The Results of 45 Years of Atmospheric Corrosion Study in the Czech Republic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
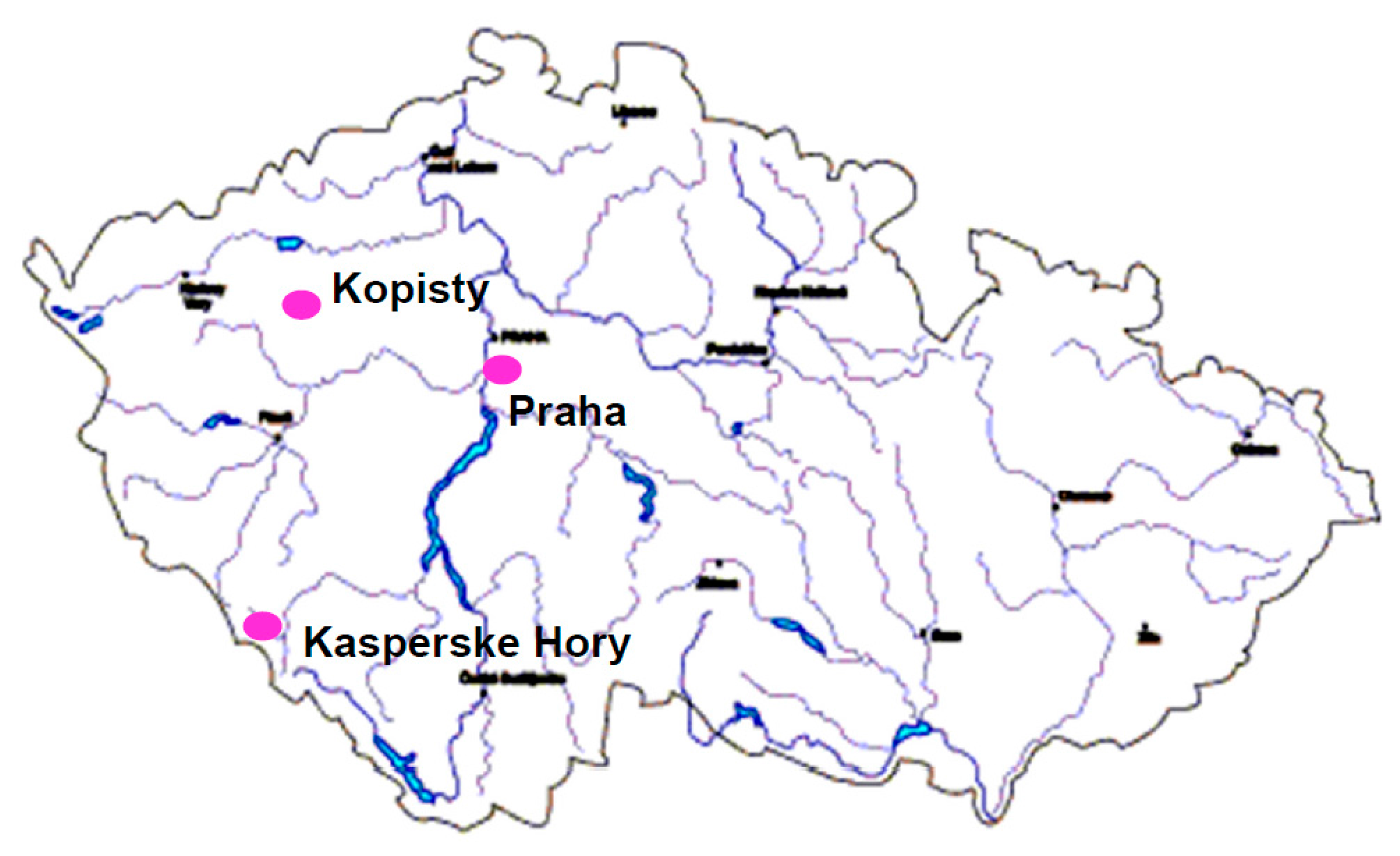
2.2. Atmospheric Test Sites
- (a)
- Prague—Urban atmospheric environment;
- (b)
- Kopisty—Industrial atmospheric environment;
- (c)
- Kasperske Hory—Rural atmospheric environment.
3. Results
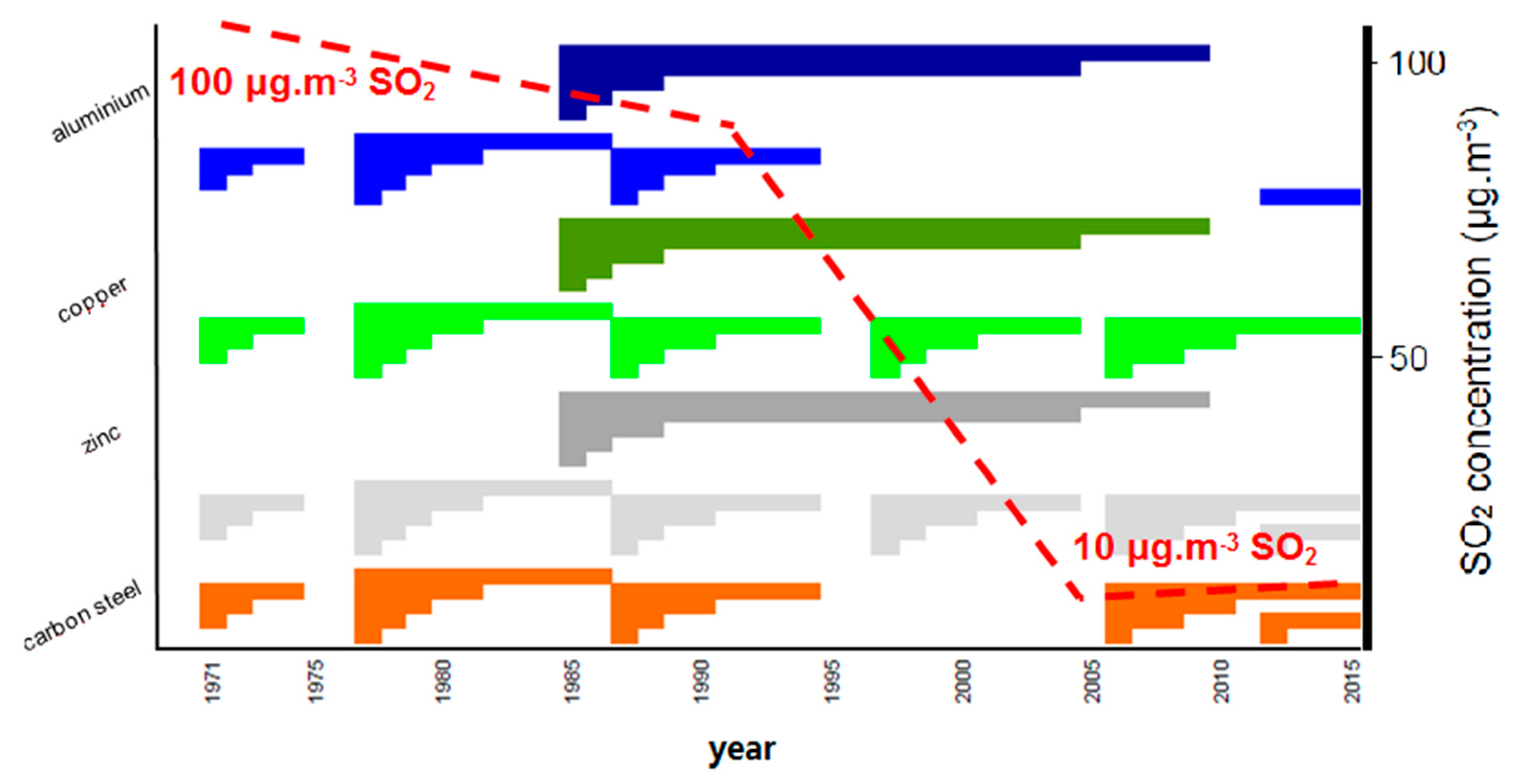
3.1. Environmental Data
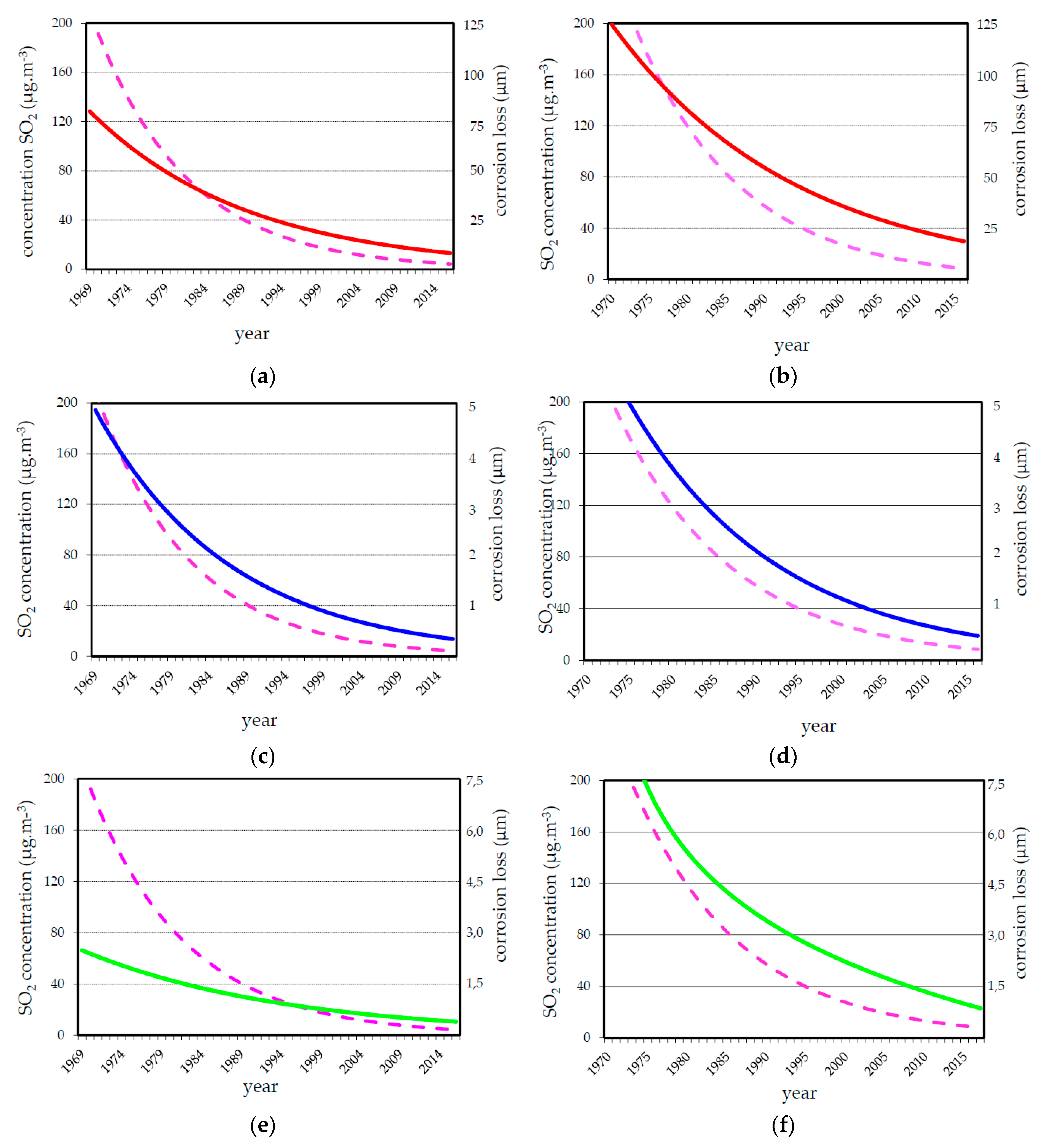
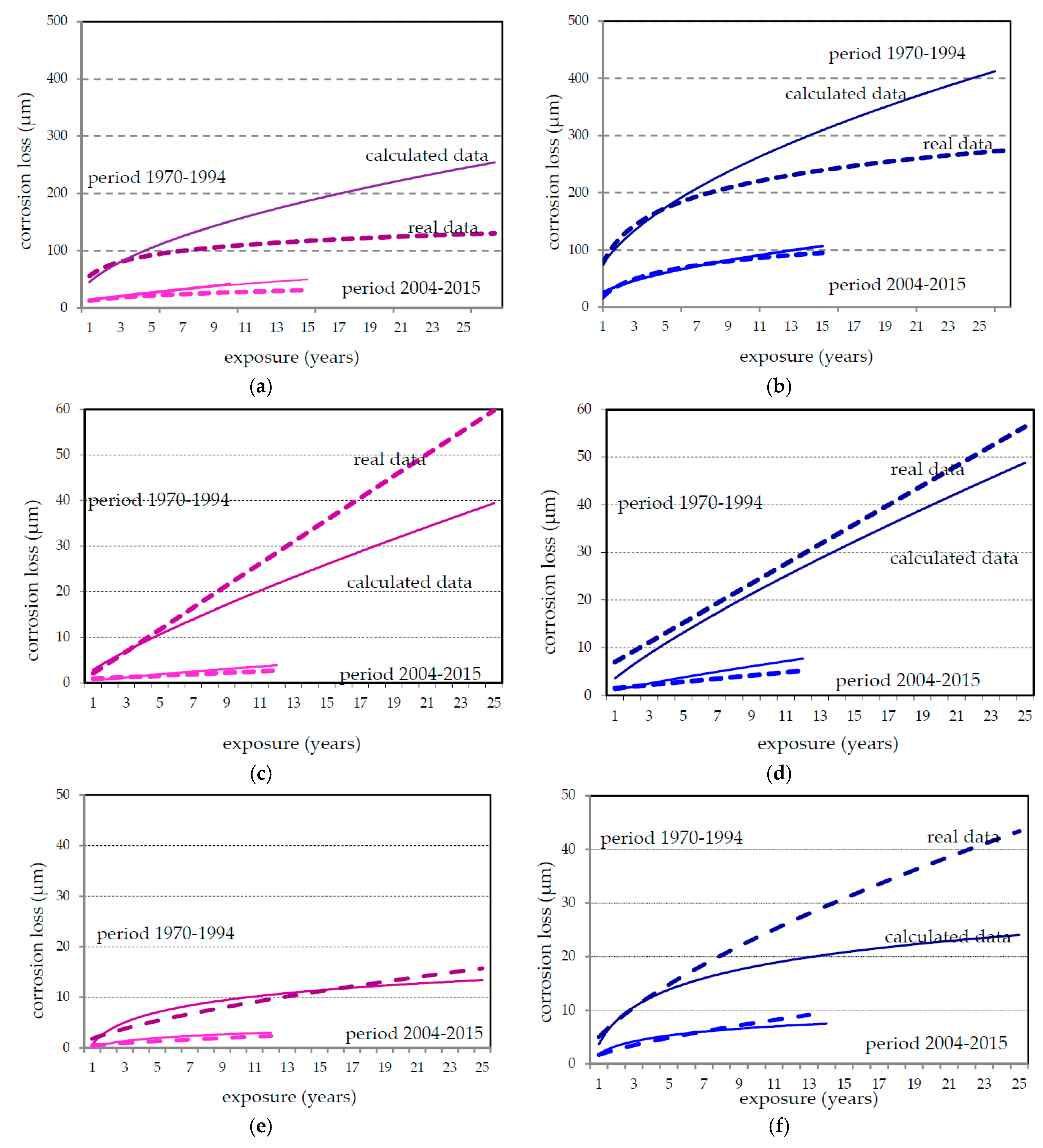
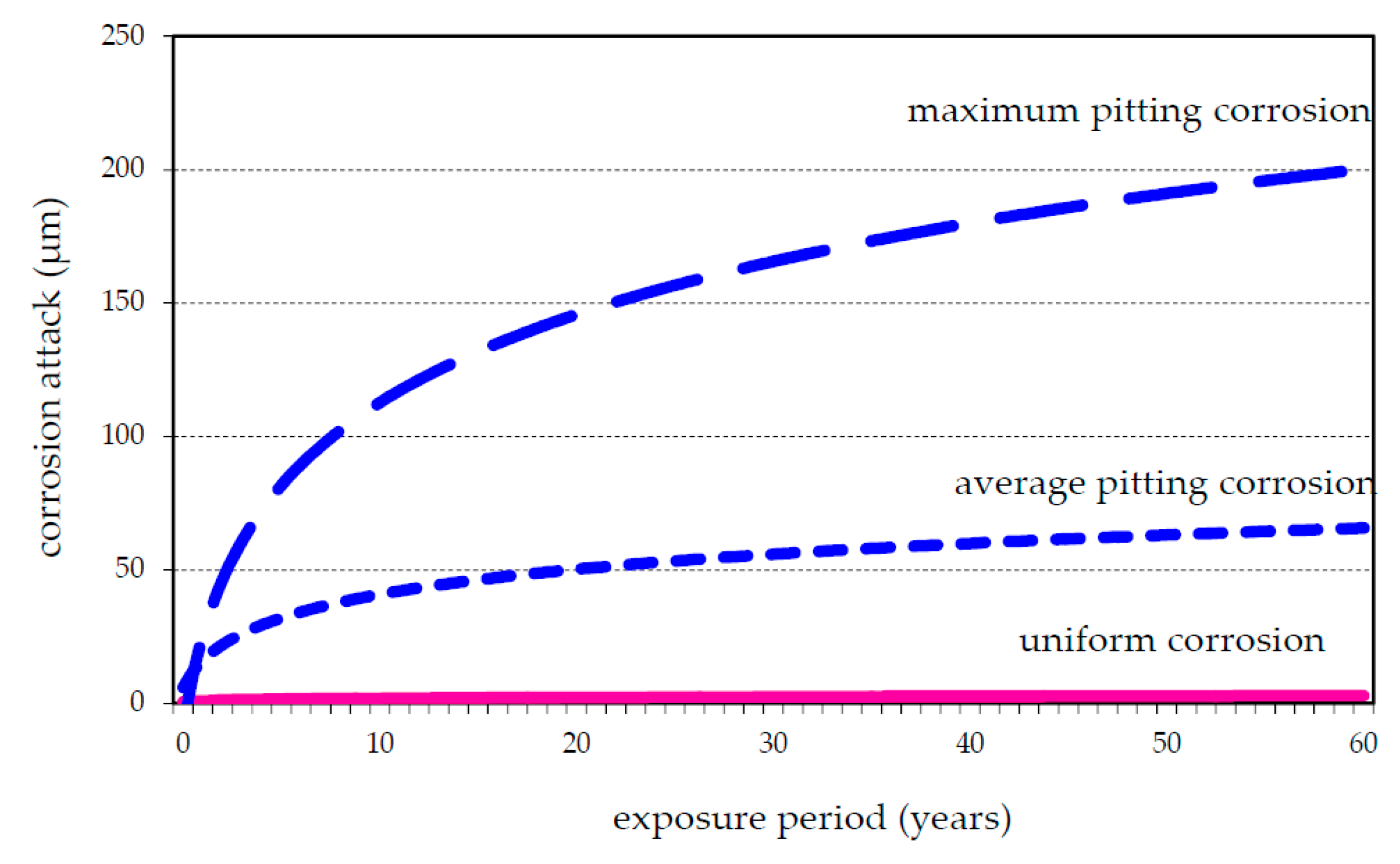
3.2. Corrosion Data
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Barton, K.; Knotkova, D.; Spanily, J. Errechnung der Geschwindigkeit der atmospharischen Korrosion nach meteorologischen und Luftverunreinigungswerten. In Vortrage des Wissenschaftlichen Kollquims Bearbeitung meteorologischer Werte in Zusammenhang mit der Erforschung der Atmospharischen Korrosion; CSSR: Kladno, Czech Republic, 1973; pp. 36–52. [Google Scholar]
- Barton, K. Protection against Atmospheric Corrosion; ISBN 0-471-01349-8; J. Wiley & Sons: Hoboken, NJ, USA, 1976. [Google Scholar]
- Knotkova, D.; Bosek, B.; Vlckova, J. Corrosion Aggressively of model regions of Czechoslovakia. In Corrosion in Natural Environments; ASTM STP 558; ASTM: West Conshohocken, PA, USA, 1974; pp. 52–74. [Google Scholar]
- Kreislova, K.; Geiplova, H.; Majtas, D. Long-term study of structural metals’ atmospheric corrosion in the Czech Republic. In Proceedings of the EUROCORR 2016, Montpellier, France, 11–15 September 2016. [Google Scholar]
- Kreislova, K.; Knotkova, D. Corrosion behaviour of structural metals in respect to long-term changes in the atmospheric environment. In Proceedings of the EUROCORR 2011, Stockholm, Sweden, 5–8 September 2011. [Google Scholar]
- Cold Rolled Low Carbon Steel Flat Products for Cold Forming—Technical Delivery Conditions; British Standards Institution: London, UK, 2006; EN 10130.
- Hot Rolled Products of Structural Steels—Part 5: Technical Delivery Conditions for Structural Steels with Improved Atmospheric Corrosion Resistance; EN 10025-5; British Standards Institution: London, UK, 2004.
- Protection against Corrosion—Atmospheric Test Stations—General Requirements (Czech Technical Standard); CSN 03 8110; Czechoslovak Standardization Institute: Prague, Czech Republic, 1978.
- Metals and Alloys—Atmospheric Corrosion Testing—General Requirements; ISO 8565; International Organization for Standardization: Geneva, Switzerland, 2011.
- Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Measurement of Environmental Parameters Affecting Corrosivity of Atmospheres; ISO 9225; International Organization for Standardization: Geneva, Switzerland, 2012.
- Kreislova, K.; Knotkova, D.; Tidblad, J.; Henriksen, J. Trends in corrosivity of atmosphere and material deterioration in Europe region in period 1987–2001. In Proceedings of the Abstracts of Conference Acid Rain 2005, Prague, Czech Republic, 12–17 June 2005; p. 697. [Google Scholar]
- Arroyave, C.; Morcillo, M. The effect of nitrogen oxides in atmospheric corrosion of metals. Corros. Sci. 1995, 37, 293–305. [Google Scholar] [CrossRef]
- Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens; ISO 8407; International Organization for Standardization: Geneva, Switzerland, 2009.
- Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation; ISO 9223; International Organization for Standardization: Geneva, Switzerland, 2012.
- Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Guiding Values for Corrosivity Categories; ISO 9224; International Organization for Standardization: Geneva, Switzerland, 2012.
- Knotkova, D.; Kreislova, K.; Dean, W.S. ISOCORRAG—International Atmospheric Exposure Program: Summary of Results; ASTM Data Serie 71; ASTM International: West Conshohocken, PA, USA, 2010; ISBN 978-0-8031-7011-7. [Google Scholar]
- Kucera, V.; Tidblad, J.; Kreislova, K.; Knotkova, D.; Faller, M.; Reiss, D.; Snethlage, R.; Yates, T.; Henriksen, J.; Schreiner, M.; et al. UN/ECE ICP Materials dose-response functions for the multi-pollutant situation. Water Air Soil Pollut. Focus 2007, 7, 249–258. [Google Scholar] [CrossRef]
- Watt, J.; Tidblad, J.; Kucera, V.; Hamilton, R. (Eds.) The Effect of Air Pollution on Cultural Heritage; Springer: Berlin, Germany, 2009; ISBN 978-0-387-84892-1. [Google Scholar]
- Geiplova, H.; Kreislova, K.; Mindos, L.; Turek, L. Evaluation of long-term exposed structures and their maintenance. Corrosion and Surface Treatment in Industry. Mater. Sci. Forum 2016, 844, 79–82. [Google Scholar] [CrossRef]
- Hatch, J.E. (Ed.) Aluminium—Properties and Physical Metallurgy; ASM: Geauga County, OH, USA, 1984; pp. 242–264. [Google Scholar]
- Vargel, C. Corrosion of Aluminium; Elsevier Science: Oxford, UK, 2004; ISBN 0-08-044495-4. [Google Scholar]
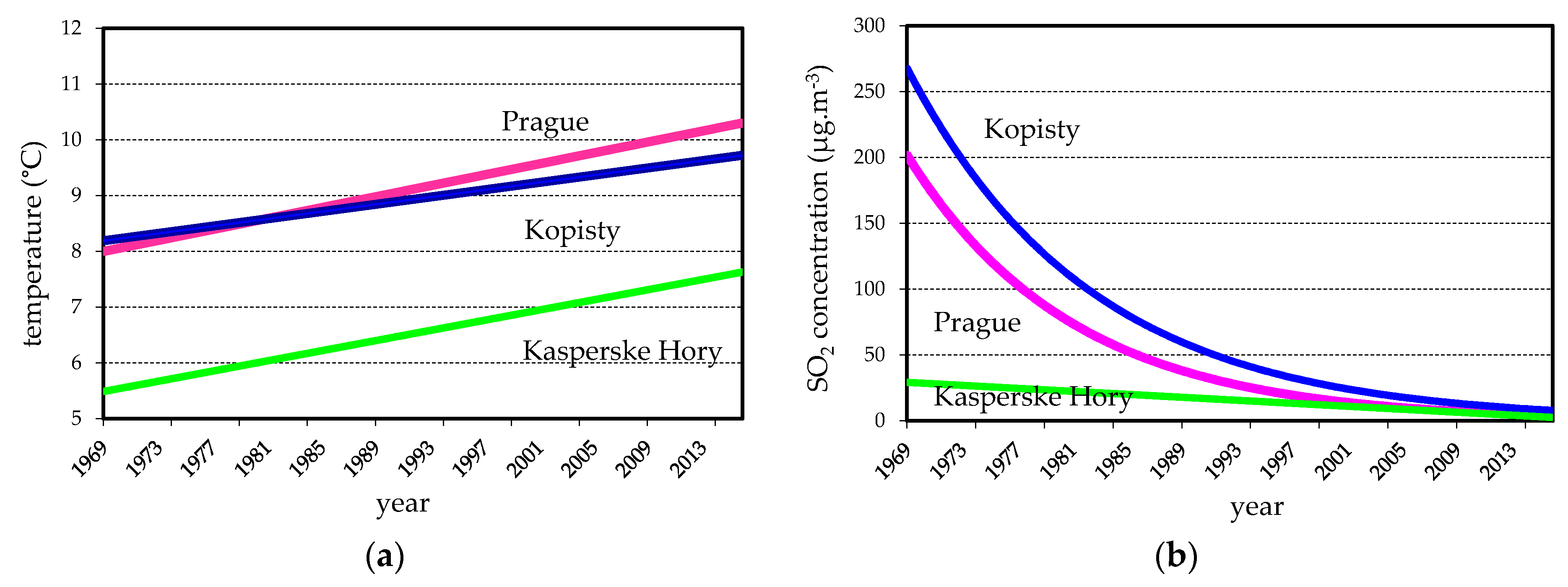

| Test Site | Year | T (°C) | RH (%) | SO2 (µg·m−3) | NOx (µg·m−3) | Rain (mm) | pH of Precipitation |
|---|---|---|---|---|---|---|---|
| Prague | 1970 | 8.6 | 76 | 92 | - | 490 | - |
| 1980 | 7.4 | 77 | 99 | - | 653 | - | |
| 1990 | 10.1 | 74 | 56 | 34 | 468 | 5.4 | |
| 2000 | 10.2 | 72 | 11 | 23 | 498 | 5.7 | |
| 2016 | 10.0 | 73 | 5 | 28 | 561 | 6.0 | |
| Kopisty | 1970 | 8.1 | 76 | 182 | - | 618 | - |
| 1980 | 7.6 | 75 | 145 | - | 520 | - | |
| 1990 | 9.7 | 71 | 67 | 32 | 398 | 4.9 | |
| 2000 | 10.2 | 76 | 16 | 28 | 486 | 4.7 | |
| 2016 | 9.9 | 76 | 18 | 23 | 567 | 6.0 | |
| Kasperske | 1970 | 5.4 | 79 | 23 | - | 637 | - |
| Hory | 1980 | 5.4 | 80 | 30 | - | 811 | - |
| 1990 | 7.0 | 78 | 26 | 9 | 722 | 4.9 | |
| 2000 | 7.8 | 76 | 8 | 9 | 798 | 5.4 | |
| 2016 | 8.7 | 74 | 11 | 24 | 841 | 6.0 |
| Test Site | Period | Environmental Data | Corrosion Rate (μm·a−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | RV (%) | SO2 (µg·m−3) | Carbon Steel | Zinc | Copper | |||||
| Real | Cal | Real | Cal | Real | Cal | |||||
| Prague | 1970–1994 | 8.8 | 77 | 87.6 | 16.1 | 15.1 | 2.82 | 2.30 | 1.30 | 0.86 |
| 2004–2015 | 10.1 | 73 | 7.3 | 2.6 | 4.0 | 0.13 | 0.45 | 0.22 | 0.23 | |
| Kopisty | 1970–1994 | 8.7 | 74 | 119.2 | 21.4 | 25.0 | 3.66 | 2.66 | 2.26 | 2.35 |
| 2004–2015 | 9.3 | 78 | 14.2 | 9.3 | 8.6 | 0.33 | 0.89 | 0.51 | 0.73 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreislova, K.; Knotkova, D. The Results of 45 Years of Atmospheric Corrosion Study in the Czech Republic. Materials 2017, 10, 394. https://doi.org/10.3390/ma10040394
Kreislova K, Knotkova D. The Results of 45 Years of Atmospheric Corrosion Study in the Czech Republic. Materials. 2017; 10(4):394. https://doi.org/10.3390/ma10040394
Chicago/Turabian StyleKreislova, Katerina, and Dagmar Knotkova. 2017. "The Results of 45 Years of Atmospheric Corrosion Study in the Czech Republic" Materials 10, no. 4: 394. https://doi.org/10.3390/ma10040394






