DFT Study of the Oxygen Reduction Reaction Activity on Fe−N4-Patched Carbon Nanotubes: The Influence of the Diameter and Length
Abstract
:1. Introduction
2. Computational Methods and Models
3. Results and Discussion
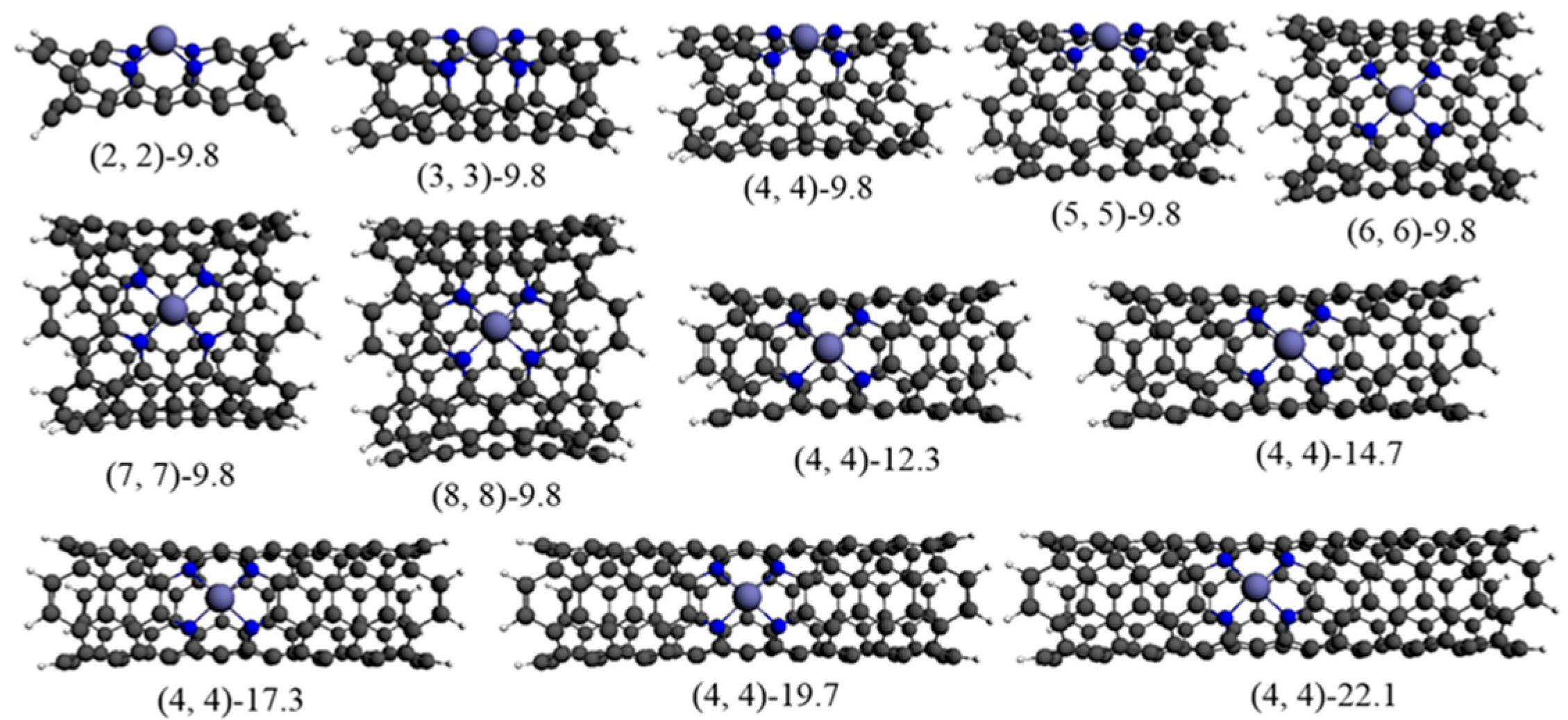
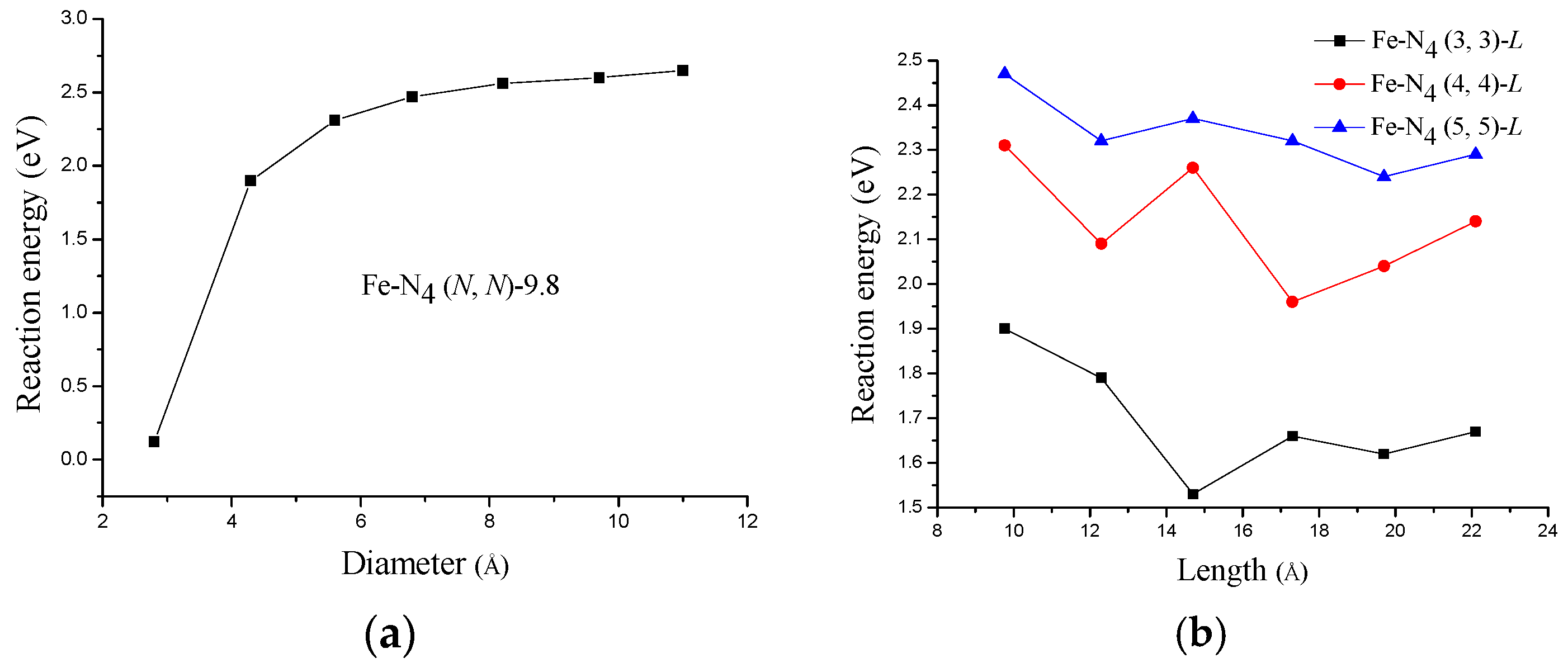
3.1. The Stability of Fe−N4 Catalytic Sites in Acid Medium
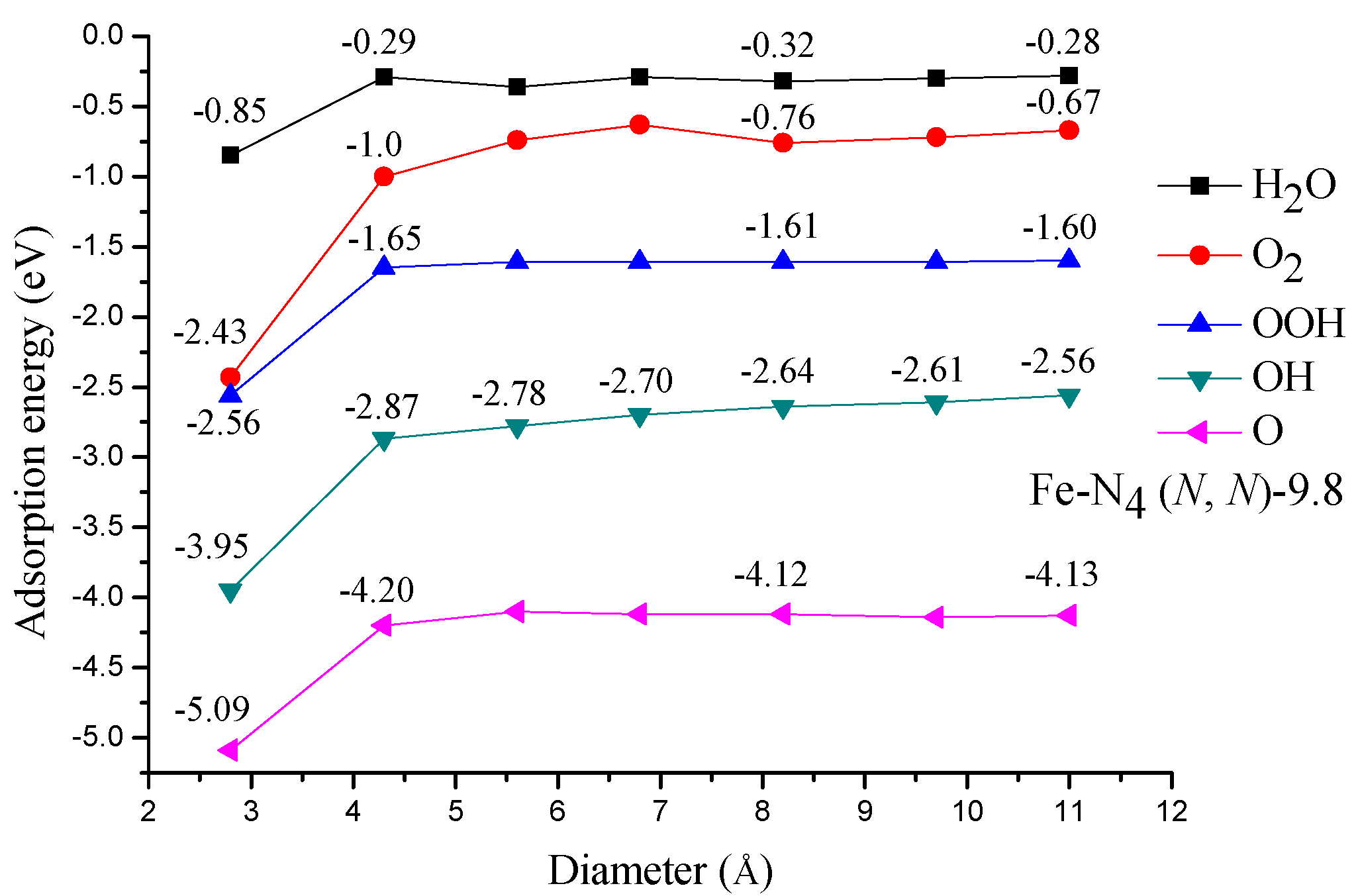
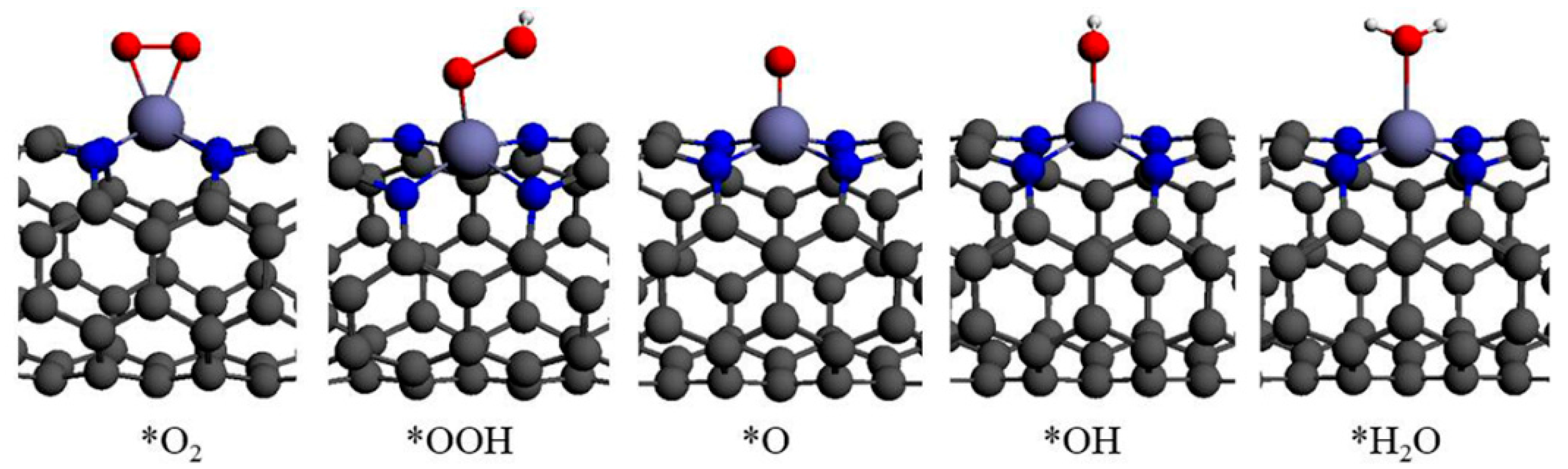
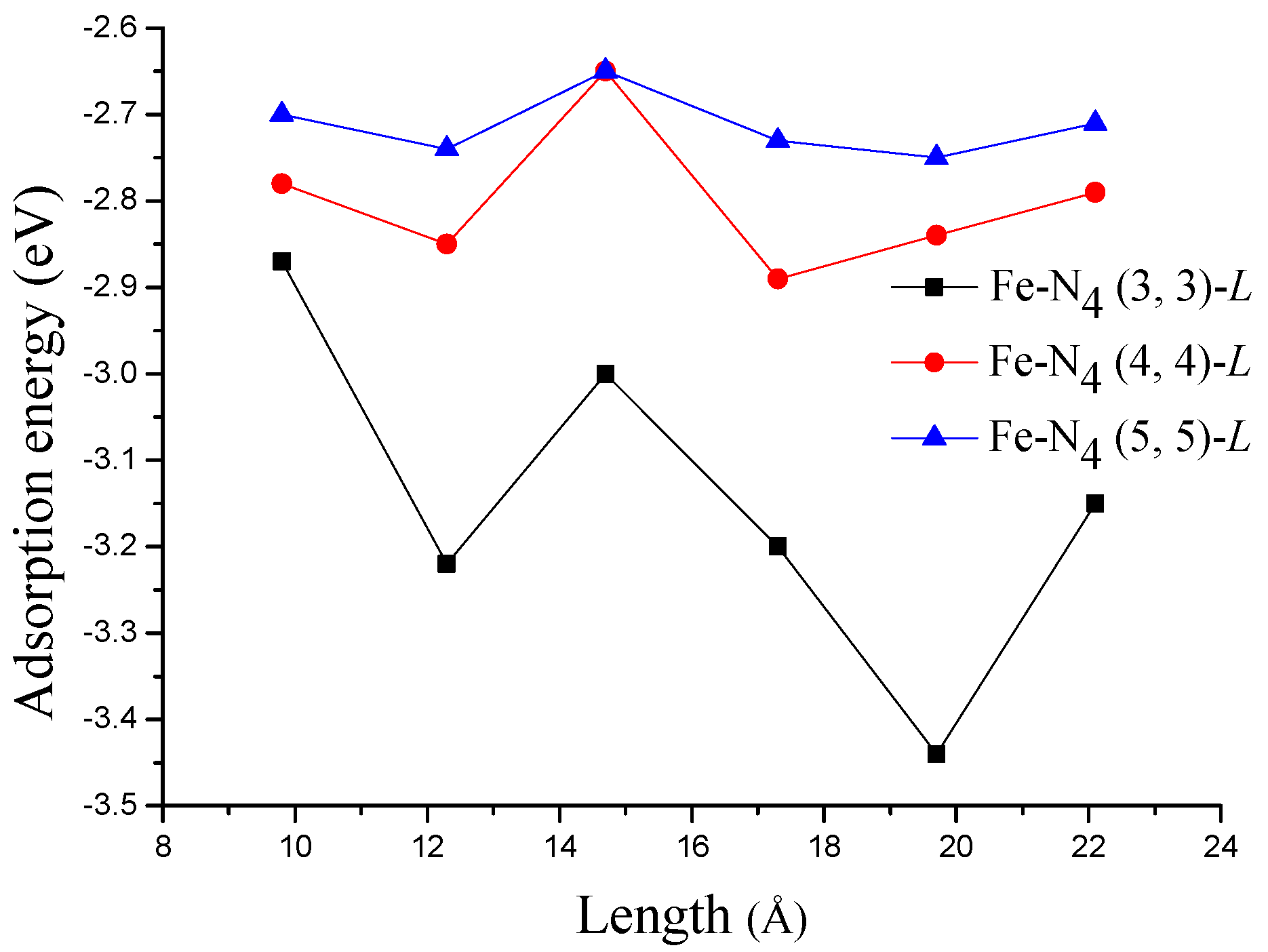
3.2. Adsorption of ORR Species
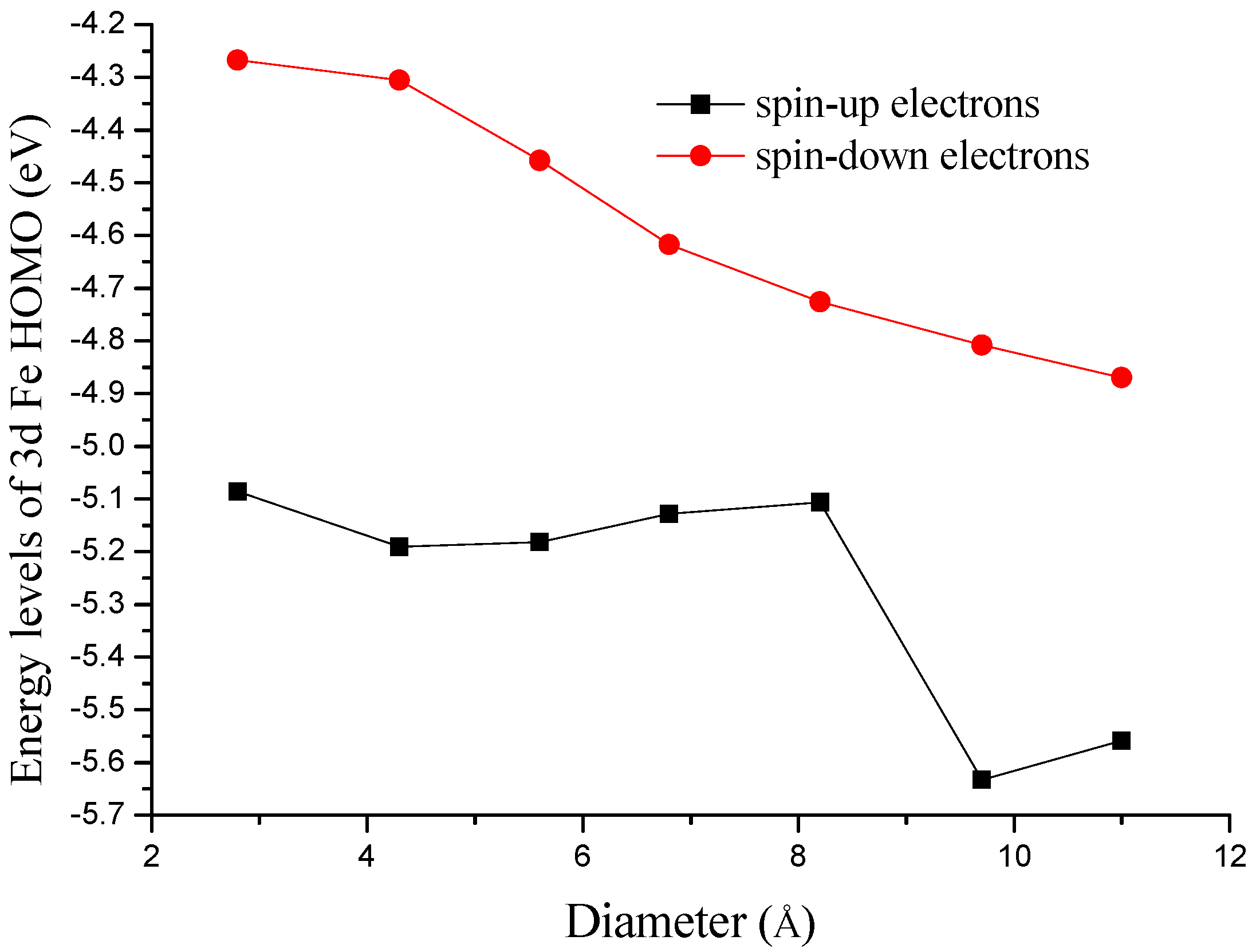
3.3. Electronic Structural Effect on the ORR Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, W.J.; Lee, W.J.; Kim, S.O.; Kim, Y.H. Theory, synthesis, and oxygen reduction catalysis of Fe-porphyrin-like carbon nanotube. Phys. Rev. Lett. 2011, 106, 175502. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, H.; Chu, D.; Wang, G. Unraveling oxygen reduction reaction mechanisms on carbon-supported Fe-phthalocyanine and Co-phthalocyanine catalysts in alkaline solutions. J. Phys. Chem. C 2009, 113, 20689–20697. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Orellana, W. Catalytic properties of transition metal-N4 moieties in graphene for the oxygen reduction reaction: Evidence of spin-dependent mechanisms. J. Phys. Chem. C 2013, 117, 9812–9818. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Malotki, C.; Arnold, L.; Koshino, N.; Higashimura, H.; Baumgarten, M.; Müllen, K. Triangular trinuclear metal-N4 complexes with high electrocatalytic activity for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 10372–10375. [Google Scholar] [CrossRef] [PubMed]
- Byon, H.R.; Suntivich, J.; Shao-Horn, Y. Graphene-based non-noble-metal catalysts for oxygen reduction reaction in acid. Chem. Mater. 2011, 23, 3421–3428. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Atanassov, P.; Kiefer, B. Density functional theory study of Ni−Nx/C electrocatalyst for oxygen reduction in alkaline and acidic medium. J. Phys. Chem. C 2012, 116, 17378–17383. [Google Scholar] [CrossRef]
- Kattel, S.; Atanassov, P.; Kiefer, B. Catalytic activity of Co−Nx/C electrocatalysts for oxygen reduction reaction: A density functional theory study. Phys. Chem. Chem. Phys. 2013, 15, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, F.; Zhang, N.; An, L.; Xia, D. Mechanism of oxygen reduction reaction catalyzed by Fe (Co)−Nx/C. Phys. Chem. Chem. Phys. 2013, 15, 19330–19336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Liu, Z.F. Oxidation of zigzag carbon nanotubes by singlet O2: Dependence on the tube diameter and the electronic structure. J. Phys. Chem. B 2004, 108, 11435–11441. [Google Scholar] [CrossRef]
- Zurek, E.; Autschbach, J. Density functional calculations of the 13C NMR chemical shifts in (9, 0) single-walled carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 13079–13088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Balbuena, P.B. Structural and reactivity properties of finite length cap-ended single-wall carbon nanotubes. J. Phys. Chem. A 2006, 110, 2771–2775. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, C.; Wu, Y.; Zhang, Z. Density functional theory study on nitrogen-doped carbon nanotubes with and without oxygen adsorption: The influence of length and diameter. New J. Chem. 2011, 35, 2601–2606. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Gland, J.L.; Sexton, B.A.; Fisher, G.B. Oxygen interactions with the Pt(111) surface. Surf. Sci. 1980, 95, 587–602. [Google Scholar] [CrossRef]
- Campbell, C.T.; Ertl, G.; Kuipers, H.; Segner, J. A molecular beam study of the adsorption and desorption of oxygen from a Pt(111) surface. Surf. Sci. 1981, 107, 220–236. [Google Scholar] [CrossRef]
- Nolan, P.D.; Lutz, B.R.; Tanaka, P.L.; Davis, J.E.; Mullins, C.B. Molecularly chemisorbed intermediates to oxygen adsorption on Pt (111): A molecular beam and electron energy-loss spectroscopy study. J. Chem. Phys. 1999, 111, 3696–3704. [Google Scholar] [CrossRef]
- Sha, Y.; Yu, T.H.; Liu, Y.; Merinov, B.V.; Goddard, W.A. Theoretical study of solvent effects on the platinum-catalyzed oxygen reduction reaction. J. Phys. Chem. Lett. 2010, 1, 856–861. [Google Scholar] [CrossRef]
- Qi, L.; Qian, X.; Li, J. Near neutrality of an oxygen molecule adsorbed on a Pt (111) surface. Phys. Rev. Lett. 2008, 101, 146101. [Google Scholar] [CrossRef] [PubMed]
- Hyman, M.P.; Medlin, J.W. Effects of electronic structure modifications on the adsorption of oxygen reduction reaction intermediates on model Pt (111)-alloy surfaces. J. Phys. Chem. C 2007, 111, 17052–17060. [Google Scholar] [CrossRef]
- Feng, Y.; Li, F.; Hu, Z.; Luo, X.; Zhang, L.; Zhou, X.F.; Wang, H.T.; Xu, J.J.; Wang, E.G. Tuning the catalytic property of nitrogen-doped graphene for cathode oxygen reduction reaction. Phys. Rev. B 2012, 85, 155454. [Google Scholar] [CrossRef]
- Lyalin, A.; Nakayama, A.; Uosaki, K.; Taketsugu, T. Theoretical predictions for hexagonal BN based nanomaterials as electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2013, 15, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Roques, J.; Anderson, A.B. Pt3Cr (111) alloy effect on the reversible potential of OOH (ads) formation from O2 (ads) relative to Pt (111). J. Fuel Cell Sci. Technol. 2005, 2, 86–93. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Wang, J. Screening of catalytic oxygen reduction reaction activity of metal-doped graphene by density functional theory. Appl. Surf. Sci. 2016, 379, 291–295. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Yu, Z.; Ke, Q. A comparative DFT study of oxygen reduction reaction on mononuclear and binuclear cobalt and iron phthalocyanines. Russ. J. Phys. Chem. A 2016, 90, 2413–2417. [Google Scholar] [CrossRef]
- Han, B.C.; Miranda, C.R.; Ceder, G. Effect of particle size and surface structure on adsorption of O and OH on platinum nanoparticles: A first-principles study. Phys. Rev. B 2008, 77, 075410. [Google Scholar] [CrossRef]
- Jacob, T.; Goddard, W.A. Water formation on Pt and Pt-based alloys: A theoretical description of a catalytic reaction. ChemPhysChem 2006, 7, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chang, J.; Yan, H.; Xia, D. Boron nitride nanocages as high activity electrocatalysts for oxygen reduction reaction: Synergistic catalysis by dual active sites. J. Phys. Chem. C 2016, 120, 28912–28916. [Google Scholar] [CrossRef]
- Chen, X. Graphyne nanotubes as electrocatalysts for oxygen reduction reaction: The effect of doping elements on the catalytic mechanisms. Phys. Chem. Chem. Phys. 2015, 17, 29340–29343. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.B.; Gland, J.L. The interaction of water with the Pt(111) surface. Surf. Sci. 1980, 94, 446–455. [Google Scholar] [CrossRef]
- Keith, J.A.; Jacob, T. Theoretical studies of potential-dependent and competing mechanisms of the electrocatalytic oxygen reduction reaction on Pt (111). Angew. Chem. Int. Ed. 2010, 49, 9521–9525. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Wang, G. Reaction pathway for oxygen reduction on FeN4 embedded graphene. J. Phys. Chem. Lett. 2014, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.L.; Hou, Z.; Shu, D.J.; Ikeda, T.; Terakura, K. Active sites and mechanisms for oxygen reduction reaction on nitrogen-doped carbon alloy catalysts: Stone−Wales defect and curvature effect. J. Am. Chem. Soc. 2014, 136, 13629–13640. [Google Scholar] [CrossRef] [PubMed]
- Vayner, E.; Anderson, A.B. Theoretical predictions concerning oxygen reduction on nitrided graphite edges and a cobalt center bonded to them. J. Phys. Chem. C 2007, 111, 9330–9336. [Google Scholar] [CrossRef]
- Chen, X.; Sun, F.; Chang, J. Cobalt or nickel doped SiC nanocages as efficient electrocatalyst for oxygen reduction reaction: A computational prediction. J. Electrochem. Soc. 2017, 164, F616–F619. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Norskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. 2006, 118, 2963–2967. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.H.B.; Zhang, J.; Shao, M.H.; Sasaki, K.; Vukmirovic, M.B.; Ticianelli, E.A.; Adzic, R.R. Catalytic activity−d-band center correlation for the O2 reduction reaction on platinum in alkaline solutions. J. Phys. Chem. C 2007, 111, 404–410. [Google Scholar] [CrossRef]
- Chen, X. Oxygen reduction reaction on cobalt–(n)pyrrole clusters from DFT studies. RSC Adv. 2016, 6, 5535–5540. [Google Scholar] [CrossRef]
| Reaction Step | (2, 2) | (3, 3) | (4, 4) | (5, 5) | (6, 6) | (7, 7) | (8, 8) | Pt(111) |
|---|---|---|---|---|---|---|---|---|
| O2 + H+ + e− → * OOH | −2.50 | −1.59 | −1.55 | −1.55 | −1.55 | −1.55 | −1.54 | −1.02 |
| * OOH + H+ + e− → * O + H2O | −1.89 | −1.90 | −1.85 | −1.86 | −1.87 | −1.88 | −1.88 | −2.01 |
| * O + H+ + e− → * OH | −1.26 | −1.07 | −1.09 | −0.99 | −0.93 | −0.88 | −0.85 | −0.77 |
| * OH + H+ + e− → H2O | +0.88 | +0.21 | +0.28 | −0.37 | −0.42 | −0.46 | −0.50 | −0.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hu, R.; Bai, F. DFT Study of the Oxygen Reduction Reaction Activity on Fe−N4-Patched Carbon Nanotubes: The Influence of the Diameter and Length. Materials 2017, 10, 549. https://doi.org/10.3390/ma10050549
Chen X, Hu R, Bai F. DFT Study of the Oxygen Reduction Reaction Activity on Fe−N4-Patched Carbon Nanotubes: The Influence of the Diameter and Length. Materials. 2017; 10(5):549. https://doi.org/10.3390/ma10050549
Chicago/Turabian StyleChen, Xin, Rui Hu, and Fan Bai. 2017. "DFT Study of the Oxygen Reduction Reaction Activity on Fe−N4-Patched Carbon Nanotubes: The Influence of the Diameter and Length" Materials 10, no. 5: 549. https://doi.org/10.3390/ma10050549






