1. Introduction
Global warming is one of the most important recent environmental concerns, which is mostly caused by anthropogenic activities. Carbon dioxide (CO
2) is believed to be the primary greenhouse gas emitted from human activities, accounting for about 96% of greenhouse gases from human activities in Taiwan [
1] and 84% in the USA [
2]. CO
2 capture and sequestration (CCS) is a set of technologies that can greatly reduce CO
2 emissions from new and existing emission sources and is considered as an effective approach in mitigating global warming. Since most anthropogenic CO
2 was a byproduct of the combustion of fossil fuels from coal-fired plants and large industrial sources, CO
2 capture technologies were commonly classified as pre-combustion capture, post-combustion capture, and oxy-combustion systems [
3]. Among the different CCS methods available, post-combustion capture appears to be the most feasible approach as it can be retrofitted to existing power plants without upgrading or modifying the existing systems [
4]. Accordingly, much effort has been expended to develop various physical and chemical methods for post-combustion CO
2 capture [
5].
The capture technology is the most important for cost-effective removal of CO
2 from the atmosphere since the expenses during capture processes account for about 70% of the overall costs in CCS [
6]. Various CO
2 capture technologies, such as absorption, adsorption, cryogenics, and membranes, have been widely studied. Among them, absorption-regeneration technologies have been recognized as the most effective methods, where the processes by using ammonia-based or amine-based absorbents have received much attention [
7]. However, the comparatively high energy demand of absorption processes restricts their applications. In 2005, the Intergovernmental Panel on Climate Change (IPCC) special report disclosed adsorption methods might be promising processes to separate CO
2 from gases by choosing appropriate adsorbents [
8]. In this respect, several possible adsorbents, including activated carbon [
9], activated carbon fibers (ACFs) [
6], zeolite [
10], silica adsorbents [
11], or carbon nanotubes (CNTs) [
12], have been considered as potential candidates for CO
2 capture.
The specific adsorption capacity may be enhanced by increasing the affinity of the adsorbent surface to CO
2. Due to the acidic role of CO
2 (a weak Lewis acid), it is expected that the introduction of Lewis bases onto the activated carbon surfaces may favor CO
2 capture performance of these materials. Nitrogen enrichment was reported to be effective in introducing basic functionalities that enhanced the specific adsorbent-adsorbate interaction for CO
2 [
13]. The N functional groups on the surface of adsorbents gave the Lewis-base active sites to the materials, inducing electron-donor properties, which might be relevant for the capture of acidic gases, such as CO
2 [
14]. The methods of obtaining N-enriched carbons included the introduction of N in precursors of carbons, the use of activation agents containing N, and the exposure to nitrogen compounds at elevated temperatures [
15].
Among of the known adsorbents, ACFs are featured with the advantages of small fiber diameter, large specific surface area, uniform pore size distribution, and rapid adsorption/desorption rate. In addition, the hierarchical composites composed of microscaled substrates decorated with nanoscaled materials have attracted much attention due to several synergistic effects being realized from multidisciplinary researchers [
16]. The ideas of growing carbon nanotubes (CNTs) onto the carbon fibers (CFs) could be traced back to the studies of Downs and Baker [
17,
18]. Direct growth of CNTs on the CF surfaces has been proposed to modify the interface of the fibers and tailor the physical properties [
19]. Grafting CNTs on the surface of carbon fibers is an effective method to create mechanical interlocking, and/or local stiffening, at the fiber/matrix interface, all of which may improve stress transfer and interfacial properties [
16,
19]. The growth of CNTs on the surface of fibers along the radial orientation could also increase the transverse reinforcement [
20]. The interfacial shear strength of the CFs is also shown to be significantly improved due to the CNTs grown on the CF surface [
21]. Greef et al. [
22] have compared two processes of growing CNTs directly onto the CFs and found that the growth temperature was the most critical parameter. They indicated that 700 °C was the optimal temperature to obtain a high number of CNTs grafted homogeneously on the CFs.
The catalytic growth of CNTs strongly depends on the process conditions, such as carbon sources and their feeding rates, types, and concentrations of the catalysts, temperature, and atmosphere. When the CNTs were doped with N atoms through a replacement of C atoms, the local physical properties around N atoms generated a significant change, resulting in a change of local chemical reactivity. This change increased the binding energy and the possibility of gas molecules interacting with N-doped CNTs (CNs). The adsorption capacity of CO
2 on KOH-modified ACFs was achieved to 250 mg/g at 25 °C and 1 atm, while that on as-received ACFs was only 140 mg/g [
6]. The adsorption amount of CO
2 on CNTs and CNTs modified by 3-aminopropyltriethoxysilane decreased with temperature, but increased with water content in air at 0–7% [
12].
The growth of CNTs directly on the CF surface has gained much attention for the applications of CF-reinforced polymer composites. The growth of CNTs has been considered as one of surface modification methods of CFs to improve interface shear strength between CFs and the matrix. However, the studies related to the characteristics and applications of the CNTs grown on the ACFs are scarce in the literature. Tzeng et al. [
23] have tried to utilize ACFs as the substrate to grow carbon nanofibers (CNFs), taking advantage of their large amounts of micropores to obtain a uniform dispersion of the nanosized catalytic metal ions for CNF growth. Kong et al. [
24] fabricated a micro-nano-scaled CNT/hollow ACF composite and attempted to propose the possible formation mechanisms. However, these studies did not show the applications of their multi-scaled composites. Moreover, the publications about multi-scaled composites have seldom discussed the applications of CNs. Therefore, the objective of this paper is to investigate the physicochemical properties of ACFs grafted with CNs (CNs/ACFs) and determine their adsorption capacity of CO
2.
2. Experimental Methods
2.1. Synthesis of CNs-Grafted ACFs
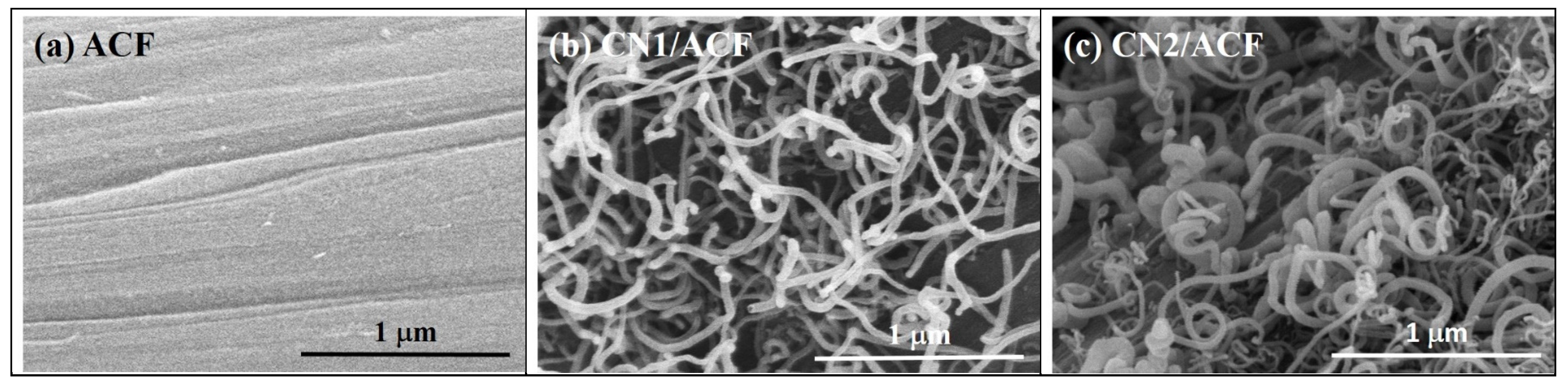
One commercial PAN-based ACF sample manufactured by Taiwan Carbon Technology Co. (Taichung, Taiwan) was used as the starting material (denoted as ACF). The chemical vapor deposition (CVD) method was used for the growth of CNs directly onto ACFs through an iron-catalyzed reaction in a tubular furnace system. The CVD system consisted of a horizontal tubular furnace with a three-zone heating and a quartz tube with a diameter of 2.54 cm. Acetonitrile was used as both the C and N sources and the ferrocene as the catalyst precursor, where the mixture of acetonitrile and ferrocene were prepared with a ratio of Fe/C = 1.3 (w/w). The ACF samples (9 cm × 8 cm, about 1.0 g) were settled inside the quartz tube and the mixture of acetonitrile and ferrocene was injected into the tubular furnace with at a rate of 1.2 mL/h using a syringe pump (KD Scientific). The process was carried out for 2 h at 900 °C in a flowing 5% H2/Ar gas mixture (500 sccm). To compare the effects of the catalyst compositions, the ACF samples were immersed in 0.05 M cobalt(II) acetate (Co(C2H3O2)2) solution and mixed for 3 h. Then the samples were dried at 100 °C for 24 h before being placed in the furnace. The products were denoted as CN1/ACF or CN2/ACF, depending on whether the ACF samples were without or with pre-immersion of the 0.05 M cobalt(II) acetate solution.
2.2. Characterization Techniques
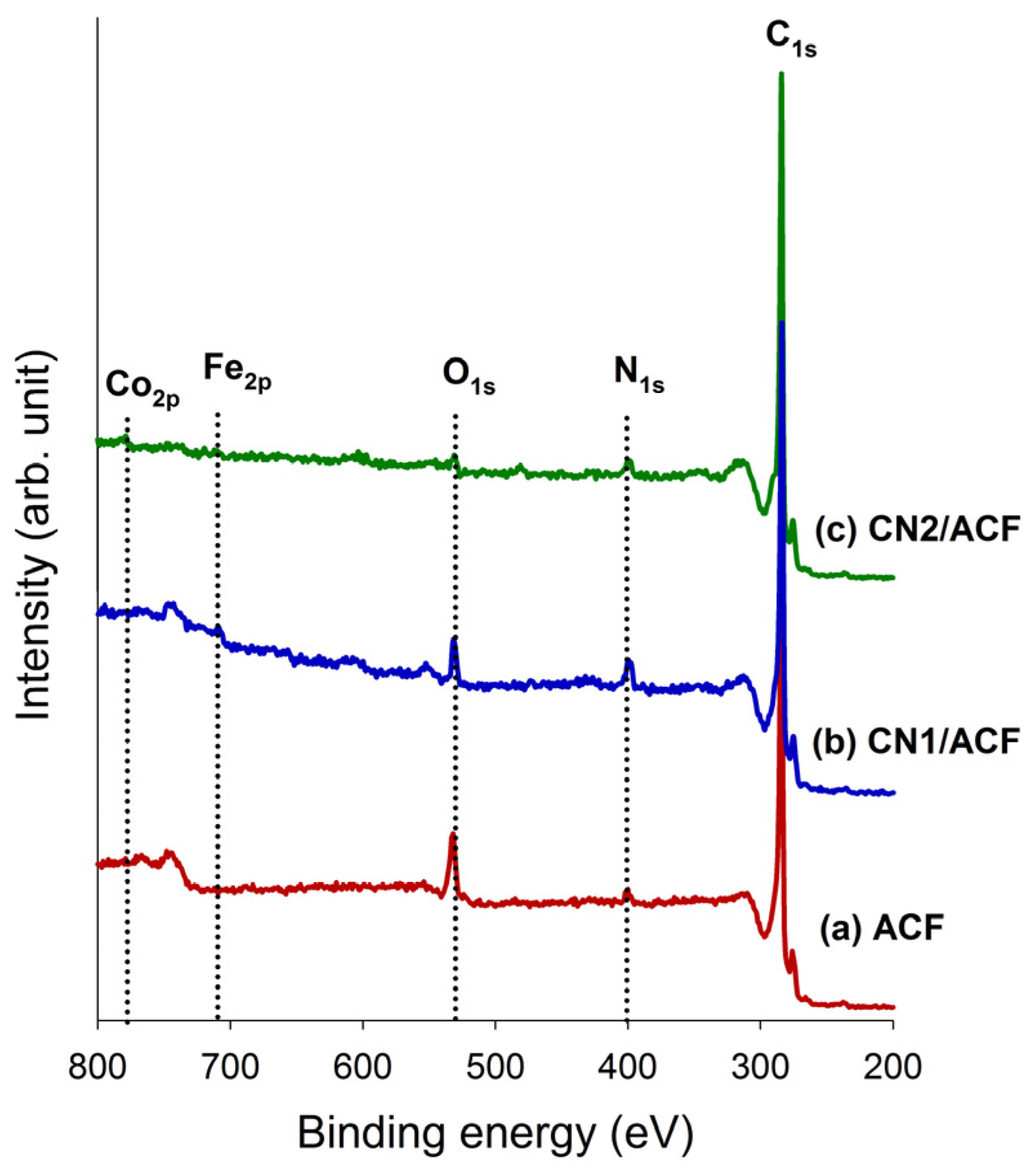
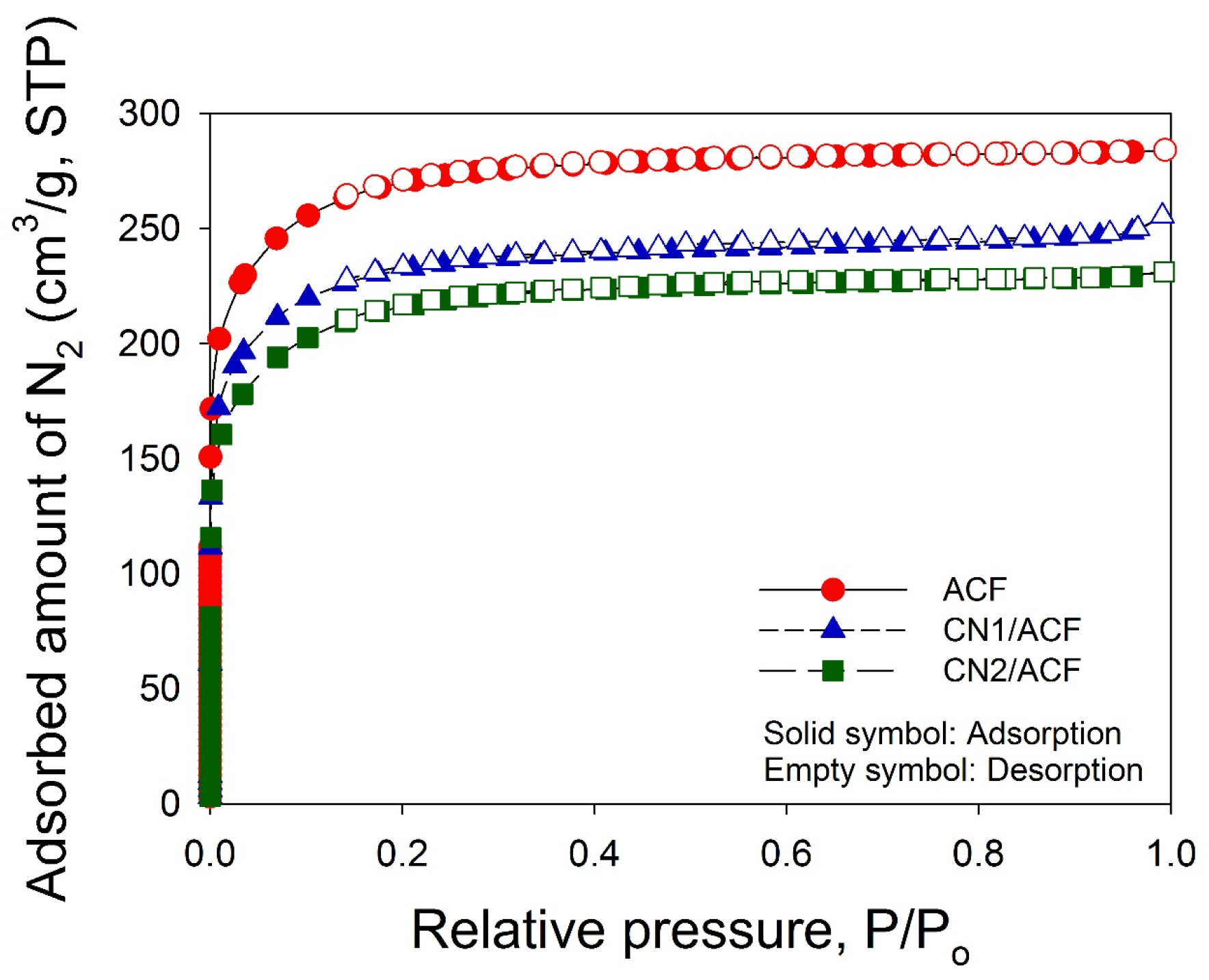
The as-received ACF and CNs-grafted ACF were characterized using several techniques. The surface morphology of the samples was inspected using field emission scanning electron microscopy (FESEM, S-4700, Hitachi, Krefeld, Germany). The oxidation resistance of the samples was determined in flowing air (60 cm3/min) with a heating rate of 10 °C/min, using a thermogravimetric analyzer (Q500, TA Instrument, New Castle, DE, USA) to measure any changes in the weight of the sample as a function of temperature (TGA plot) and the rate of weight loss versus temperature (differential thermogravimetry, DTG, plot). X-ray photoelectron spectroscopy (XPS) was used to determine the number and type of functional groups present on the surface of the samples. The XPS spectra of all samples were obtained using a spectrophotometer (PHI 1600 ESCA, Perkin-Elmer, Waltham, MA, USA). A twin anode Mg X-ray source (hν = 1253.6 eV) was used, at a voltage of 15 kV and a power of 400 W. For calibration purposes, the C1s electron binding energy that corresponds to graphitic carbon was set at 284.6 eV. A nonlinear least squares curve-fitting program (XPSPEAK software, version 4.1, The Chinese University of Hong Kong, Hong Kong, China) was used for deconvolution of the XPS spectra. The surface feature of the samples was probed by N2 adsorption/desorption isotherms measured at –196 °C using an ASAP 2020 (Micromeritics, Norcross, GA, USA) accelerated surface area and porosimetry system.
2.3. CO2 Adsorption
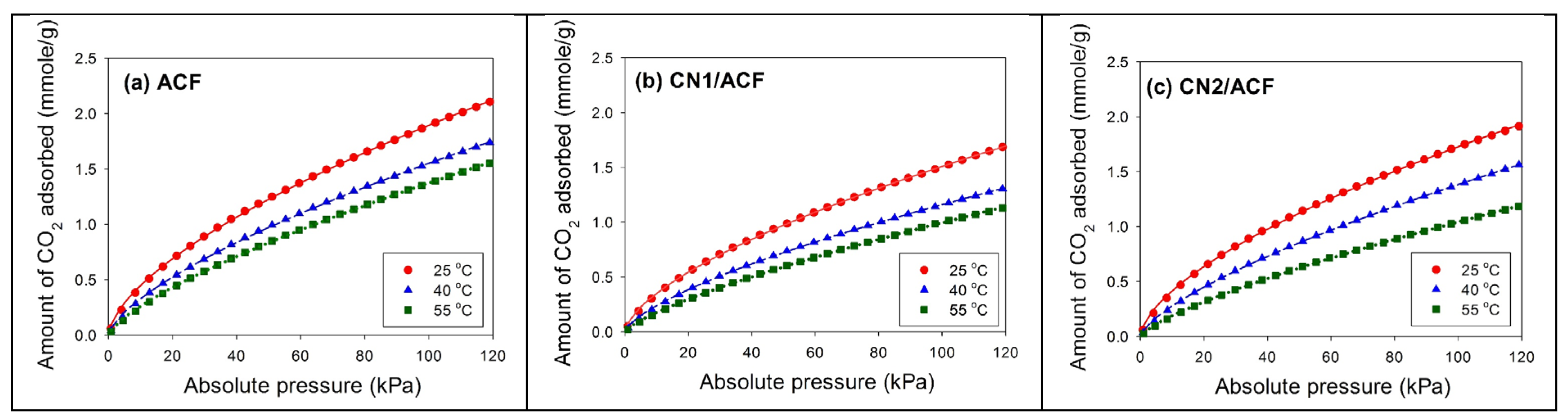
The CO
2 adsorption isotherms on all samples were measured using a Micromeritics ASAP 2020 accelerated surface and porosimetry analysis system at 25 °C, 40 °C, or 55 °C. The sample (~0.08 g) was outgassed at 350 °C overnight to remove adsorbed contaminants prior to the measurement. The equilibration interval for each pressure point was 30 s. The temperature during the CO
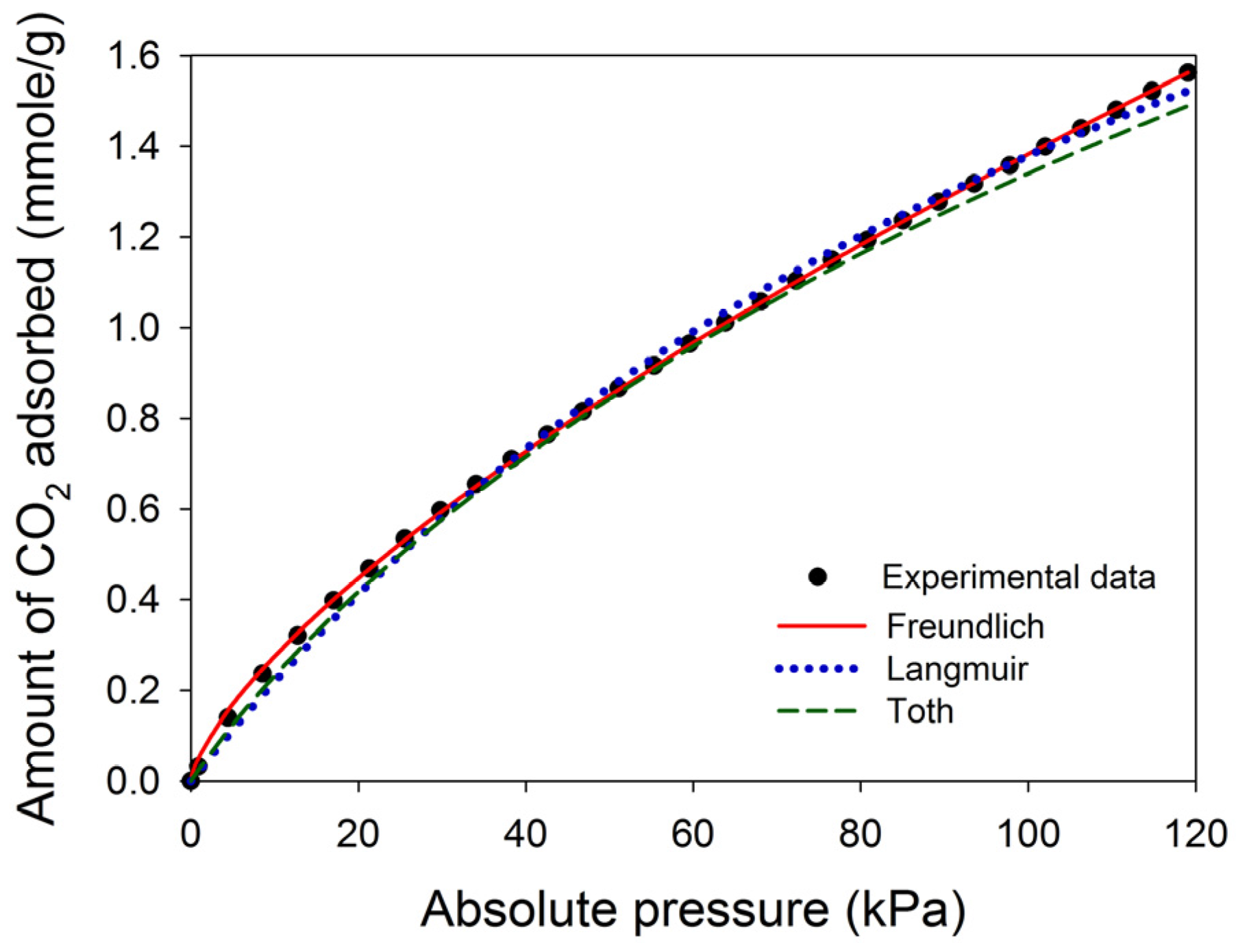
2 adsorption process was maintained by a circulating water bath thermostat. In this study, three common adsorption isotherms, including the Freundlich equation, Langmuir equation, and Toth equation, were used to fit the experimental data [
25]. The Freundlich isotherm (Equation (1)) is an empirical equation that assumes heterogeneous adsorption due to the diversity of adsorption sites:
where
qe (mmol/g, STP) is the equilibrium adsorption capacity,
P (kPa) is the equilibrium gas pressure,
KF [(mmol/g)(1/kPa)
1/n] is the Freundlich adsorption coefficient, and
n is a constant indicating the isotherm curvature. The parameter
n is usually greater than unity, and the larger this value is, the more nonlinear the adsorption isotherm becomes. The parameters
n and
KF both usually decrease with increasing temperature.
The Langmuir isotherm assumes that the adsorption involves the attachment of only one layer of molecules to the surface, i.e., monolayer adsorption, which is shown in Equation (2):
where
qm (mmol/g) is the monolayer adsorption capacity and
KL (1/kPa) is the Langmuir adsorption equilibrium coefficient. Equation (3) shows the Toth isotherm, a three-parameter model that has been particularly used to describe gas-phase adsorption data. It reduces to the Henry type at low pressures and approaches the saturation limit at high pressures:
where
qT (mmol/g) is the saturation adsorption capacity,
KT (1/kPa) is the Toth adsorption equilibrium coefficient, and
t is the degree of the heterogeneity of the adsorbent. When
t equals unity, the above equation is identical to the Langmuir equation.
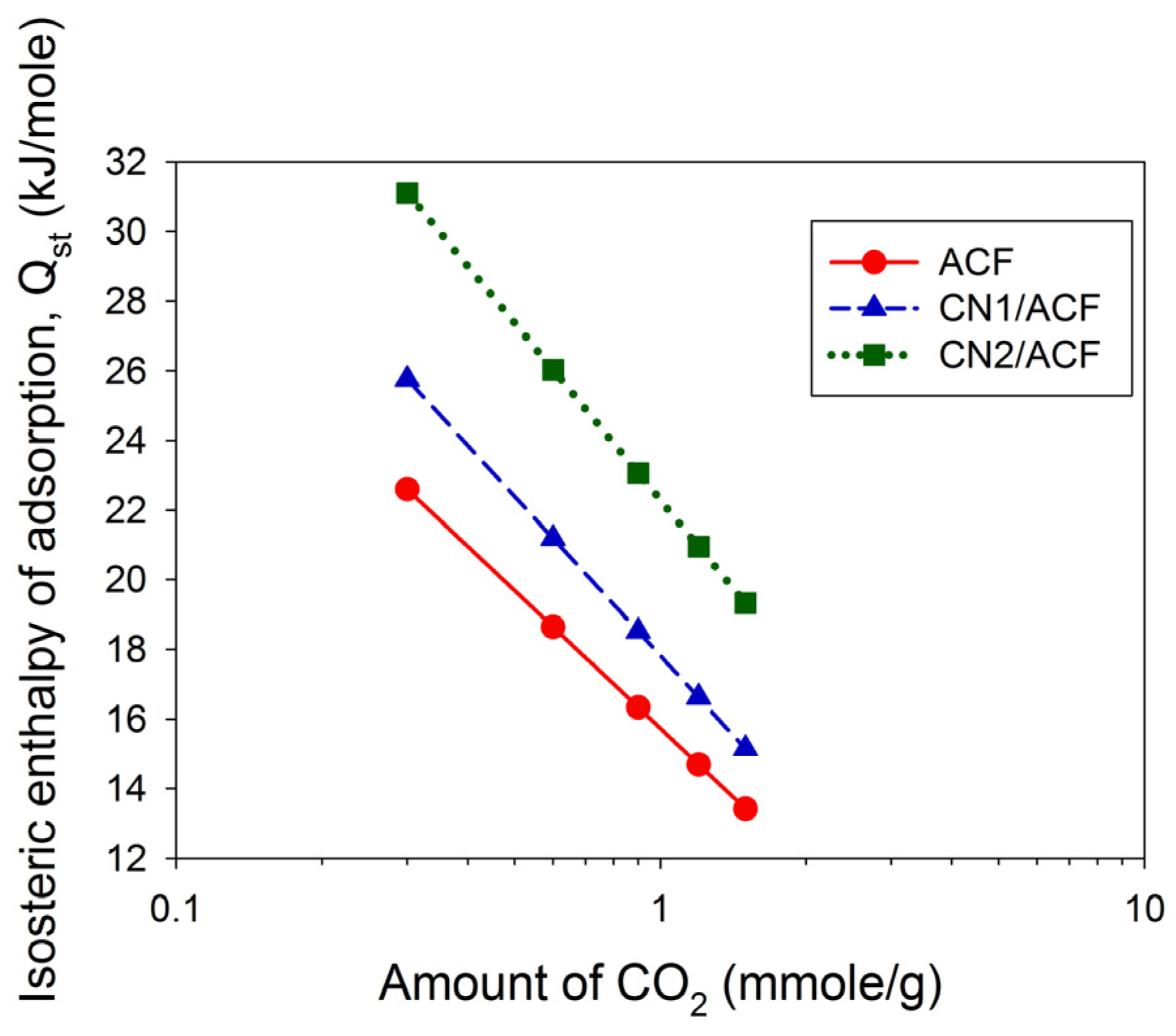
Finally, the Clausius–Clapeyron equation, Equation (4), was used to calculate the isosteric enthalpy of adsorption:
where
Qst (kJ/mol) is the isosteric enthalpy of adsorption,
R (8.314 J/mol/K) is the gas constant,
T (K) is the adsorption temperature, and
A is the coefficient.