Recovery of Silver Using Adsorption Gels Prepared from Microalgal Residue Immobilized with Functional Groups Containing Sulfur or Nitrogen
Abstract
:1. Introduction
2. Experimental
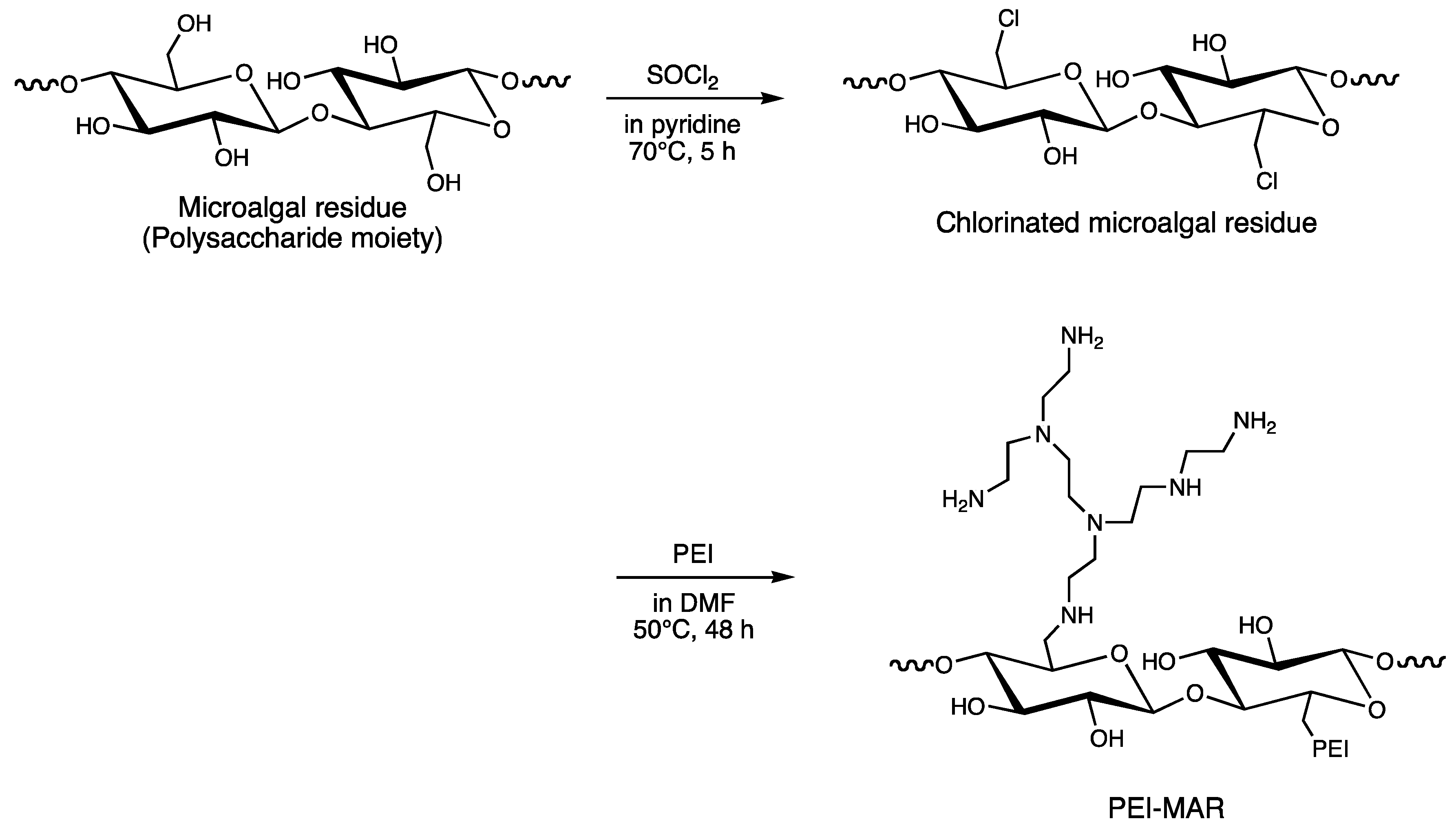
2.1. Preparation of the Adsorption Gels
2.2. Preparation of Test Solutions
2.3. Batch Adsorption Tests
3. Results and Discussion
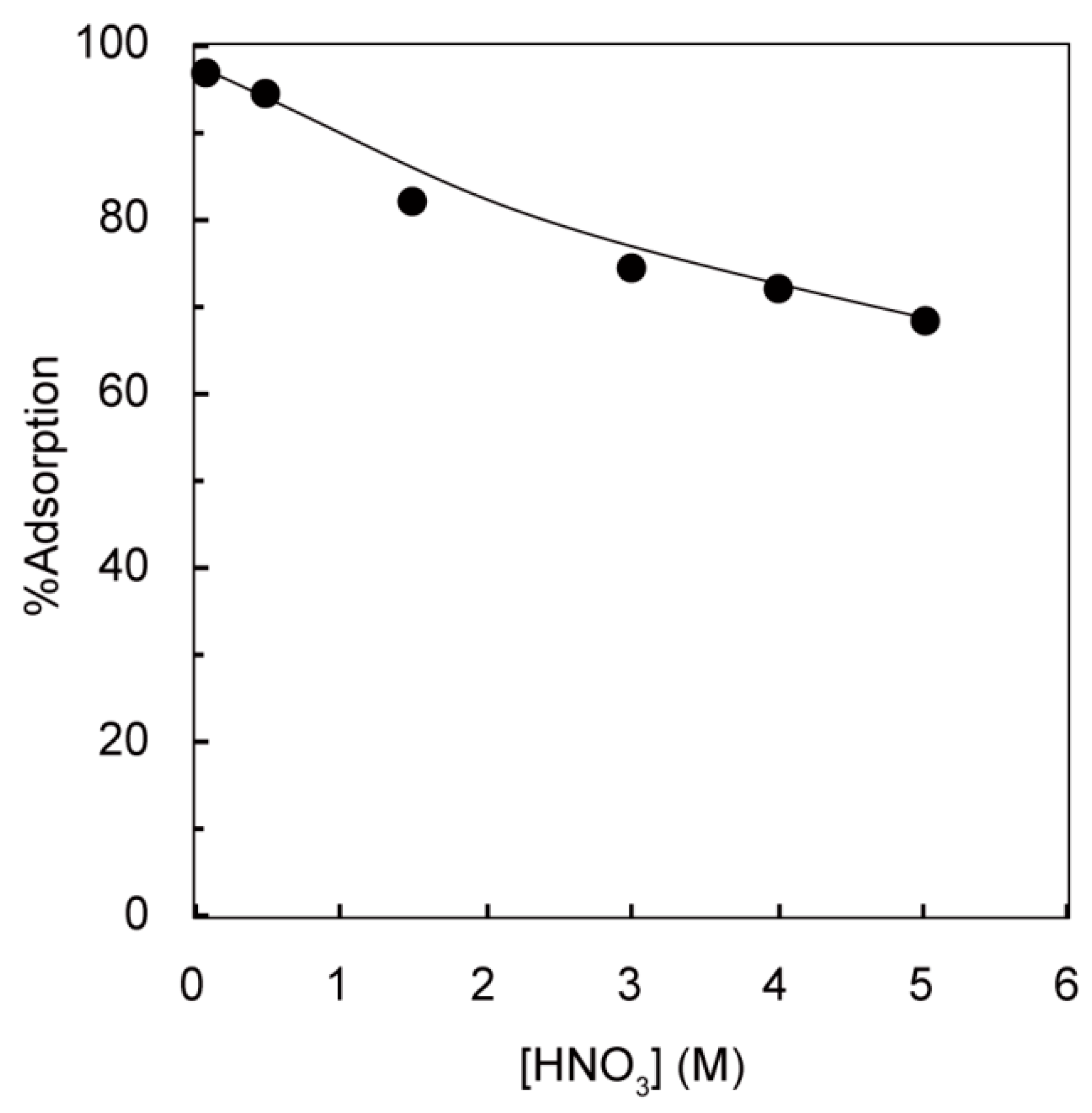
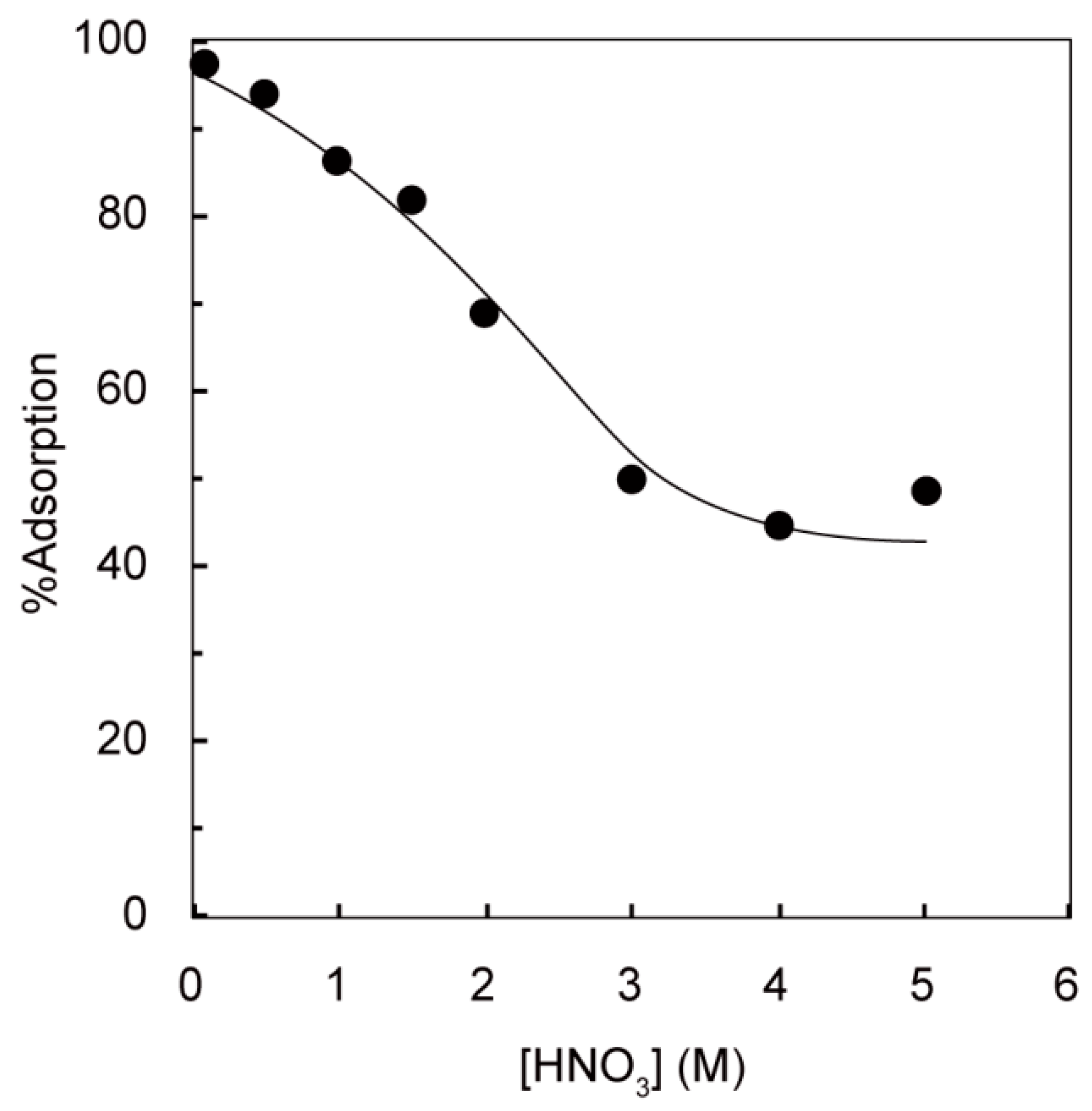
3.1. Effect of Nitric Acid Concentration
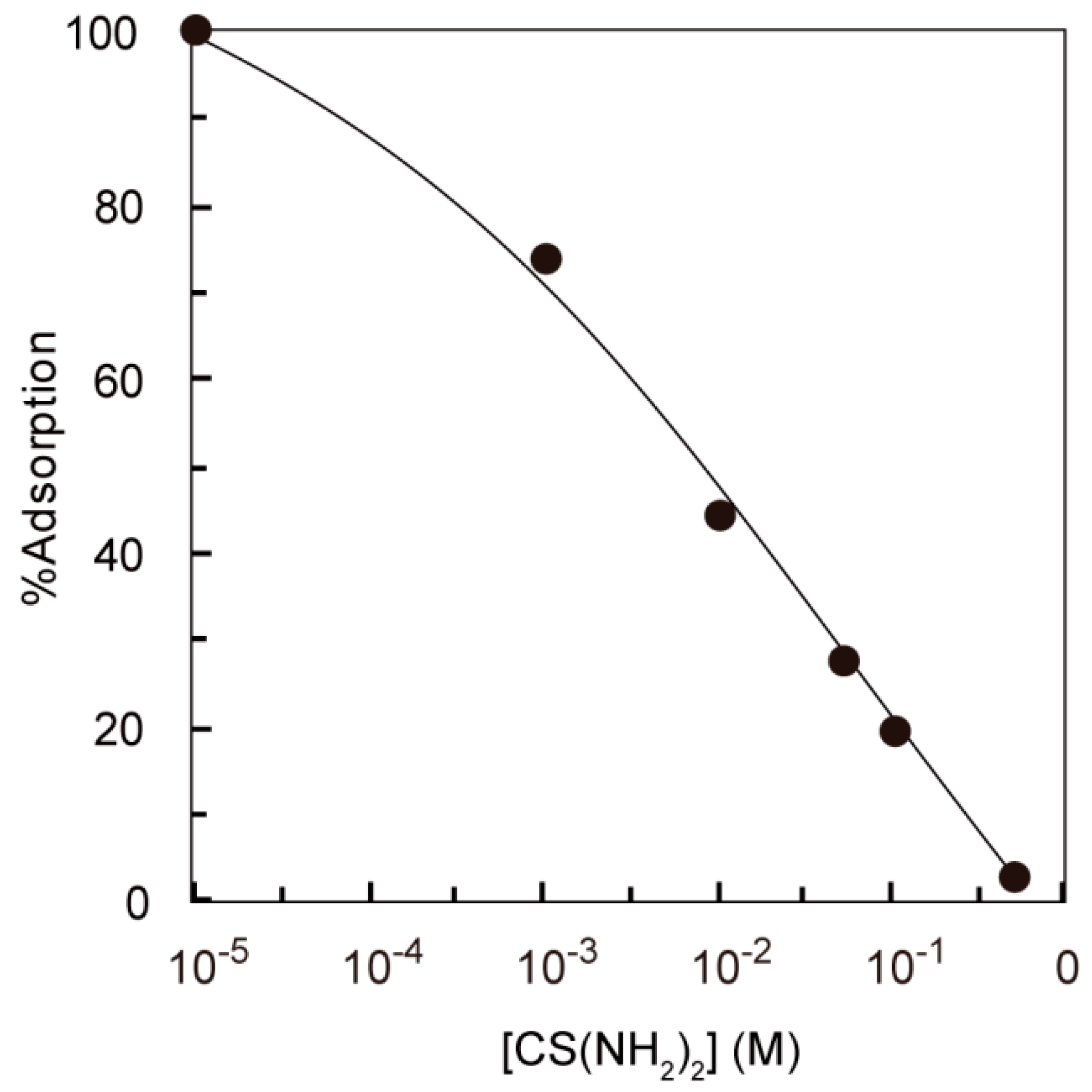
3.2. Effect of Thiourea Concentration on the Adsorption of Silver(I) from Acidothiourea Solution
3.3. Effect of Sulfuric Acid Concentration on the Adsorption of Silver(I) from Acidothiourea Solution
3.4. Adsorption Isotherms
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbras, A. Importance of biodiesel as transportation. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuel from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Kato, M.; Yamato, K.; Ishibashi, M.; Sekiguchi, H.; Kurano, N.; Miyachi, S. Characterization of the lipid accumulation in a new microalgal species, Pseudochoricystis ellipsoidea (Trebouxiophyceae). J. Jpn. Inst. Energy 2010, 89, 909–913. [Google Scholar] [CrossRef]
- Khunathai, K.; Xiong, Y.; Biswas, B.K.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, H.; Kato, H.; Kurata, M.; Atsumi, K. Selective recovery of gold by simultaneous adsorption-reduction using microalgal residues generated from bioconversion processes. J. Chem. Technol. Biotechnol. 2012, 87, 393–401. [Google Scholar] [CrossRef]
- Khunathai, K.; Inoue, K.; Ohto, K.; Kawakita, H.; Kurata, M.; Atsumi, K.; Fukuda, H.; Alam, S. Adsorptive recovery of palladium(II) and platinum(IV) on the chemically modified-microalgal residue. Solvent Extr. Ion Exch. 2013, 31, 320–334. [Google Scholar] [CrossRef]
- Khunathai, K.; Inoue, K.; Ohto, K.; Kawakita, H.; Kurata, M.; Atsumi, K.; Alam, S. Dithiooxamide-immobilized microalgal residue for the selective recovery of Pd(II) and Pt(IV). Sep. Sci. Technol. 2012, 47, 1185–1193. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Chen, C.K.; Lung, T.N.; Wan, C.C. A study of the leaching of gold and silver by acidothioureation. Hydrometallurgy 1980, 5, 207–212. [Google Scholar] [CrossRef]
- Balaz, P.; Ficeriova, J.; Sepelak, V.; Kammel, R. Thiourea leaching of silver from mechanically activated tetrahedrite. Hydrometallurgy 1996, 43, 367–377. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Recovery of gold and silver from spent mobile phones by means of acidothiourea leaching followed by adsorption using bioadsorbent prepared from persimmon tannin. Hydrometallurgy 2013, 133, 84–93. [Google Scholar] [CrossRef]
- Baba, Y.; Umrzaki, Y.; Inoue, K. Extraction equilibrium of silver(I) with triisobutylphosphine sulfide from nitrate media. J. Chem. Eng. Jpn. 1986, 19, 27–30. [Google Scholar] [CrossRef]
- Karabakan, A.; Karabalut, S.; Denizil, A.; Yurum, Y. Removal of silver(I) from aqueous solutions with low-rank turkish coals. Adsorp. Sci. Technol. 2004, 22, 135–144. [Google Scholar] [CrossRef]
- Ghassabzadeli, H.; Mohadespour, A.; Torab-Mostaedi, M.; Zaheri, P.; Maragheh, M.G.; Taheri, H. Adsorption of Ag, Cu and Hg from aqueous solutions using expanded perlite. J. Hazard. Mater. 2010, 177, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Akgul, M.; Karabakan, A.; Acar, O.; Yurum, Y. Removal of silver(I) from aqueous solutions with clinoptilolite. Micropor. Mesopor. Mater. 2006, 94, 99–104. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Xue, X.; Li, F.; Li, G. Hybrid surfactant-templatedmesoporous silica formed in ethanol and its application for heavy metal removal. J. Hazard. Mater. 2008, 152, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.I.; Su, H.J.; Tan, T.W. Adsorption of Ag+ by a surface molecular-imprinted biosorbent. Chem. Eng. J. 2009, 150, 139–144. [Google Scholar] [CrossRef]
- Li, X.G.; Ma, X.L.; Sun, J.; Huang, M.R. Powerful reactive sorption of silver(I) and mercury(II) onto poly(O-phenylenediamine) microparticles. Langmuir 2009, 25, 1675–1684. [Google Scholar] [CrossRef] [PubMed]






| Adsorbent | Maximum Adsorption Capacity (mmol/g) | Aqueous Media | Literature |
|---|---|---|---|
| Coal | 0.02 | pH = 4 | [12] |
| Expanded perlite | 0.08 | pH = 6.5 | [13] |
| Clinoptilolite | 0.31 | pH = 4 | [14] |
| Mesoporous silica | 0.43 | pH = 7 | [15] |
| Ag(I)-imprinted chitosan | 1.85 | pH = 7 | [16] |
| Poly(O-phenylenediamine) | 4.94 | pH = 5 | [17] |
| DTO-MAR | 2.40 | 0.1 M nitric acid | This work |
| DTO-MAR | 0.98 | 50 mM sulfuric acid + 50 mM thiourea | This work |
| PEI-MAR | 2.70 | 0.1 M nitric acid | This work |
| PEI-MAR | 0.49 | 50 mM sulfuric acid + 50 mM thiourea | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khunathai, K.; Inoue, K.; Ohto, K.; Kawakita, H.; Kurata, M.; Atsumi, K.; Fukuda, H.; Alam, S. Recovery of Silver Using Adsorption Gels Prepared from Microalgal Residue Immobilized with Functional Groups Containing Sulfur or Nitrogen. Materials 2017, 10, 636. https://doi.org/10.3390/ma10060636
Khunathai K, Inoue K, Ohto K, Kawakita H, Kurata M, Atsumi K, Fukuda H, Alam S. Recovery of Silver Using Adsorption Gels Prepared from Microalgal Residue Immobilized with Functional Groups Containing Sulfur or Nitrogen. Materials. 2017; 10(6):636. https://doi.org/10.3390/ma10060636
Chicago/Turabian StyleKhunathai, Kanjana, Katsutoshi Inoue, Keisuke Ohto, Hidetaka Kawakita, Minoru Kurata, Kinya Atsumi, Hiroaki Fukuda, and Shafiq Alam. 2017. "Recovery of Silver Using Adsorption Gels Prepared from Microalgal Residue Immobilized with Functional Groups Containing Sulfur or Nitrogen" Materials 10, no. 6: 636. https://doi.org/10.3390/ma10060636





