Badawi, M.; Soudki, K. Control of corrosion-induced damage in reinforced concrete beams using carbon fiber-reinforced polymer laminates. J. Compos. Constr. 2005, 9, 195–201. [Google Scholar] [CrossRef]
- Soudki, K.; El-Salakawy, E.; Craig, B. Behavior of cfrp strengthened reinforced concrete beams in corrosive environment. J. Compos. Constr. 2007, 11, 291–298. [Google Scholar] [CrossRef]
- Masoud, S.; Soudki, K. Evaluation of corrosion activity in frp repaired rc beams. Cem. Concr. Compos. 2006, 28, 969–977. [Google Scholar] [CrossRef]
- Rousakis, T.C. Reusable and recyclable nonbonded composite tapes and ropes for concrete columns confinement. Compos. B: Eng. 2016, 103, 15–22. [Google Scholar] [CrossRef]
- Callens, M.; Gorbatikh, L.; Verpoest, I. Ductile steel fibre composites with brittle and ductile matrices. Compos. A: Appl. Sci. Manuf. 2014, 61, 235–244. [Google Scholar] [CrossRef]
- Callens, M.G.; De Cuyper, P.; Gorbatikh, L.; Verpoest, I. Effect of fibre architecture on the tensile and impact behaviour of ductile stainless steel fibre polypropylene composites. Compos. Struct. 2015, 119, 528–533. [Google Scholar] [CrossRef]
- Callens, M.G.; Gorbatikh, L.; Bertels, E.; Goderis, B.; Smet, M.; Verpoest, I. Tensile behaviour of stainless steel fibre/epoxy composites with modified adhesion. Compos. A: Appl. Sci. Manuf. 2015, 69, 208–218. [Google Scholar] [CrossRef]
- Allaer, K.; De Baere, I.; Lava, P.; Van Paepegem, W.; Degrieck, J. On the in-plane mechanical properties of stainless steel fibre reinforced ductile composites. Compos. Sci. Technol. 2014, 100, 34–43. [Google Scholar] [CrossRef]
- Faes, J.C.; Rezaei, A.; Van Paepegem, W.; Degrieck, J. Influence of matrix toughness and interfacial strength on the toughness of epoxy composites with ductile steel fabric reinforcement. Compos. Interfaces 2015, 22, 779–793. [Google Scholar] [CrossRef]
- Mosleh, Y.; Clemens, D.; Gorbatikh, L.; Verpoest, I.; van Vuure, A.W. Penetration impact resistance of novel tough steel fibre-reinforced polymer composites. J. Reinf. Plast. Compos. 2015, 34, 624–635. [Google Scholar] [CrossRef]
- Breuer, U.; Schmeer, S.; Eberth, U. Carbon and metal fibre reinforced airframe structrues—A new approach to composite multifunctionality. In Proceedings of the Deutscher Luft- und Raumfahrtkongress 2013, Stuttgart, Germany, 10–12 September 2013. [Google Scholar]
- Shirvanimoghaddam, K.; Hamim, S.U.; Akbari, M.K.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. A: Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Naebe, M.; Shirvanimoghaddam, K. Functionally graded materials: A review of fabrication and properties. Appl. Mater. Today 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Fiber-Reinforced Polymer Composites: Pursuing the Promise; Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, February 2014.
- Agarwal, A.; Garg, S.; Rakesh, P.; Singh, I.; Mishra, B. Tensile behavior of glass fiber reinforced plastics subjected to different environmental conditions. Indian J. Eng. Mater. Sci. 2010, 17, 471–476. [Google Scholar]
- Center, A.R. (Ed.) Handbook of Stainless Steel; Outokumpu Oyj: Avesta, Sweden, 2013. [Google Scholar]
- Ma, F.Y. Corrosive Effects of Chlorides on Metals. In Pitting Corrosion; Bensalah, N., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0275-5. [Google Scholar]
- ArcelorMittal. Stainless Steel and Corrosion; ArcelorMittal Paris: Paris, France, 2010. [Google Scholar]
- Stainless Steel Comparator; AK Steel Corporation: West Chester, OH, USA, 2007.
- Corrosion Mechanisms in Stainless Steel. Available online: http://www.bssa.org.uk/topics.php?article=95 (accessed on 9 June 2017).
- Pistorius, P.; Burstein, G. Metastable pitting corrosion of stainless steel and the transition to stability. Philos. Trans. R. Soc. Lond. A: Math. Phys. Eng. Sci. 1992, 341, 531–559. [Google Scholar] [CrossRef]
- Vermilyea, D.A. Concerning the critical pitting potential. J. Electrochem. Soc. 1971, 118, 529–531. [Google Scholar] [CrossRef]
- Böhni, H. Metastable Pitting—Occurrence and Significance for Passive Metals; Swiss Federal Institute of Technology: Zürich, Switzerland, 2002. [Google Scholar]
- Petrović, Z.C. Catastrophes caused by corrosion. Mil. Tech. Cour. Vojnoteh. Glas. 2016, 64, 1048–1064. [Google Scholar] [CrossRef]
- Burstein, G.; Pistorius, P.; Mattin, S. The nucleation and growth of corrosion pits on stainless steel. Corros. Sci. 1993, 35, 57–62. [Google Scholar] [CrossRef]
- Muslim, Z.R.; Abbas, A.A. The effect of ph and temperature on corrosion rate stainless steel 316l used as biomaterial. Int. J. Basic Appl. Sci. 2015, 4, 17–20. [Google Scholar]
- Prawoto, Y.; Ibrahim, K.; Wan Nik, W. Effect of ph and chloride concentration on the corrosion of duplex stainless steel. Arab. J. Sci. Eng. 2009, 34, 115. [Google Scholar]
- Burstein, G.; Pistorius, P. Surface roughness and the metastable pitting of stainless steel in chloride solutions. Corrosion 1995, 51, 380–385. [Google Scholar] [CrossRef]
- Wang, J.; Su, C.; Szklarska-Smialowska, Z. Effects of cl- concentration and temperature on pitting of aisi 304 stainless steel. Corrosion 1988, 44, 732–737. [Google Scholar] [CrossRef]
- Liebhafsky, H.; Newkirk, A. Corrosion of stainless steel in ferric chloride solution★. Corrosion 1956, 12, 48–54. [Google Scholar] [CrossRef]
- Pistorius, P.; Burstein, G. Growth of corrosion pits on stainless steel in chloride solution containing dilute sulphate. Corros. Sci. 1992, 33, 1885–1897. [Google Scholar] [CrossRef]
- Streicher, M. Pitting corrosion of 18cr-8ni stainless steel. J. Electrochem. Soc. 1956, 103, 375–390. [Google Scholar] [CrossRef]
- Stewart, J.; Williams, D. The initiation of pitting corrosion on austenitic stainless steel: On the role and importance of sulphide inclusions. Corros. Sci. 1992, 33, 457465–463474. [Google Scholar] [CrossRef]
- Dai, J.; Yao, X.; Liang, X.; Yeh, H. Experimental study of micro-cracks in stress corrosion of fiber reinforced composites. Polym. Test. 2006, 25, 758–765. [Google Scholar] [CrossRef]
- Kumosa, L.; Armentrout, D.; Kumosa, M. An evaluation of the critical conditions for the initiation of stress corrosion cracking in unidirectional e-glass/polymer composites. Compos. Sci. Technol. 2001, 61, 615–623. [Google Scholar] [CrossRef]
- Kumosa, L.; Kumosa, M.; Armentrout, D. Resistance to stress corrosion cracking of unidirectional ecr-glass/polymer composites for high voltage composite insulator applications. Compos. A: Appl. Sci. Manuf. 2003, 34, 1–15. [Google Scholar] [CrossRef]
- Kumosa, L.; Armentrout, D.; Kumosa, M. The effect of sandblasting on the initiation of stress corrosion cracking in unidirectional e-glass/polymer composites used in high voltage composite (non-ceramic) insulators. Compos. Sci. Technol. 2002, 62, 1999–2015. [Google Scholar] [CrossRef]
- Megel, M.; Kumosa, L.; Ely, T.; Armentrout, D.; Kumosa, M. Initiation of stress-corrosion cracking in unidirectional glass/polymer composite materials. Compos. Sci. Technol. 2001, 61, 231–246. [Google Scholar] [CrossRef]
- Hogg, P. Factors affecting the stress corrosion of grp in acid environments. Composites 1983, 14, 254–261. [Google Scholar] [CrossRef]
- Ely, T.; Armentrout, D.; Kumosa, M. Evaluation of stress corrosion properties of pultruded glass fiber/polymer composite materials. J. Compos. Mater. 2001, 35, 751–773. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Tensile behavior contrast of basalt and glass fibers after chemical treatment. Mater. Des. 2010, 31, 4244–4250. [Google Scholar] [CrossRef]
- Gu, H. Tensile behaviours of some high performance filaments after naoh treatment. Mater. Des. 2008, 29, 1893–1896. [Google Scholar] [CrossRef]
- Naebe, M.; Abolhasani, M.M.; Khayyam, H.; Amini, A.; Fox, B. Crack damage in polymers and composites: A review. Polym. Rev. 2016, 56, 31–69. [Google Scholar] [CrossRef]
- Shokrieh, M.; Nasir, V.; Karimipour, H. A micromechanical study on longitudinal strength of fibrous composites exposed to acidic environment. Mater. Des. 2012, 35, 394–403. [Google Scholar] [CrossRef]
- ASTM G48–11, Standard Test Methods for Pitting and Crevice Corrosion Resistance of Stainless Steels and Related Alloys by Use of Ferric Chloride Solution; ASTM: West Conshohocken, PA, USA, 2015.
- Stainless Grades. Available online: http://www.pennstainless.com/stainless-grades/ (accessed on 9 June 2017).
- Geerinck, S. (Ed.) Stainless Steel Filler Materials for Plastics; Bekaert Fiber Technologies: Zwevegem, Belgium, 2013. [Google Scholar]
- Ahmed, T. Hybrid Composite Structures: Multifunctionality Through Metal Fibres; Delft University of Technology: Delt, The Netherlands, 2009. [Google Scholar]
- Derakane 411-350 Epoxy Vinyl Ester Resin Technical Datasheet; Ashland Inc.: Covington, KY, USA, 2004.
- Epon Resin 828 Technical Datasheet; Hexion Inc.: Batesville, AR, USA, 2005.
- ASTM D5766, Standard Test Method for Tensile Properties of Plastics; ASTM: West Conshohocken, PA, USA, 2002.
- Loctite Armstrong A-12 Epoxy Resin Adhesive Laboratory Data Sheet; Henkel Inc.: Rocky Hill, CT, USA, 2015.
- ASTM D5766/d5766m-11, Standard Test Method for Open-Hole Tensile Strength of Polymer Matrix Composite Laminates; ASTM: West Conshohocken, PA, USA, 2011; Volume ASTM D5766.
- Blaber, J. Ncorr v1.2. Available online: http://ncorr.com/ (accessed on 9 June 2017).

| Fe | Cr | Ni | Mo | Mn | Si | Cu | V | Co |
|---|---|---|---|---|---|---|---|---|
| 65.4% | 20.2% | 10.5% | 2.4% | 0.57% | 0.49% | 0.17% | 0.14% | 0.10% |
| Sample * | % of Original Weight | Maximum Stress MPa (ksi) | Maximum Strain (%) | Percent Strength Lost (%) | Percent Strain Lost (%) | No. Failures Outside Gage Length |
|---|---|---|---|---|---|---|
| 430-0-1 | - | 485 (70.3) | 18.2 | - | - | 0 |
| 430-0-2 | - | 501 (72.6) | 27.2 | - | - | |
| 430-0-3 | - | 469 (68.0) | 25.6 | - | - | |
| 430-144-1 | 91.5 | 304 (44.1) | 3.1 | 37.3 | 83.0 | 1 |
| 430-144-2 | 92.9 | 262 (38.0) | 4.4 | 45.9 | 75.7 | |
| 430-144-3 | 90.8 | 267 (38.7) | 0.6 | 44.9 | 96.8 | |
| 430-288-1 | 84.3 | 265 (38.5) | 1.5 | 45.2 | 91.6 | 2 |
| 430-288-2 | 86.2 | 242 (35.1) | 1.4 | 50.0 | 92.6 | |
| 430-288-3 | 83.3 | 243 (35.2) | 0.5 | 49.9 | 97.1 | |
| 17-4-0-1 | - | 1038 (150.6) | 4.2 | - | - | 0 |
| 17-4-0-2 | - | 1042 (151.2) | 4.7 | - | - | |
| 17-4-144-1 | 97.2 | 636 (92.2) | 0.7 | 38.9 | 84.6 | 2 |
| 17-4-144-2 | 97.6 | 944 (137.0) | 0.7 | 9.2 | 84.4 | |
| 17-4-144-3 | 96.7 | 398 (57.7) | 0.1 | 61.7 | 97.8 | |
| 17-4-288-1 | 96.6 | 175 (25.3) | 0.3 | 83.2 | 93.3 | 0 |
| 17-4-288-2 | 96.3 | 473 (68.5) | 0.2 | 54.6 | 95.2 | |
| 316-0-1 | - | 500 (72.6) | 30.3 | - | - | 0 |
| 316-0-3 | - | 513 (74.4) | 40.1 | - | - | |
| 316-144-1 | 99.3 | 410 (59.4) | 10.3 | 19.1 | 65.9 | 2 |
| 316-144-2 | 98.8 | 354 (51.4) | 8.1 | 30.0 | 73.3 | |
| 316-144-3 | 99.0 | 389 (56.5) | 10.3 | 23.1 | 66.2 | |
| 316-288-1 | 99.4 | 330 (47.9) | 8.6 | 34.8 | 71.7 | 2 |
| 316-288-2 | 99.6 | 486 (70.4) | 17.7 | 4.1 | 41.7 | |
| 304-0-1 | - | 596 (86.4) | 37.9 | - | - | 0 |
| 304-0-2 | - | 604 (87.6) | 39.3 | - | - | |
| 304-144-1 | 96.2 | 300 (43.5) | 13.9 | 50.0 | 63.4 | 2 |
| 304-144-2 | 96.8 | 403 (58.5) | 12.7 | 32.7 | 66.4 | |
| 304-144-3 | 97.1 | 388 (56.3) | 13.1 | 35.2 | 65.5 | |
| 304-288-1 | 93.6 | 324 (46.9) | 9.4 | 46.1 | 75.3 | 2 |
| 304-288-2 | 95.0 | 325 (47.1) | 9.4 | 45.9 | 75.3 | |
| 304-288-3 | 97.1 | 542 (78.7) | 9.0 | 9.6 | 76.2 | |
| Comp.-0-1 | - | 111 (16.2) | 3.5 | - | - | 0 |
| Comp.-0-2 | - | 109 (15.8) | 2.6 | - | - | |
| Comp.-144-1 | 98.87 | 84 (12.2) | 2.6 | 23.6 | −1.5 | 0 |
| Comp.-144-2 | 99.43 | 104 (15.0) | 2.3 | 6.1 | 12.0 | |
| Comp.-288-1 | 93.07 | 96 (14.0) | 2.3 | 12.6 | 12.4 | 0 |
| Comp.-288-2 | 96.75 | 106 (15.3) | 2.4 | 4.3 | 9.1 | |
| Comp.-288-3 | 96.50 | 110 (15.9) | 2.3 | 0.5 | 11.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, C.; McBride, A.; E. Zaghi, A.; Burke, K.A.; Hill, A. Mechanical Behavior of Stainless Steel Fiber-Reinforced Composites Exposed to Accelerated Corrosion. Materials 2017, 10, 772. https://doi.org/10.3390/ma10070772
O’Brien C, McBride A, E. Zaghi A, Burke KA, Hill A. Mechanical Behavior of Stainless Steel Fiber-Reinforced Composites Exposed to Accelerated Corrosion. Materials. 2017; 10(7):772. https://doi.org/10.3390/ma10070772
Chicago/Turabian StyleO’Brien, Caitlin, Amanda McBride, Arash E. Zaghi, Kelly A. Burke, and Alex Hill. 2017. "Mechanical Behavior of Stainless Steel Fiber-Reinforced Composites Exposed to Accelerated Corrosion" Materials 10, no. 7: 772. https://doi.org/10.3390/ma10070772




