Effect of the SiCl4 Flow Rate on SiBN Deposition Kinetics in SiCl4-BCl3-NH3-H2-Ar Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation Method
2.2. Characterization Method
3. Results and Discussion
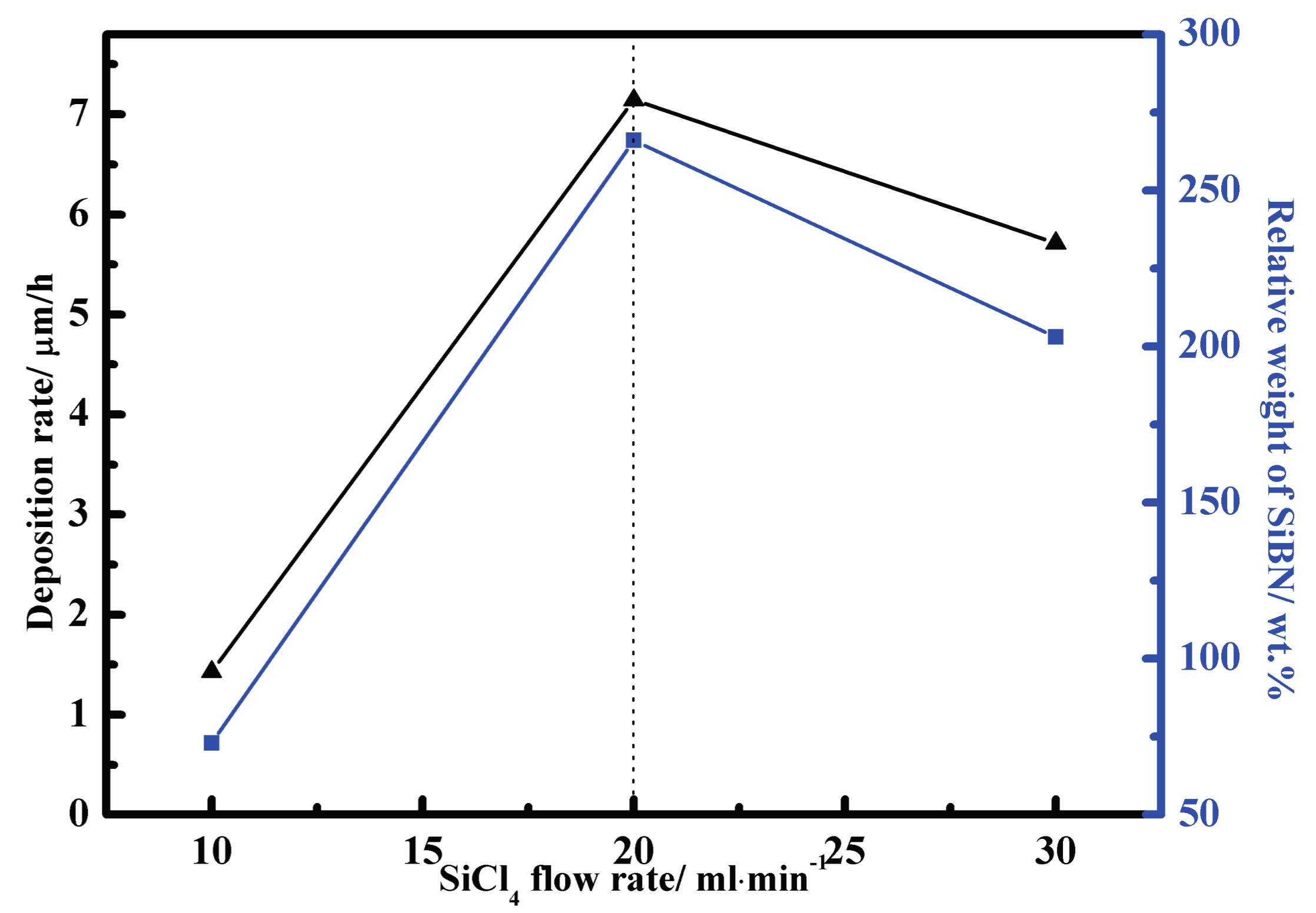
3.1. Deposition Rate
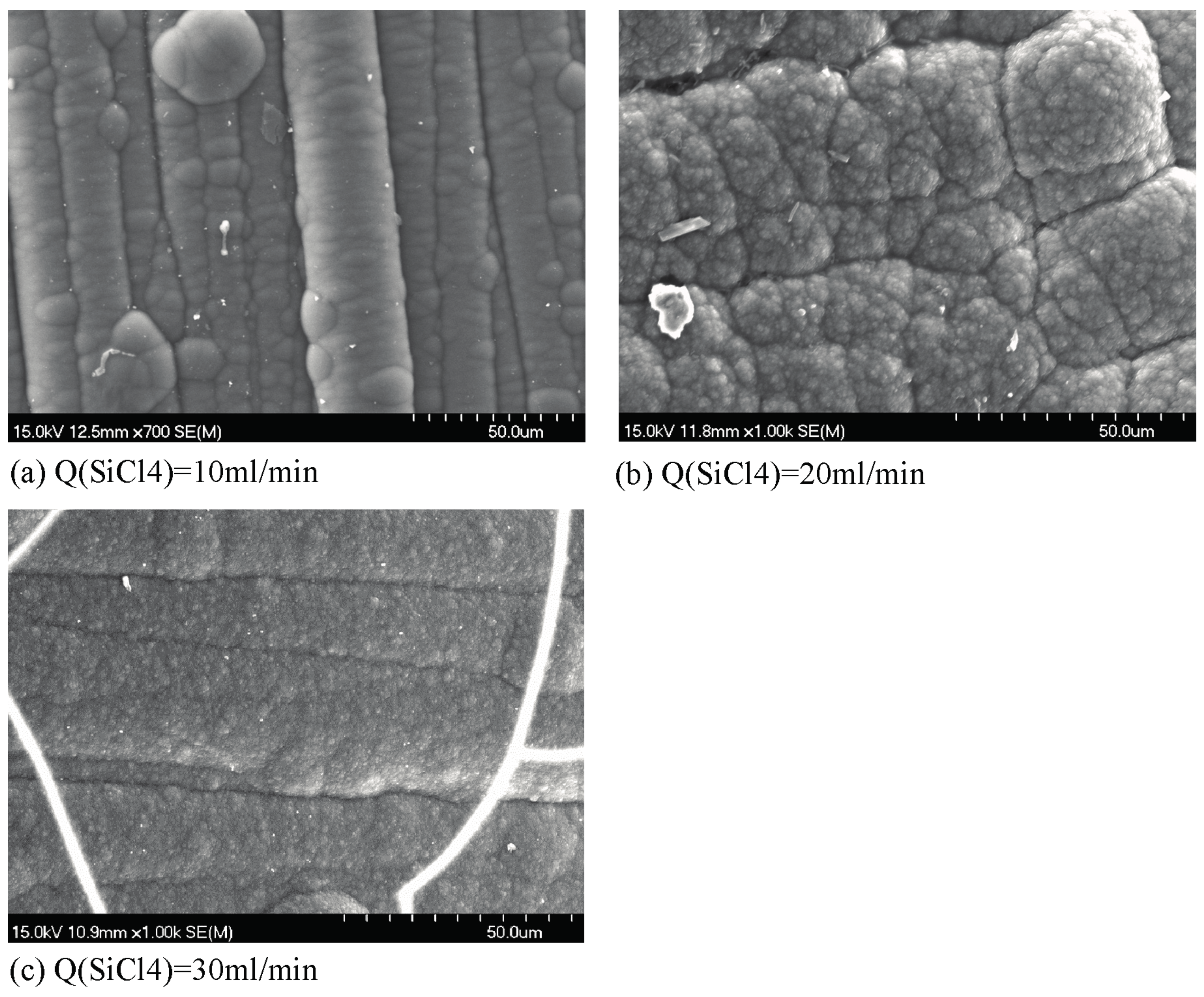
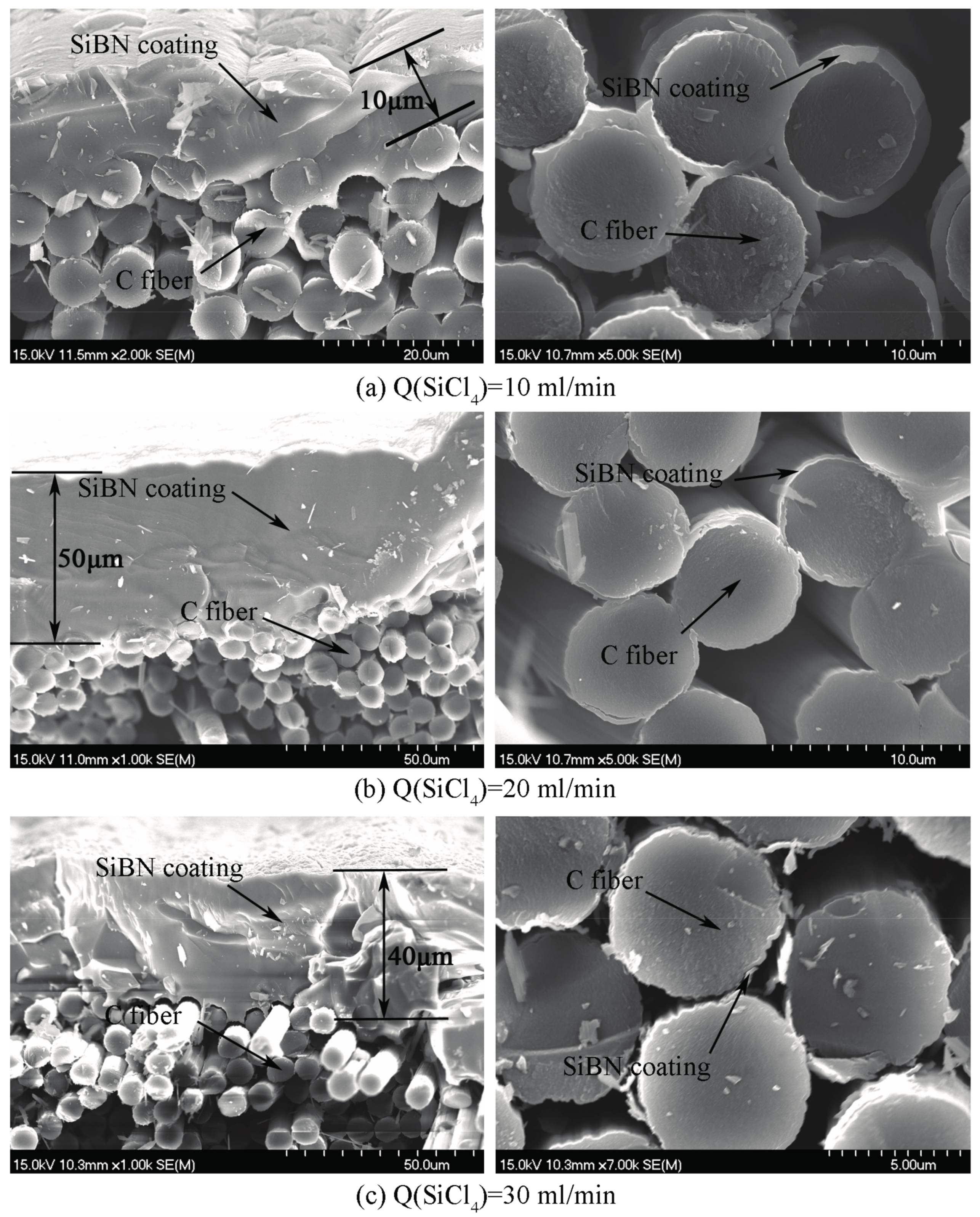
3.2. Morphology
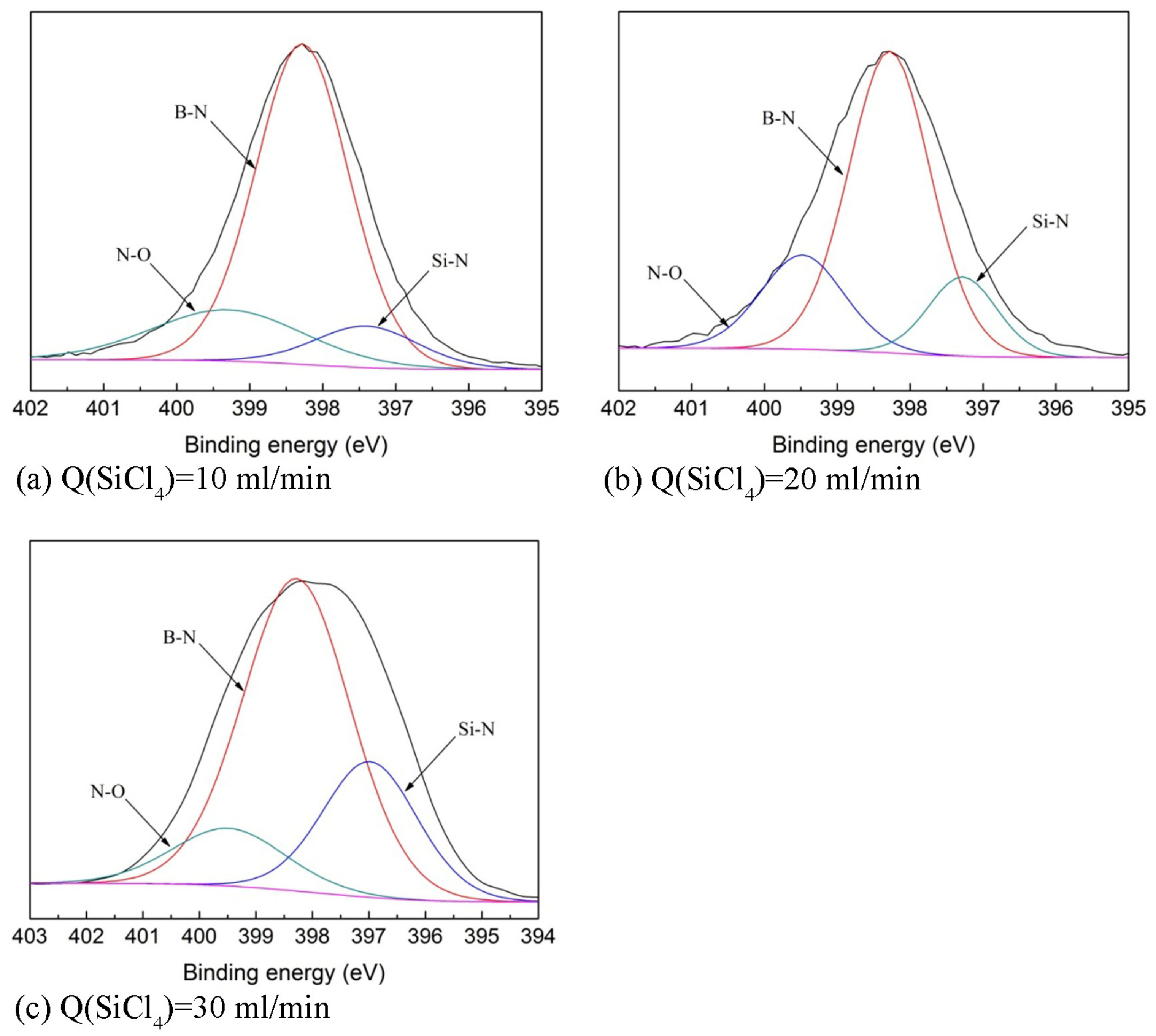
3.3. Phase Composition, Chemical Composition, and Bonding States
3.4. Deposition Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naslain, R. Design, preparation and properties of non-oxide cmcs for application in engines and nuclear reactors: An overview. Compos. Sci. Technol. 2004, 64, 155–170. [Google Scholar] [CrossRef]
- Rosso, M. Ceramic and metal matrix composites: Routes and properties. J. Mater. Process. Technol. 2006, 175, 364–375. [Google Scholar] [CrossRef]
- Morscher, G.N. The effect of static and cyclic tensile stress and temperature on fatlure for precracked hi-nicalon/bn/cvd sic minicomposites in air. In Proceedings of the 21st Annual Conference on Composites, Advanced Ceramics, Materials, and Structures—A: Ceramic Engineering and Science Proceedings, Cocoa Beach, FL, USA, 12–16 January 1997; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 737–745. [Google Scholar]
- Morscher, G.N. Tensile stress rupture of sicf/sicm minicomposites with carbon and boron nitride interphases at elevated temperatures in air. J. Am. Ceram. Soc. 1997, 80, 2029–2042. [Google Scholar] [CrossRef]
- Dugne, O.; Prouhet, S.; Guette, A.; Naslain, R.; Fourmeaux, R.; Khin, Y.; Sevely, J.; Rocher, J.P.; Cotteret, J. Interface characterization by tem, aes and sims in tough SiC (ex-PCS) fibre-SiC (CVI) matrix composites with a bn interphase. J. Mater. Sci. 1993, 28, 3409–3422. [Google Scholar] [CrossRef]
- Hurwitz, F.I.; Scott, J.M.; Chayka, P.V. Progress on bn and doped-bn coatings on woven fabrics. In Proceedings of the 25th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: A: Ceramic Engineering and Science Proceedings, Cocoa Beach, FL, USA, 21–27 January 2001; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 389–397. [Google Scholar]
- Bryant, D.R. Oxidation and Volatilization of Bn Interphases in Sic Fiber-Reinforced Sic Matrix Composites. Master’s Thesis, The Pennsylvania State University—University Park, State College, PA, USA, 1997. [Google Scholar]
- Moore, A.W.; Sayir, H.; Fanner, S.C.; Morscher, G.N. Improved interface coatings for sic fibers in ceramic composites. In Proceedings of the 19th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures-A: Ceramic Engineering and Science Proceedings, Cocoa Beach, FL, USA, 8–12 January 1995; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 409–416. [Google Scholar]
- Nakamura, K.; Sasaki, T. Preparation of sibn films deposited by MOCVD. J. Solid State Chem. 2004, 177, 542–546. [Google Scholar] [CrossRef]
- Essafti, A.; Go’mez-Aleixandre, C.; Albella, J.M. Preparation of SINB films by cvd techniques: Effect of SIH4 addition to B2H6 and NH3 gas mixtures. Diam. Relat. Mater. 1996, 5, 580–583. [Google Scholar] [CrossRef]
- Li, Z. Process and Structure of SIBN Interphase Manufactured by CVD/CVI. Master’s Thesis, Northwestern Polytechnical University, Xi’an, China, 2014. (In Chinese). [Google Scholar]
- Li, Z.; Cheng, L.; Liu, Y.; Ye, F. Thermodynamic analysis of chemical vapor deposition of BCL3-NH3-SICL4-H2-Ar system. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2015, 30, 951–958. [Google Scholar] [CrossRef]
- Liu, Y.; Chai, N.; Qin, H.; Li, Z.; Ye, F.; Cheng, L. Tensile fracture behavior and strength distribution of SiCf/SiC composites with different sibn interface thicknesses. Ceram. Int. 2015, 41, 1609–1616. [Google Scholar] [CrossRef]
- Liu, Y.; Chai, N.; Li, Z.; Ye, F.; Liu, X.; Cheng, L. Effect of deposition temperature on deposition kinetics and mechanism of silicon boron nitride coating deposited from BLC3-NH3-SICL14-H2-Ar mixture using low pressure chemical vapor deposition. Surf. Coat. Technol. 2015, 261, 295–303. [Google Scholar] [CrossRef]
- Meng, G. Chemical Vapor Deposition and New Inorganic Materials; Science Press Ltd.: Beijing, China, 1984; pp. 78–79. (In Chinese) [Google Scholar]

| Substrate | T (°C) | T (h) | QSiCl4 | QBCl3 | QNH3 | QH2 (Dilution) | QAr | P (kPa) |
|---|---|---|---|---|---|---|---|---|
| (mL/min) | (mL/min) | (mL/min) | (mL/min) | (mL/min) | ||||
| Carbon cloth | 900 | 7 | 102030 | 20 | 60 | 100 | 100 | 1 |
| SiCl4 Flow Rate (mL/min) | Element Content (at.%) | |||
|---|---|---|---|---|
| B | N | Si | O | |
| 10 | 42.10 | 36.88 | 6.33 | 14.69 |
| 20 | 31.09 | 38.73 | 14.30 | 15.88 |
| 30 | 30.38 | 33.82 | 18.81 | 16.98 |
| SiCl4 Flow Rate (mL/min) | Bonding Content (at.%) | ||
|---|---|---|---|
| N-B (398.28 eV) | N-Si (397.40 eV) | N-O (399.28 eV) | |
| 10 | 71.11 | 10.27 | 18.62 |
| 20 | 65.01 | 14.34 | 20.65 |
| 30 | 63.45 | 24.25 | 12.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Qin, H.; Liu, Y.; Ye, F.; Li, Z.; Cheng, L.; Zhang, L. Effect of the SiCl4 Flow Rate on SiBN Deposition Kinetics in SiCl4-BCl3-NH3-H2-Ar Environment. Materials 2017, 10, 627. https://doi.org/10.3390/ma10060627
Li J, Qin H, Liu Y, Ye F, Li Z, Cheng L, Zhang L. Effect of the SiCl4 Flow Rate on SiBN Deposition Kinetics in SiCl4-BCl3-NH3-H2-Ar Environment. Materials. 2017; 10(6):627. https://doi.org/10.3390/ma10060627
Chicago/Turabian StyleLi, Jianping, Hailong Qin, Yongsheng Liu, Fang Ye, Zan Li, Laifei Cheng, and Litong Zhang. 2017. "Effect of the SiCl4 Flow Rate on SiBN Deposition Kinetics in SiCl4-BCl3-NH3-H2-Ar Environment" Materials 10, no. 6: 627. https://doi.org/10.3390/ma10060627





