Design and Preparation of Carbon Based Composite Phase Change Material for Energy Piles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CPCMs
2.3. Characterization Tests for CPCMs
2.3.1. Micromorphology of CPCMs
2.3.2. Chemical Compatibility of CPCMs
2.3.3. Thermal Capacity of the CPCMs
2.3.4. Thermal Stability of CPCMs
2.3.5. Thermal Reliability of CPCMs
2.3.6. Compressive Strength of Cement Paste Composite Containing CPCMs
3. Results and Discussions
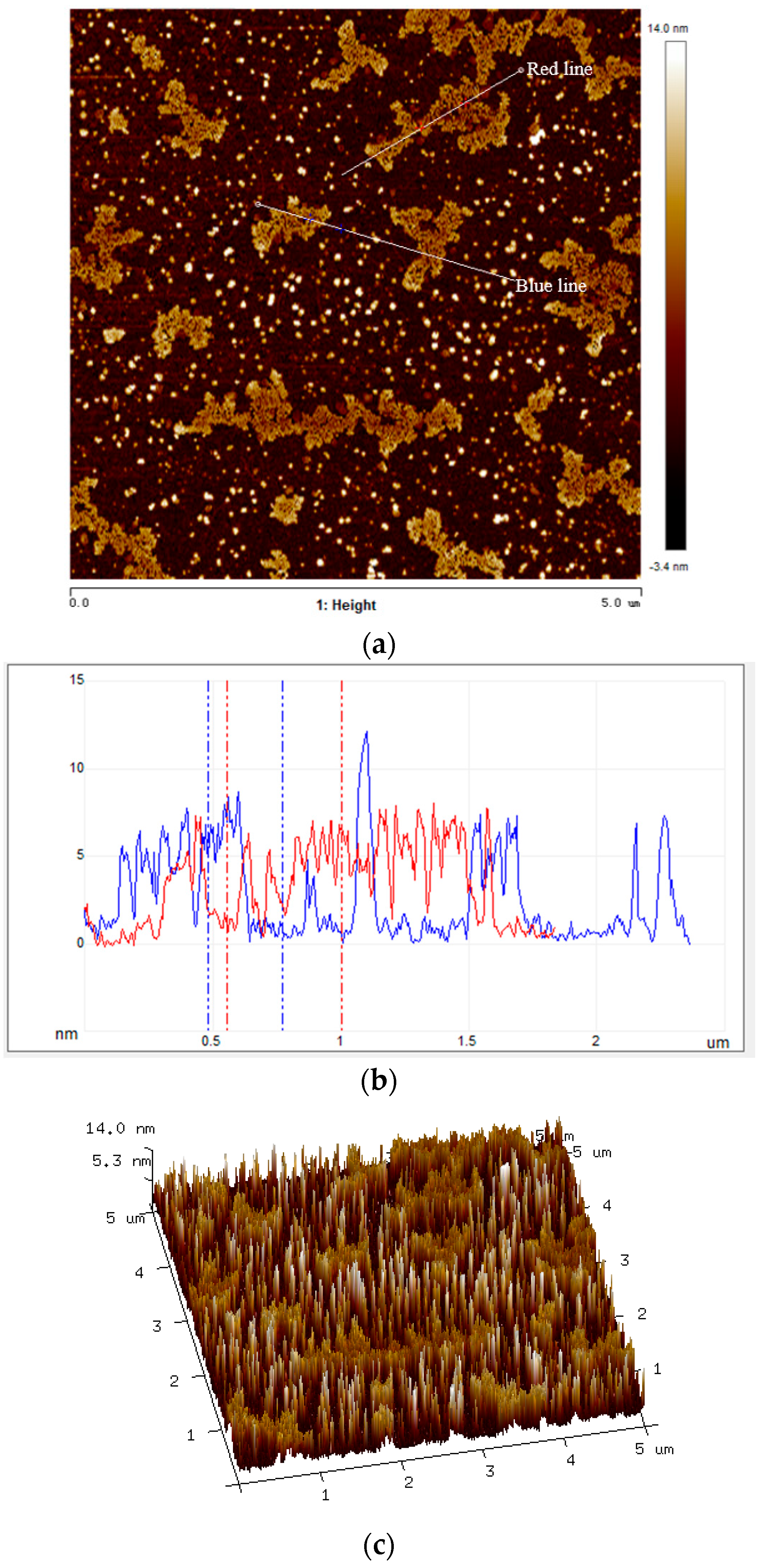
3.1. Macro- and Micro-Morphology of Composite PCM
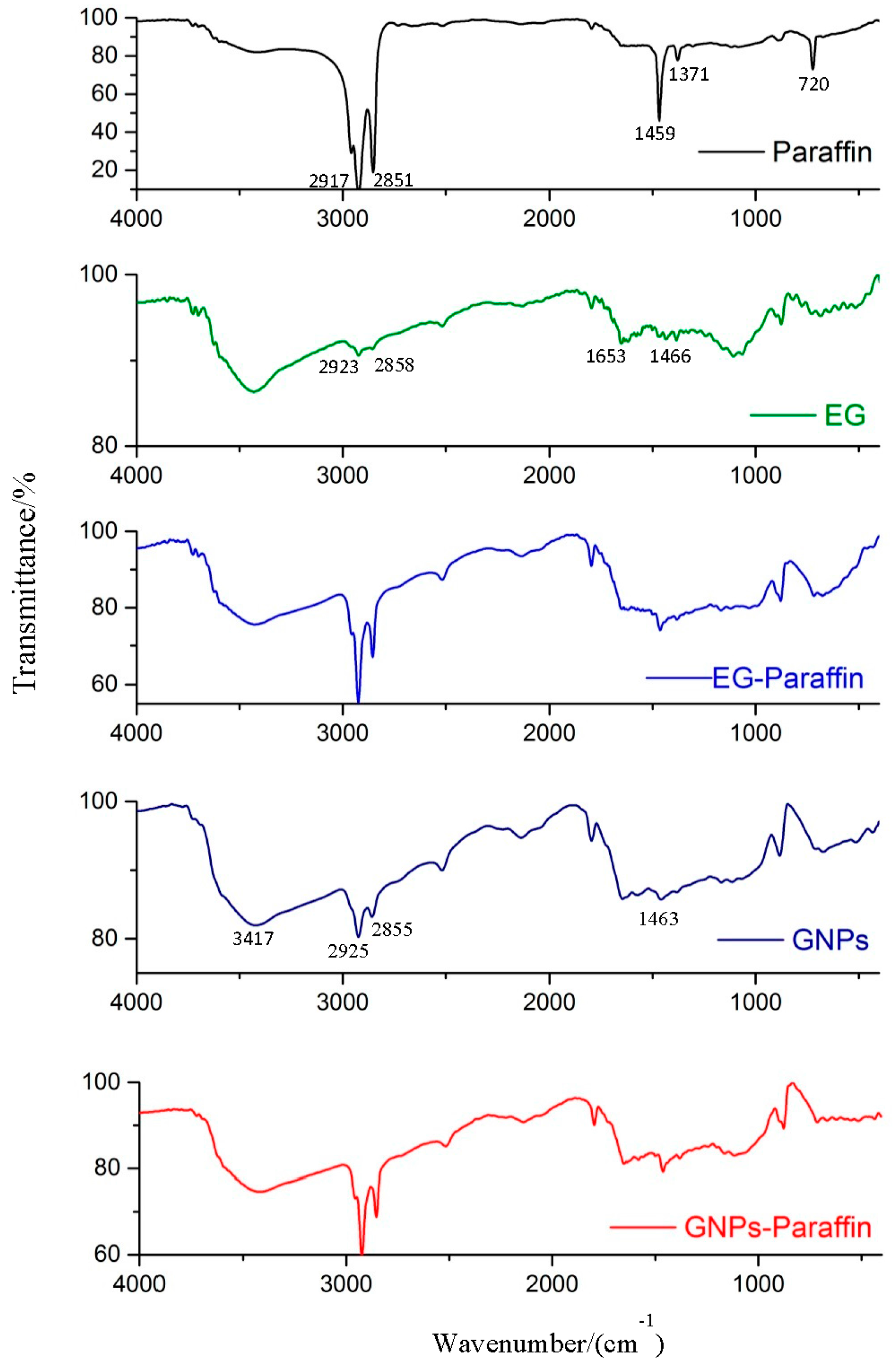
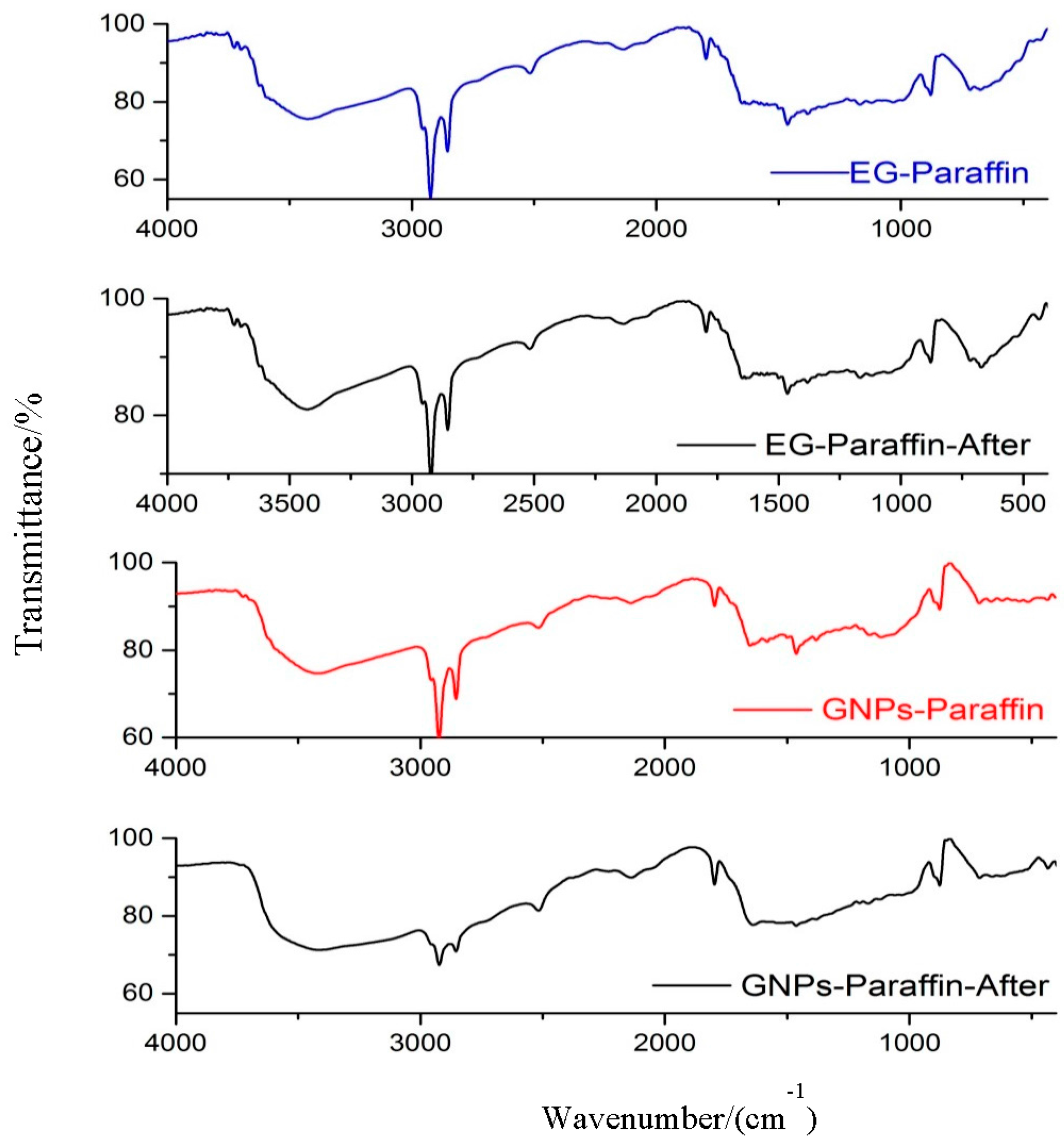
3.2. Chemical Compatibility of the CPCMs
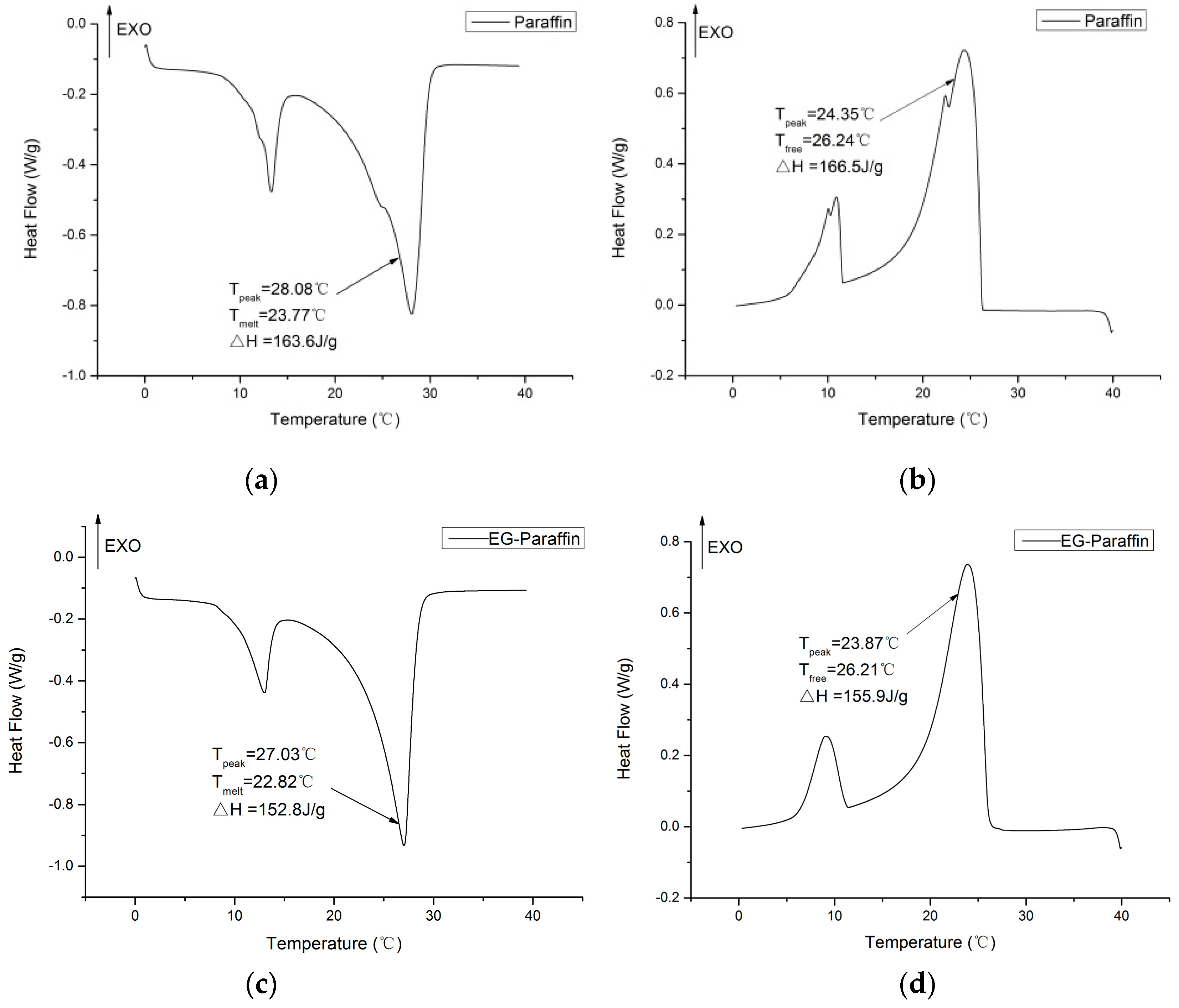
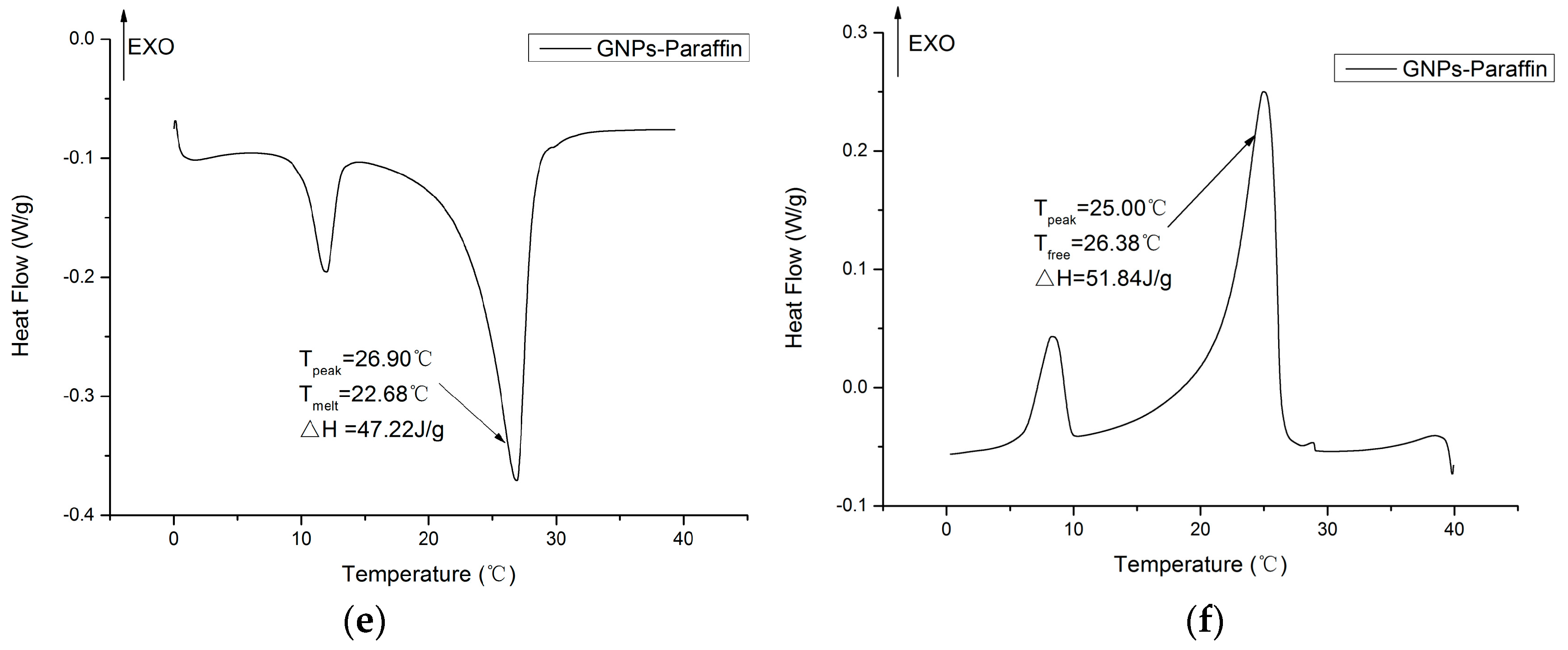
3.3. Thermal Properties of CPCMs
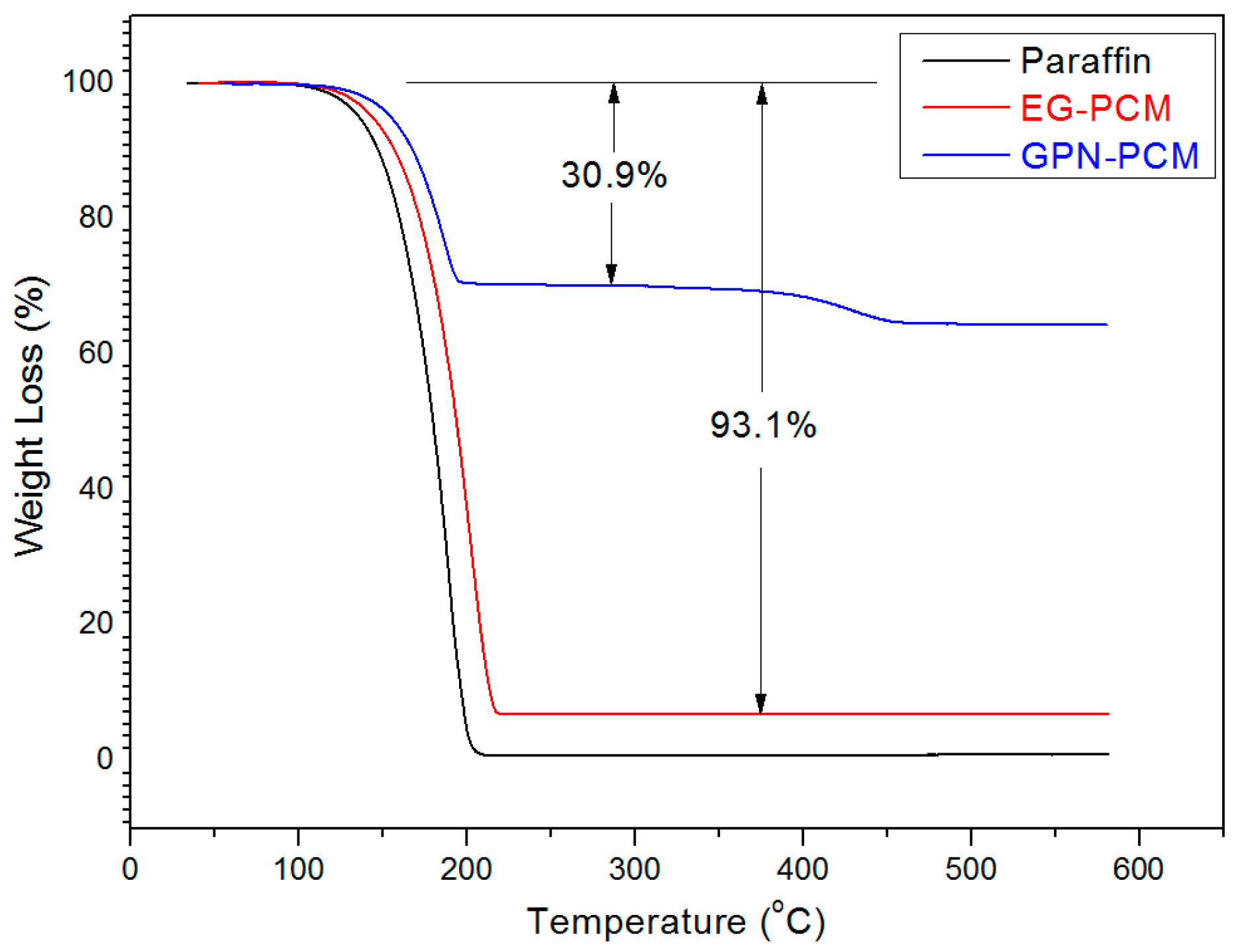
3.4. Thermal Stability of the CPCMs
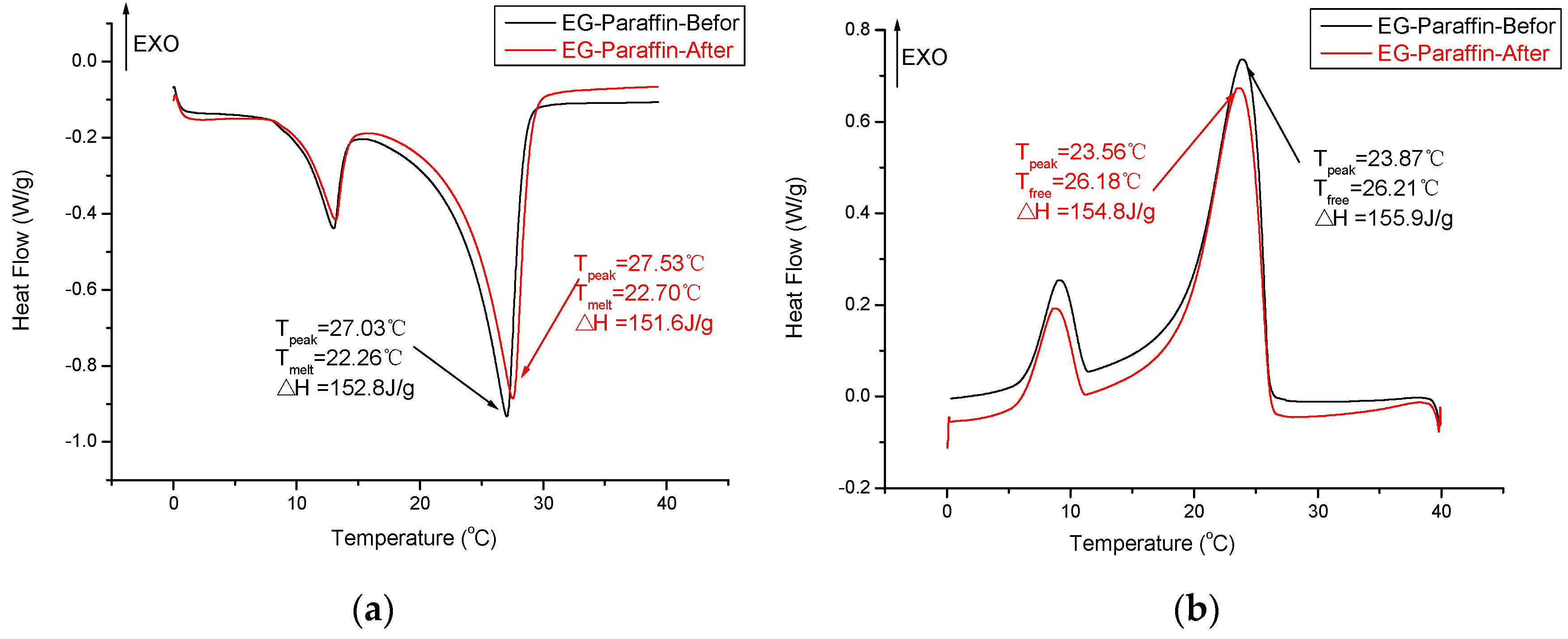
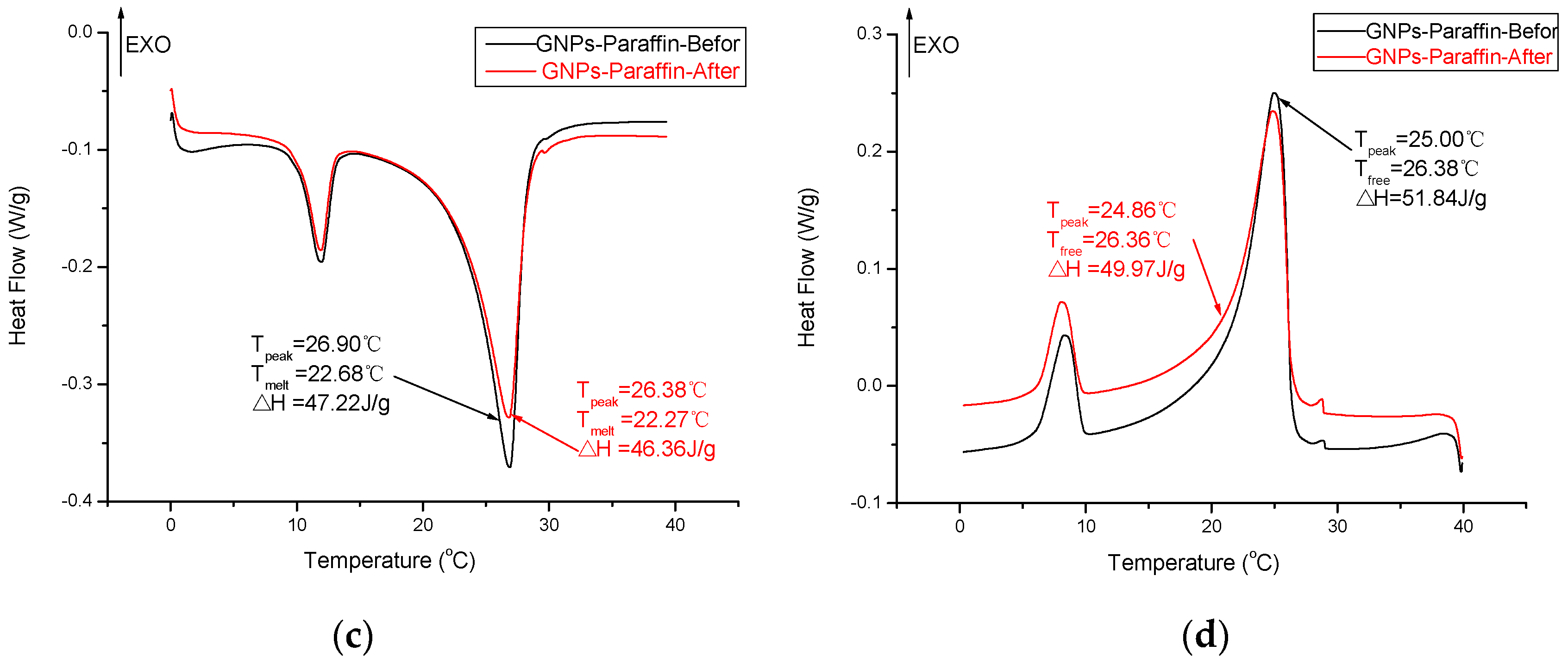
3.5. Thermal Reliability of CPCMs
3.6. Compressive Strength of Cement Paste Containing CPCMs
4. Conclusions
- (1)
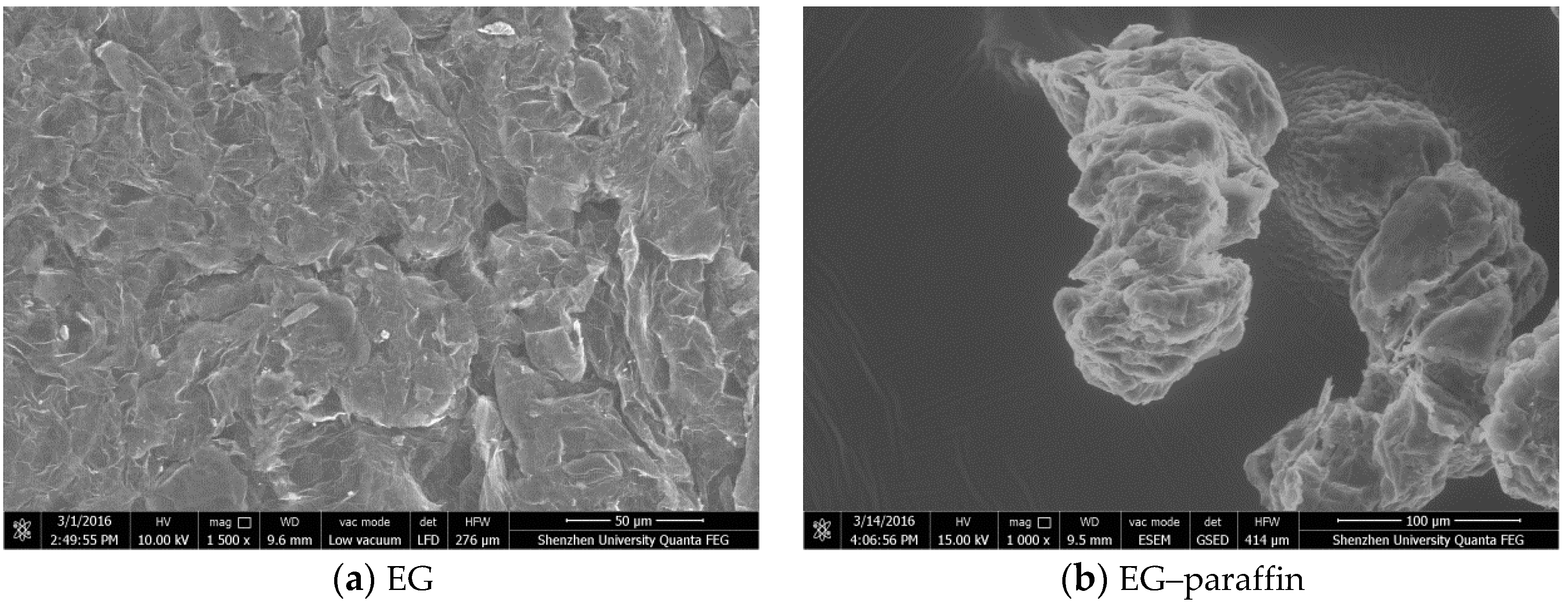
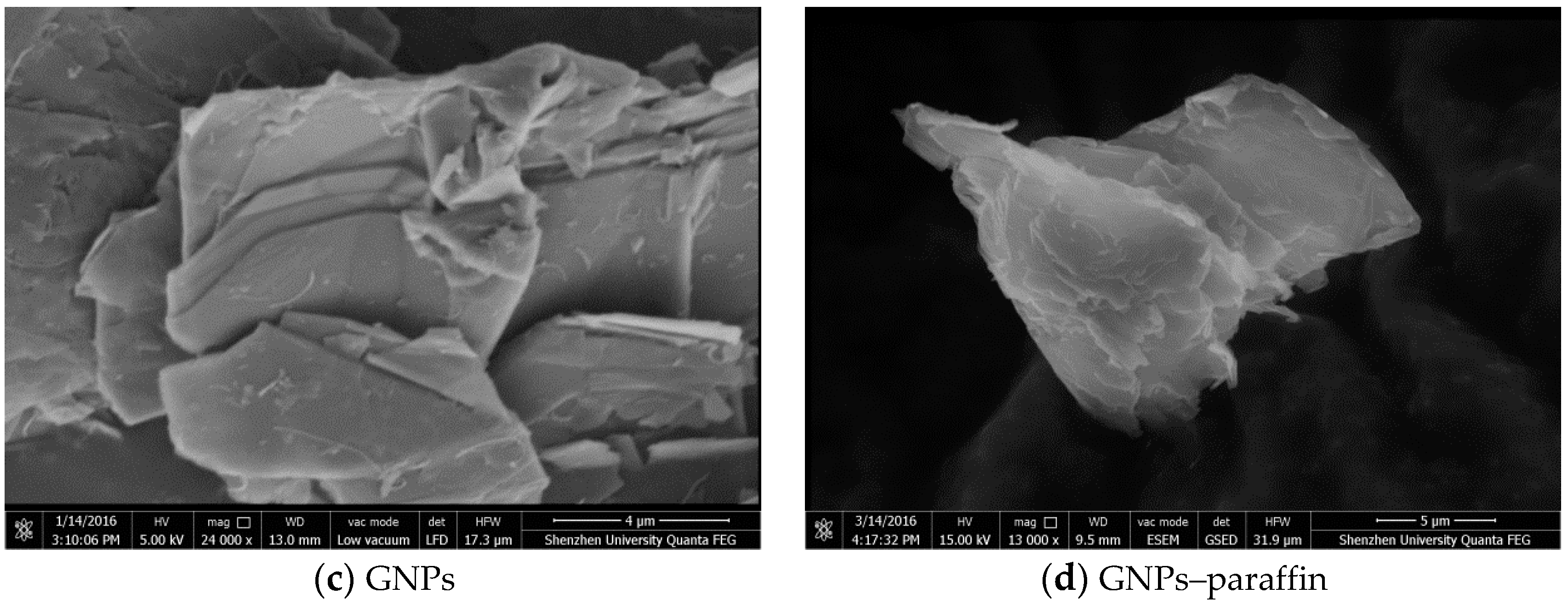
- The maximum percentage of paraffin retained by EG and GNPs through vacuum impregnation was 92.3% and 31.5% respectively. This data is similar to the data obtained from TGA results (93.1% and 30.9%), thus testifying the homogenous preparation of composite PCM. From micro-morphology, it was found that PCM possessed a honeycomb structure of EG due to capillary and surface tension forces while for GNPs, the larger surface area provided favourable conditions to absorb PCM.
- (2)
- From the FT-IR analysis, the interaction between components of composite PCM was physical in nature and therefore the components of prepared carbon-based composites are chemically compatible with each other.
- (3)
- The DSC analysis showed that the developed carbon-based PCMs possess considerable latent heat and can therefore be a potential candidate for energy piles.
- (4)
- From thermal stability results, it was found that the incorporation of carbon-based materials in PCM shifted the initial decomposition to a higher temperature, indicating an increase in the thermal stability of composite PCM. Furthermore, the developed composite PCM did not show any sign of degradation below 100 °C. Hence, it is thermally stable and can be utilized for thermal energy storage applications.
- (5)
- The chemical structure and thermal properties of developed carbon-based composite PCMs were not affected by thermal cycling. Therefore, the composite PCM is thermally reliable and can be used for latent heat storage applications.
- (6)
- The compressive strength of cement paste containing 10 wt % GNP-PCM was found to be 37 MPa and it therefore has potential application for structural purposes.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PCM | Phase change materials |
| CPCM | Composite phase change material |
| EG | Expanded graphite |
| GNP | Graphene nanoplatelet |
| EG-PCM | Expanded graphite-based PCM |
| GNP-PCM | Graphene nanoplatelets-based PCM |
References
- Da Cunha, J.P.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Memon, S.; Liao, W.; Yang, S.; Cui, H.; Shah, S. Development of Composite PCMs by Incorporation of Paraffin into Various Building Materials. Materials 2015, 8, 499–518. [Google Scholar] [CrossRef]
- International Energy Outlook; U.S. Energy Information Administration: Washington, DC, USA, 2016; Volume 1.
- Dong, Z.; Cui, H.; Tang, W.; Chen, D.; Wen, H. Development of Hollow Steel Ball Macro-Encapsulated PCM for Thermal Energy Storage Concrete. Materials 2016, 9, 59. [Google Scholar] [CrossRef]
- Cui, H.; Liao, W.; Memon, S.; Dong, B.; Tang, W. Thermophysical and Mechanical Properties of Hardened Cement Paste with Microencapsulated Phase Change Materials for Energy Storage. Materials 2014, 7, 8070–8087. [Google Scholar] [CrossRef]
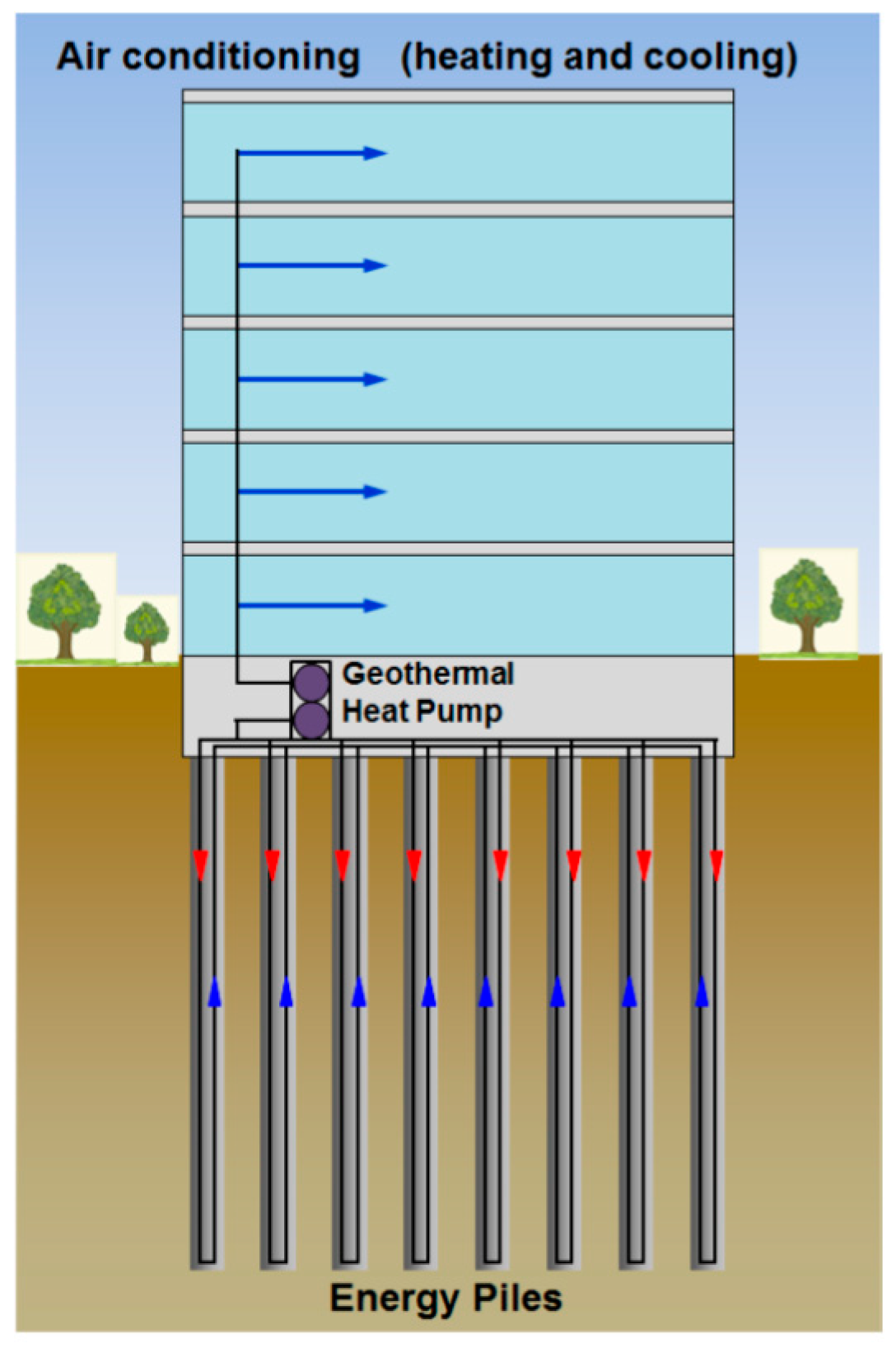
- Olgun, C.G. Energy Piles: Background and Geotechnical Engineering Concepts. In Proceedings of the 16th Annual George F. Sowers Symposium, Atlanta, GA, USA, 7 May 2013. [Google Scholar]
- Barla, M.; Perino, A. Energy from geo-structures: A topic of growing interest. Environ. Geotech. 2015, 2, 3–7. [Google Scholar] [CrossRef]
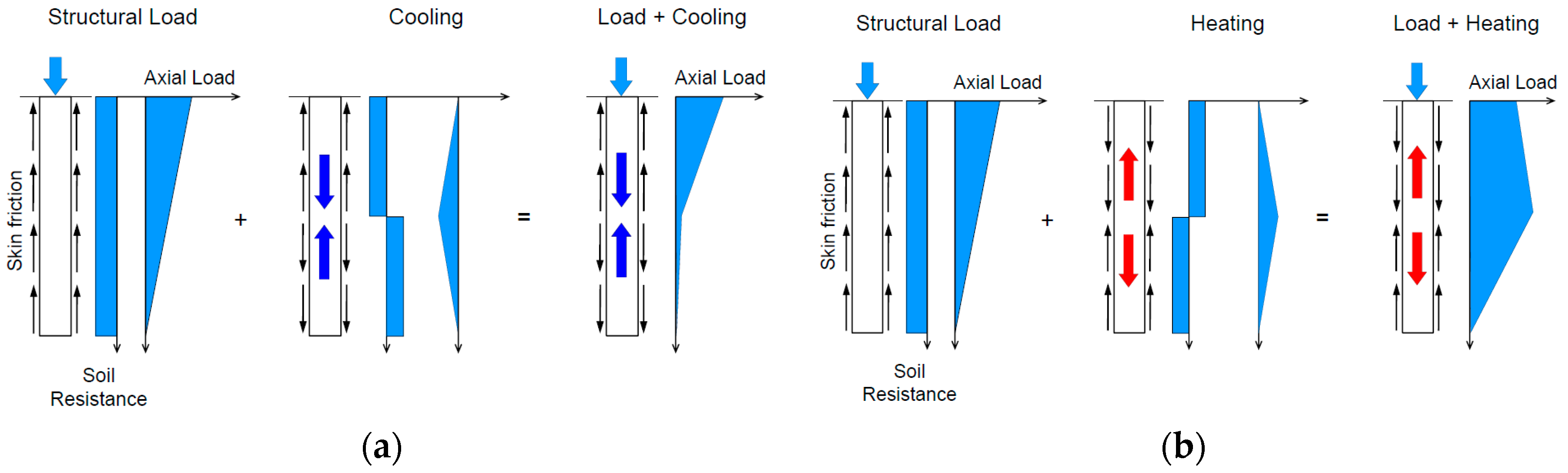
- Donna, A.D.; Dupray, F.; Laloui, L. Numerical study of the heating-cooling effects on the geotechnical behaviour of energy piles. In Proceedings of the International Symposium on Coupled Phenoma in Environmental Geotechnics, Torino, Italy, 1–3 July 2013. [Google Scholar]
- Mimouni, T.; Laloui, L. Behaviour of a group of energy piles. Can. Geotech. J. 2015, 52, 1913–1929. [Google Scholar] [CrossRef]
- Knellwolf, C.; Péron, H.; Laloui, L. Geotechnical Analysis of Heat Exchanger Piles. J. Geotech. Geoenviron. Eng. 2011, 137, 890–902. [Google Scholar] [CrossRef]
- Abdelaziz, S.L.; Ozudogru, T.Y. Non-uniform thermal strains and stresses in energy piles. Geoenviron. Geotech. 2016, 3, 237–252. [Google Scholar] [CrossRef]
- Abdelaziz, S.L.; Ozudogru, T.Y. Selection of the design temperature change for energy piles. Appl. Therm. Eng. 2016, 107, 1036–1045. [Google Scholar] [CrossRef]
- Chen, Y.J.; Nguyen, D.D.; Shen, M.Y.; Yip, M.C.; Tai, N.H. Thermal characterizations of the graphite nanosheets reinforced paraffin phase-change composites. Compos. Part A Appl. Sci. Manuf. 2013, 44, 40–46. [Google Scholar] [CrossRef]
- Yu, Z.T.; Fang, X.; Fan, L.W.; Wang, X.; Xiao, Y.Q.; Zeng, Y.; Xu, X.; Hu, Y.C.; Cen, K.F. Increased thermal conductivity of liquid paraffin-based suspensions in the presence of carbon nano-additives of various sizes and shapes. Carbon 2013, 53, 277–285. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl. Therm. Eng. 2007, 27, 1271–1277. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Preparation and thermal properties of polyethylene glycol/expanded graphite blends for energy storage. Appl. Energy 2009, 86, 1479–1483. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R.Z. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon 2010, 48, 2538–2548. [Google Scholar] [CrossRef]
- Mills, A.; Farid, M.; Selman, J.R.; Al-Hallaj, S. Thermal conductivity enhancement of phase change materials using a graphite matrix. Appl. Therm. Eng. 2006, 26, 1652–1661. [Google Scholar] [CrossRef]
- Zeng, J.L.; Gan, J.; Zhu, F.R.; Yu, S.B.; Xiao, Z.L.; Yan, W.P.; Zhu, L.; Liu, Z.Q.; Sun, L.X.; Cao, Z. Tetradecanol/expanded graphite composite form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 127, 122–128. [Google Scholar] [CrossRef]
- Silakhori, M.; Fauzi, H.; Mahmoudian, M.R.; Metselaar, H.S.C.; Mahlia, T.M.I.; Khanlou, H.M. Preparation and thermal properties of form-stable phase change materials composed of palmitic acid/polypyrrole/graphene nanoplatelets. Energy Build. 2015, 99, 189–195. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Tang, Y.; Jia, Y.; Alva, G.; Huang, X.; Fang, G. Synthesis, characterization and properties of palmitic acid/high density polyethylene/graphene nanoplatelets composites as form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2016, 155, 421–429. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, G.; Wang, S.; Fang, X.; Liu, X. Thermal energy storage cement mortar containing n-octadecane/expanded graphite composite phase change material. Renew. Energy 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Coates, J. Intrepretation of Infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Severcan, F.; Toyran, N.; Kaptan, N.; Turan, B. Fourier transform infrared study of the effect of diabetes on rat liver and heart tissues in the CH region. Talanta 2000, 53, 55–59. [Google Scholar] [CrossRef]
- Gokulakumar, B.; Narayanasyamy, R. Fourier Transform-Infrared Spectra (FT-IR) Analysis of Root Rod Disease in Sesame (Sesamum Indicum). Roman. J. Biophys. 2008, 18, 217–223. [Google Scholar]
- Memon, S.A.; Lo, T.Y.; Barbhuiya, S.A.; Xu, W. Development of form-stable composite phase change material by incorporation of dodecyl alcohol into ground granulated blast furnace slag. Energy Build. 2013, 62, 360–367. [Google Scholar] [CrossRef]
- Hamizi, N.A.; Johan, M.R. Optical and FTIR studies of CdSe quantum dots. In Proceedings of the 3rd International Nanoelectronics Conference (INEC), Hong Kong, China, 3–8 January 2010. [Google Scholar]
- Cheng, Q.Q.; Cao, Y.; Yang, L.; Zhang, P.P.; Wang, K.; Wang, H.J. Synthesis and photocatalytic activity of titania microspheres with hierarchical structures. Mater. Res. Bull. 2011, 46, 372–377. [Google Scholar] [CrossRef]
- Qi, G.Q.; Liang, C.L.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Polyethylene glycol based shape-stabilized phase change material for thermal energy storage with ultra-low content of graphene oxide. Sol. Energy Mater. Sol. Cells 2014, 123, 171–177. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C.; Silakhori, M. Shape-stabilized phase change materials with high thermal conductivity based on paraffin/graphene oxide composite. Energy Convers. Manag. 2013, 67, 275–282. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Hawes, D.W. Development and Application of Organic-Phase Change Mixtures in Thermal Storage Gypsum Wallboard. Sol. Energy Mater. Sol. Cells 1995, 36, 147–157. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Capric-myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323–332. [Google Scholar] [CrossRef]
- Hawes, D.W.; Feldman, D.; Banu, D. Latent heat storage in building materials. Energy Build. 1993, 20, 77–86. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D. DSC analysis for the evaluation of an energy storing wallboard. Thermochim. Acta 1996, 272, 243–251. [Google Scholar] [CrossRef]
- Shilei, L.; Neng, Z.; Guohui, F. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage. Energy Build. 2006, 38, 708–711. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Kaygusuz, K. Capric acid and myristic acid for latent heat thermal energy storage. Energy Sources Part A 2008, 30, 1498–1507. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Preparation and characterization of fatty acid ester/building material composites for thermal energy storage in buildings. Energy Build. 2011, 43, 1952–1959. [Google Scholar] [CrossRef]
- Shilei, L.; Guohui, F.; Neng, Z.; Li, D. Experimental study and evaluation of latent heat storage in phase change materials wallboards. Energy Build. 2007, 39, 1088–1091. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A. Capric acid and palmitic acid eutectic mixture applied in building wallboard for latent heat thermal energy storage. J. Sci. Ind. Res. 2007, 66, 470–476. [Google Scholar]
- Li, M.; Kao, H.; Wu, Z.; Tan, J. Study on preparation and thermal property of binary fatty acid and the binary fatty acids/diatomite composite phase change materials. Appl. Energy 2011, 88, 1606–1612. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Z. A novel montmorillonite-based composite phase change material and its applications in thermal storage building materials. Energy Build. 2006, 38, 377–380. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E.; Ozay, S.; Ozkilic, Y. Preparation of phase change material–montmorillonite composites suitable for thermal energy storage. Thermochim. Acta 2011, 524, 39–46. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailor-made for thermal energy storage cement-based composites. Energy 2014, 72, 371–380. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of capric acid/expanded perlite composite for thermal energy storage. Mater. Chem. Phys. 2008, 109, 459–464. [Google Scholar] [CrossRef]
- Kim, D.; Jung, J.; Kim, Y.; Lee, M.; Seo, J.; Khan, S.B. Structure and thermal properties of octadecane/expanded graphite composites as shape-stabilized phase change materials. Int. J. Heat Mass Transf. 2016, 95, 735–741. [Google Scholar] [CrossRef]
- Mochane, M.J.; Luyt, A.S. The effect of expanded graphite on the thermal stability, latent heat, and flammability properties of EVA/wax phase change blends. Polym. Eng. Sci. 2015, 55, 1255–1262. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Meshgin, P.; Xi, Y. Effect of Phase-Change Materials on Properties of Concrete. ACI Mater. J. 2012, 109, 71–80. [Google Scholar]
- Meshgin, P.; Xi, Y.; Li, Y. Utilization of phase change materials and rubber particles to improve thermal and mechanical properties of mortar. Constr. Build. Mater. 2012, 28, 713–721. [Google Scholar] [CrossRef]


| Type | Particle Size | Diameter | Expanded Time | Nanosheet Number |
|---|---|---|---|---|
| Expanded graphite | ~0.5 mm | / | 300 | / |
| Graphene | / | 5–7 μm | / | <20 |
| Cement Paste | Cement | Water | CPCMs | Superplasticizer (wt %) |
|---|---|---|---|---|
| Control specimen (OPC) | 1 | 0.35 | 0 | 0.15 |
| EG–paraffin-10 | 1 | 0.35 | 0.1 | 0.3 |
| GNP–paraffin-10 | 1 | 0.35 | 0.1 | 0.3 |
| Composite PCMs | Melting Point (°C) | Latent Heat (J/g) | Reference |
|---|---|---|---|
| Dodecanol (25–30 wt %)/gypsum | 20 | 17 | [33] |
| Capric-myristic acid (20 wt %)/Vermicuilite | 19.8 | 27 | [34] |
| Capric-lauric acid + fire retardant (25–30 wt %)/gypsum | 17 | 28 | [35] |
| Butyl stearate (25–30 wt %)/gypsum | 18 | 30 | [35] |
| Emersest2326/gypsum | 16.9 | 35 | [36] |
| Capric-lauric acid (26 wt %)/gypsum | 19 | 35.2 | [37] |
| Capric-myristic acid (25 wt %)/gypsum | 21.1 | 36.2 | [38] |
| Erythritol tetrapalmitate ester (18 wt %)/ cement | 21.9 | 37.2 | [39] |
| Capric-lauric acid (26 wt %)/gypsum | 18.49 | 39.13 | [40] |
| Propyl palmitate (25–30 wt %)/gypsum | 19 | 40 | [33] |
| Capric-palmitic acid (25 wt %)/gypsum | 22.9 | 42.5 | [41] |
| Paraffin/GNPs | 22.68 | 47.22 | This study |
| Decanoic/Dodecanoic acid/Diatomite | 16.7 | 66.8 | [42] |
| RT20 (58%)/Montmorillonite | 23 | 79.3 | [43] |
| PCM-clay composite (PMMT1-4) | 16–17 | 82–128 | [44] |
| Paraffin/Diatomite/CNTs | 27.12 | 89.40 | [45] |
| Capric acid (55%)/Expanded perlite | 31.8 | 98.1 | [46] |
| Octadecane (70%)/Expanded graphite | 29.6 | 138.8 | [47] |
| Paraffin/Expanded graphite | 22.82 | 152.8 | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Memon, S.A.; Bao, X.; Cui, H.; Li, D. Design and Preparation of Carbon Based Composite Phase Change Material for Energy Piles. Materials 2017, 10, 391. https://doi.org/10.3390/ma10040391
Yang H, Memon SA, Bao X, Cui H, Li D. Design and Preparation of Carbon Based Composite Phase Change Material for Energy Piles. Materials. 2017; 10(4):391. https://doi.org/10.3390/ma10040391
Chicago/Turabian StyleYang, Haibin, Shazim Ali Memon, Xiaohua Bao, Hongzhi Cui, and Dongxu Li. 2017. "Design and Preparation of Carbon Based Composite Phase Change Material for Energy Piles" Materials 10, no. 4: 391. https://doi.org/10.3390/ma10040391






