Characterization and Thermal Stability of Acetylated Slicewood Production by Alkali-Catalyzed Esterification
Abstract
:1. Introduction
2. Materials & Methods
2.1. Materials
2.2. Acetylation
2.3. Flexural Properties
2.4. Dynamic Mechanical Analysis
2.5. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectral Measurements
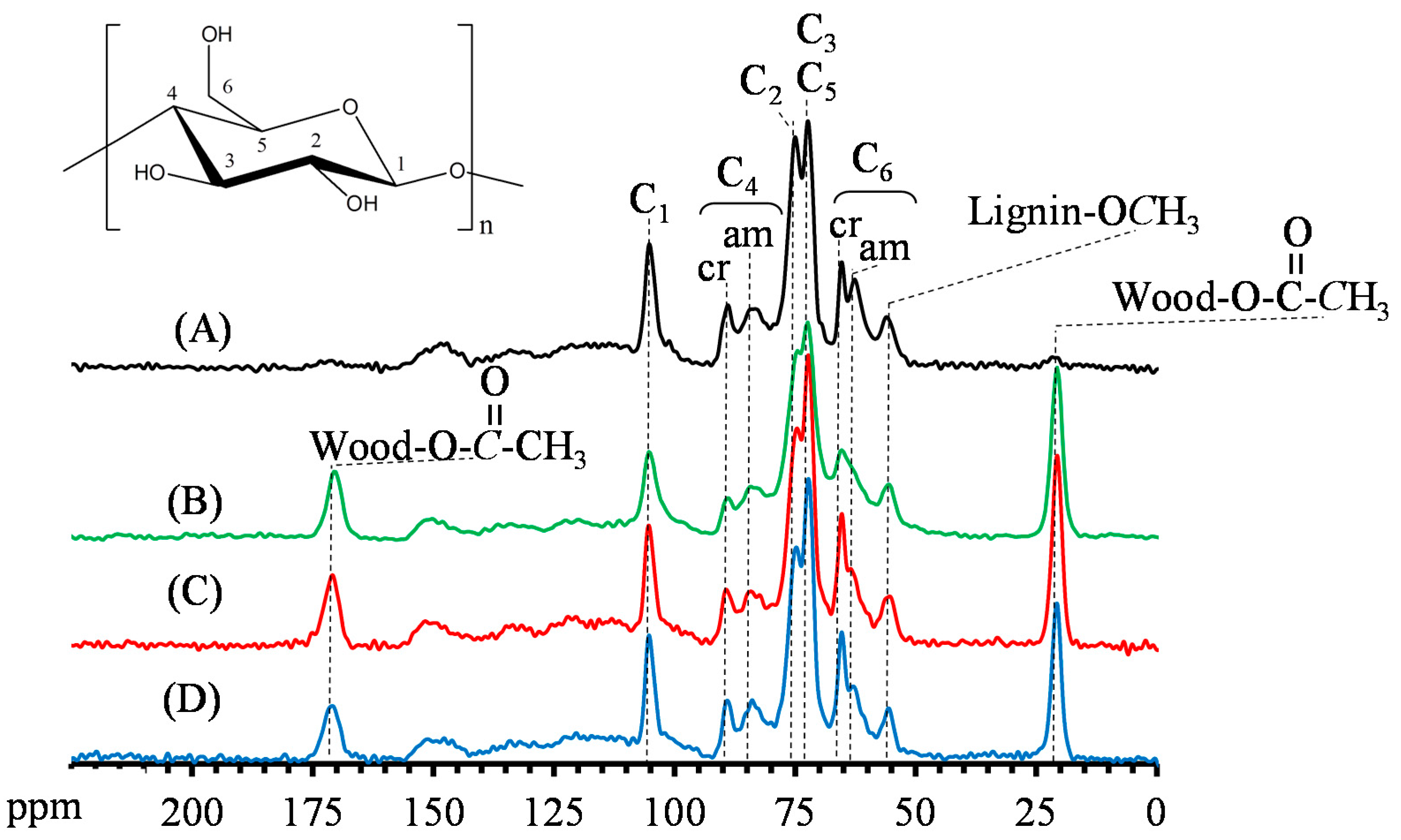
2.6. Solid-State Cross-Polarization Magic Angle Spin (CP/MAS) Carbon-13 Nuclear Magnetic Resonance (13C-NMR) Analysis
2.7. Thermogravimetric Analysis (TGA)
2.8. Statistical Analyses
3. Results and Discussion
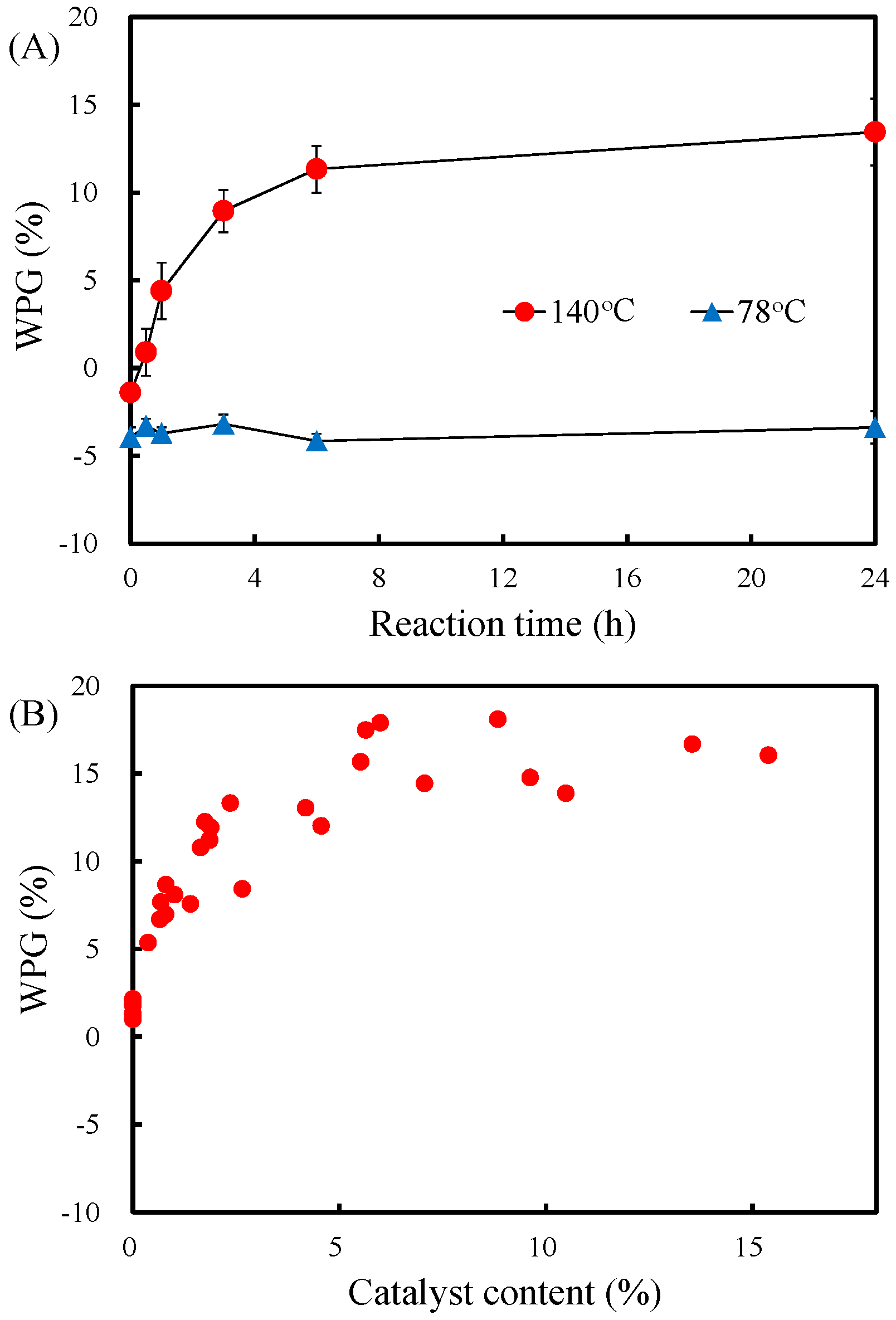
3.1. Effects of the Reaction Temperature, Reaction Time, and Catalyst Content
3.2. WPG, MOE, and MOR of VA-Acetylated Slicewood
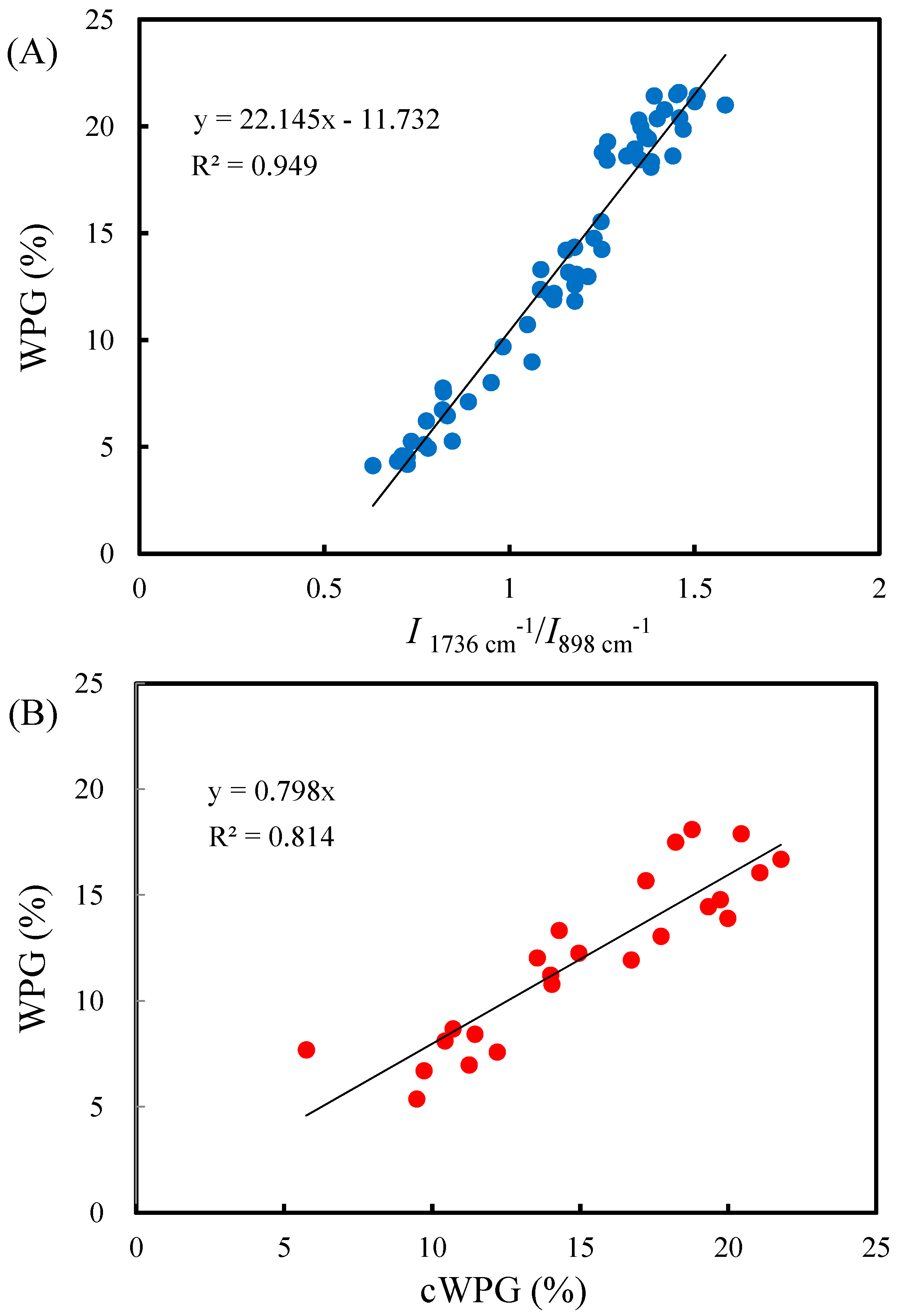
3.3. Functional Groups and Calibrated WPG of VA-Acetylated Slicewood
3.4. Reactive Characteristics of VA-Acetylated Slicewood
3.5. Viscoelastic Properties of Acetylated Slicewood
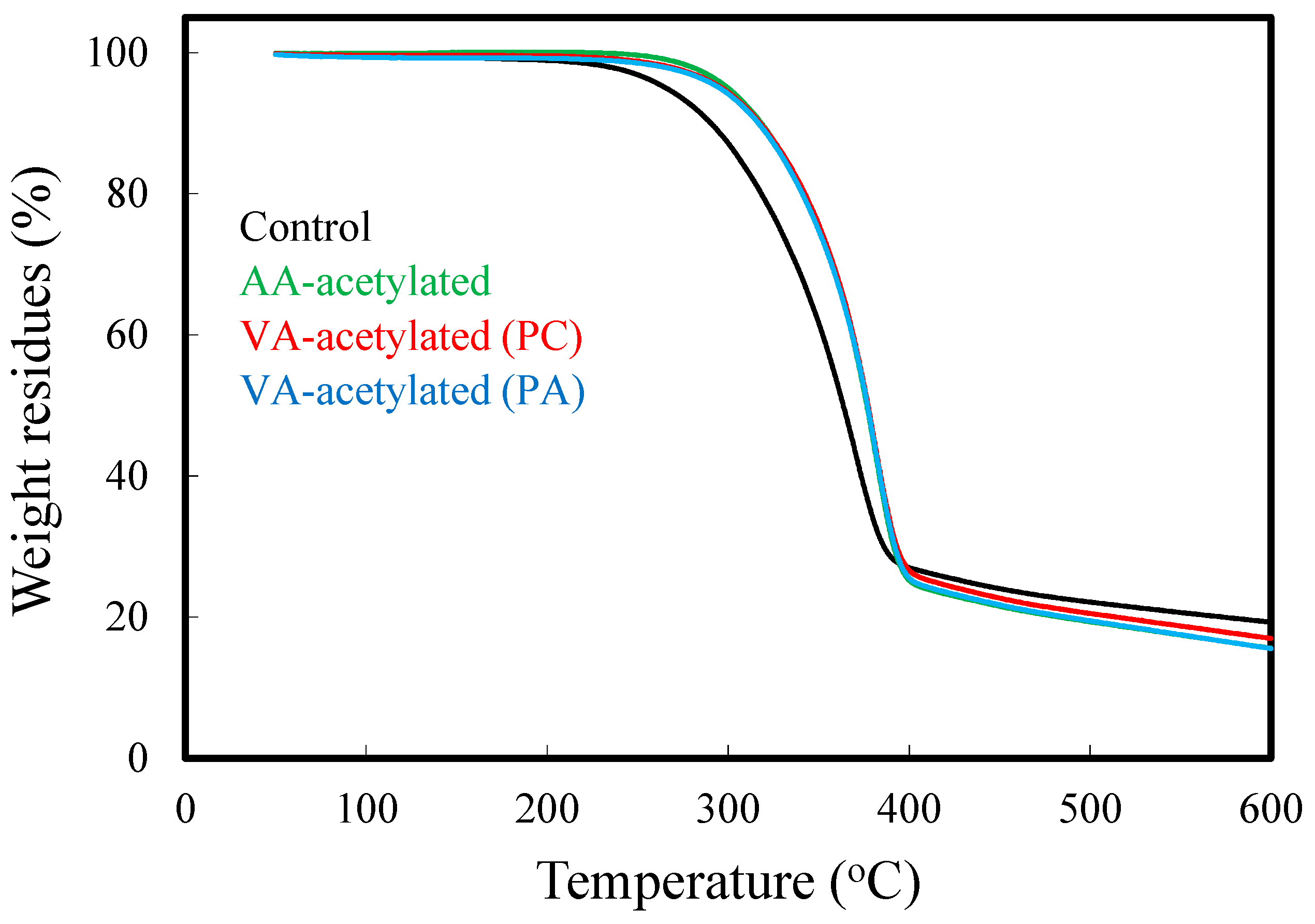
3.6. Thermal Decomposition Kinetics of Acetylated Slicewood
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoadley, R.B. Understanding Wood: A Craftsman’s Guide to Wood Technology; The Taunton Press: Newtown, CT, USA, 2000. [Google Scholar]
- Srinivas, K.; Pandey, K.K. Effect of heat treatment on color changes, dimensional stability, and mechanical properties of wood. J. Wood Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons Ltd: Chichester, UK, 2006. [Google Scholar]
- Wang, X.; Liu, J.; Chai, Y. Thermal, mechanical, and moisture absorption properties of wood-TiO2 composites prepared by a sol-gel process. Bioresources 2012, 7, 893–901. [Google Scholar]
- Wu, J.-H.; Hsieh, T.-Y.; Lin, H.-Y.; Shiau, I.-L.; Chang, S.-T. Properties of wood plasticization with octanoyl chloride in a solvent-free system. Wood Sci. Technol. 2004, 37, 363–372. [Google Scholar] [CrossRef]
- Jebrane, M.; Harper, D.; Labbé, N.; Sèbe, G. Comparative determination of the grafting distribution and viscoelastic properties of wood blocks acetylated by vinyl acetate or acetic anhydride. Carbohydr. Polym. 2011, 84, 1314–1320. [Google Scholar] [CrossRef]
- Jebrane, M.; Pichavant, F.; Sèbe, G. A comparative study on the acetylation of wood by reaction with vinyl acetate and acetic anhydride. Carbohydr. Polym. 2011, 83, 339–345. [Google Scholar] [CrossRef]
- Minato, K.; Shimizu, R.; Kawaguchi, S. Contribution of lignin to the reactivity of wood in chemical modification I: Influence of delignification on actylation. J. Wood Sci. 2007, 53, 218–222. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood: A short review. Wood Mater. Sci. Eng. 2006, 1, 29–33. [Google Scholar] [CrossRef]
- Yang, C.-N.; Hung, K.-C.; Wu, T.-L.; Yang, T.-C.; Chen, Y.-L.; Wu, J.-H. Comparisons and characteristics of slicewood acetylation with acetic anhydride by liquid phase, microwave, and vapor phase reactions. Bioresources 2014, 9, 6463–6475. [Google Scholar] [CrossRef]
- Çetin, N.S.; Ozmen, N. Acetylation of wood components and Fourier transform infra-red spectroscopy studies. Afr. J. Biotechnol. 2011, 10, 3091–3096. [Google Scholar]
- Jebrane, M.; Sèbe, G. A novel simple route to wood acetylation by transesterification with vinyl acetate. Holzforschung 2007, 61, 143–147. [Google Scholar] [CrossRef]
- Xu, C.; Leppӓnen, A.-S.; Eklund, P.; Holmlund, P.; Sjöholm, R.; Sundberg, K.; Willför, S. Acetylation and characterization of spruce (Picea abies) galactoglucomannans. Carbohyd. Res. 2010, 345, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Özmen, N.; Çetin, N.S.; Mengeloğlu, F.; Birinci, E.; Karakuș, K. Effect of wood acetylation with vinyl acetate and anhydride on the properties of wood-plastic composites. Bioresources 2013, 8, 753–767. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G.; Freitag, C.; Morrell, J.J. Effects of wood fiber esterification on properties, weatherability and biodurability of wood plastic composites. Polym. Degrad. Stabil. 2013, 98, 1348–1361. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stabil. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour. Technol. 2013, 127, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, L.; Kai, C.; Huang, R.; Wu, Q. Bamboo and high density polyethylene composite with heat-treated bamboo fiber: Thermal decomposition properties. Bioresources 2013, 8, 900–912. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Meterials; ASTM D790; ASTM: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Çetin, N.S.; Ozmen, N.; Birinci, E. Acetylation of wood with various catalysts. J. Wood Chem. Technol. 2011, 31, 142–153. [Google Scholar] [CrossRef]
- Birkinshaw, C.; Hale, M.D. Mechanical properties and fungal resistance of acetylated fast grown softwoods. I. Small specimens. Ir. For. 2002, 59, 49–58. [Google Scholar]
- Rowell, R.M.; Banks, W.B. Tensile strength and toughness of acetylated pine and lime flakes. Polym. Int. 1987, 19, 479–482. [Google Scholar] [CrossRef]
- Fávaro, S.L.; Lopes, M.S.; Neto, A.G.V.C.; Santana, R.R.; Radovanovic, E. Chemical, morphological, and mechanical analysis of rice husk/post-consumer polyethylene composites. Compos. A Appl. Sci. Manuf. 2010, 41, 154–160. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, J.-H. Mechanical and interfacial properties of plastic composite panels made from esterified bamboo particles. J. Wood Sci. 2010, 56, 216–221. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Bakare, I.O.; Khairul, A.; Issam, A.M.; Bhat, I. Effect of anhydride modification on the thermal stability of cultivated Acacia mangium. J. Wood Chem. Technol. 2011, 31, 154–171. [Google Scholar] [CrossRef]
- Boonstra, M.G.; Pizzi, A.; Tekely, P.; Pendlebury, J. Chemical modification of Norway spruce and Scots pine. A 13C NMR CP-MAS study of the reactivity and reactions of polymeric wood components with acetic anhydride. Holzforschung 1996, 50, 215–220. [Google Scholar] [CrossRef]
- Obataya, E.; Furuta, Y.; Gril, J. Dynamic viscoelastic properties of wood acetylated with acetic anhydride solution of glucose pentaacetate. J. Wood Sci. 2003, 49, 152–157. [Google Scholar] [CrossRef]
- Backamn, A.C.; Lindberg, K.A.H. Differences in wood material responses for radial and tangential direction as measured by dynamic mechanical thermal analysis. J. Mater. Sci. 2001, 36, 3777–3783. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, J. Dynamic viscoelastic behavior of wood under drying conditions. Front. For. China 2009, 4, 374–379. [Google Scholar] [CrossRef]
- Gao, J.; Kim, J.S.; Terziev, N.; Daniel, G. Decay resistance of softwoods and hardwoods thermally modified by the thermovouto type thermo-vacuum process to brown rot and white rot fungi. Holzforschung 2016, 70, 877–884. [Google Scholar] [CrossRef]
- Brown, M.E.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A. Computational aspects of kinetic analysis. Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Vuthaluru, H.B. Investigations into the pyrolytic behavior of coal/biomass blends using thermogravimetric analysis. Bioresour. Technol. 2004, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]


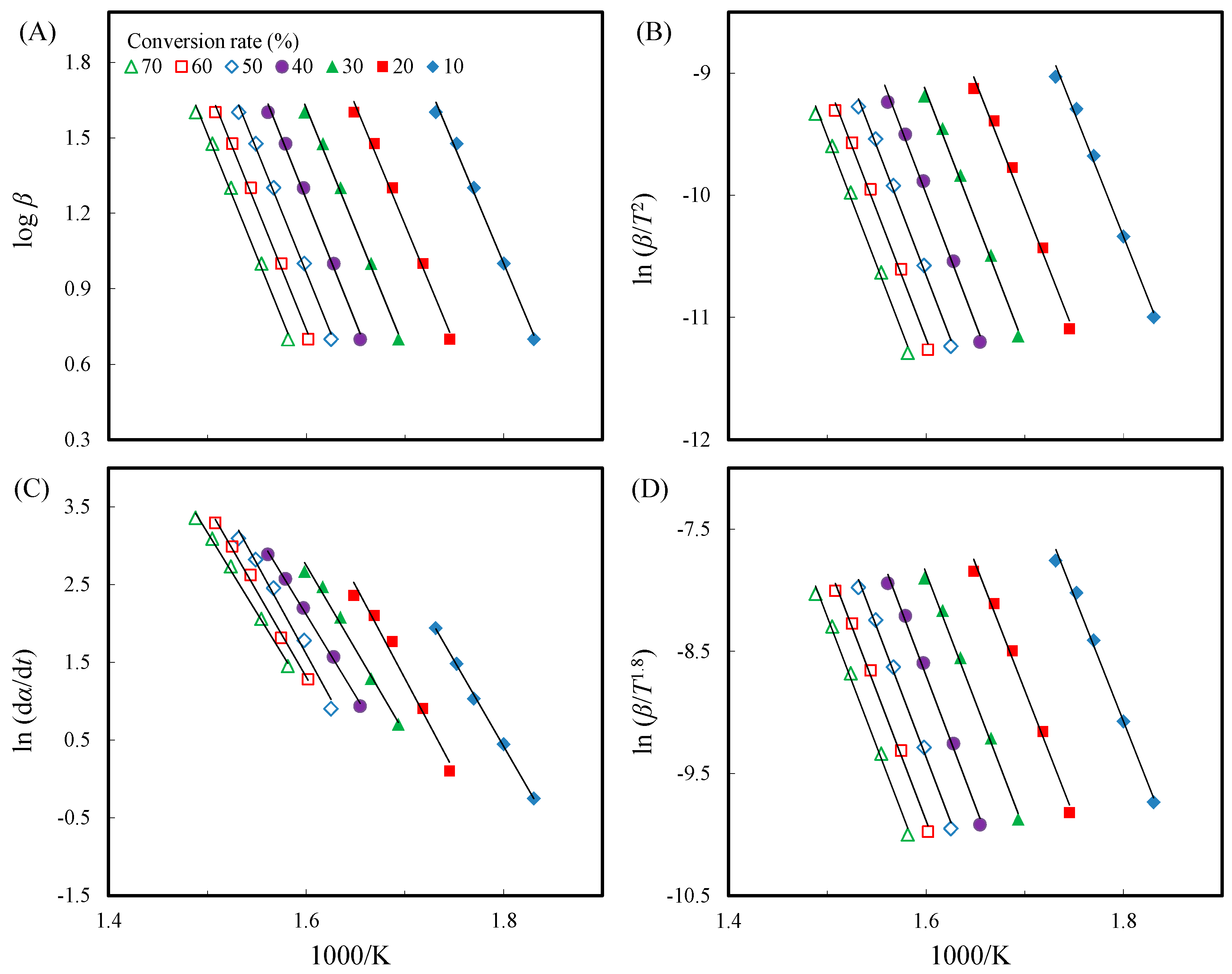
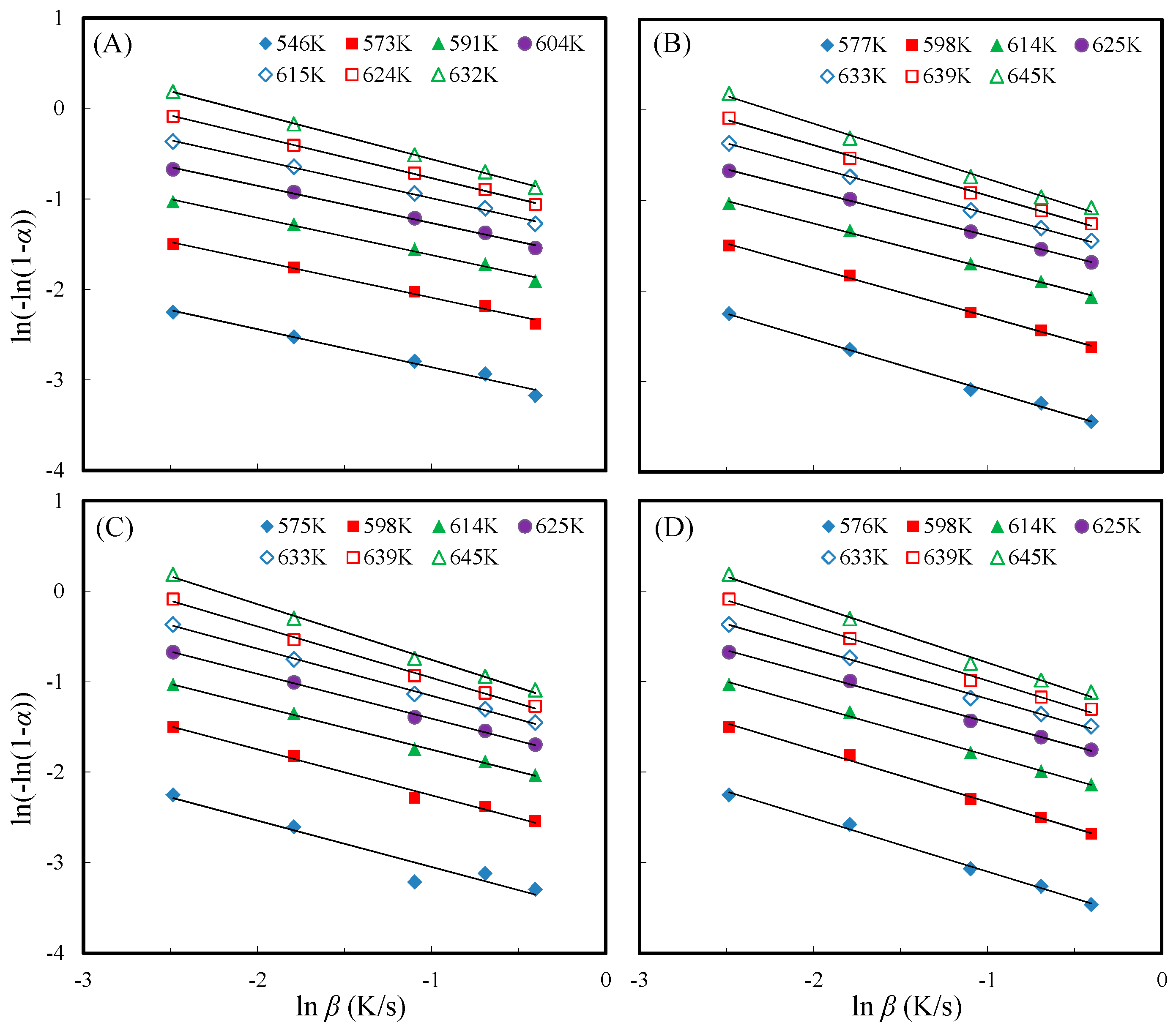
| Reaction Time (h) | Potassium Carbonate (5%) | Potassium Acetate (5%) | ||||
|---|---|---|---|---|---|---|
| WPG (%) | MOE (GPa) | MOR (MPa) | WPG (%) | MOE (GPa) | MOR (MPa) | |
| Control | − | 6.5 ± 0.4 a | 79 ± 14 a | − | 6.5 ± 0.4 a | 79 ± 14 a |
| 0 | −1.4 ± 0.4 e | 6.6 ± 1.3 a | 91 ± 18 a | −3.5 ± 1.1 e | 6.9 ± 1.0 a | 100 ± 20 a |
| 0.5 | 0.9 ± 1.3 d | 5.8 ± 0.6 a | 80 ± 2 a | −0.7 ± 0.8 d | 6.1 ± 1.1 a | 78 ± 18 a |
| 1 | 4.4 ± 1.6 c | 5.8 ± 0.9 a | 80 ± 14 a | 0.2 ± 0.8 d | 6.5 ± 0.9 a | 105 ± 17 a |
| 3 | 9.0 ± 1.2 b | 5.6 ± 0.6 a | 94 ± 33 a | 3.6 ± 1.3 c | 5.1 ± 2.5 a | 70 ± 33 a |
| 6 | 11.3 ± 1.2 a | 6.7 ± 1.2 a | 89 ± 24 a | 9.4 ± 1.1 b | 5.1 ± 1.3 a | 78 ± 13 a |
| 24 | 13.4 ± 1.9 a | 5.5 ± 0.8 a | 76 ± 20 a | 12.9 ± 1.1 a | 6.6 ± 1.4 a | 88 ± 23 a |
| Slicewood | Methods | Units | Conversion Rates | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | Mean | |||
| Unmodified | F-W-O | Ea (kJ/mol) | 170 | 172 | 175 | 178 | 177 | 176 | 177 | 175 |
| (Control) | R2 | 0.994 | 0.992 | 0.995 | 0.995 | 0.996 | 0.996 | 0.996 | – | |
| Modified C-R | Ea (kJ/mol) | 169 | 171 | 174 | 176 | 176 | 175 | 175 | 174 | |
| R2 | 0.993 | 0.991 | 0.995 | 0.995 | 0.996 | 0.996 | 0.995 | – | ||
| Friedman | Ea (kJ/mol) | 183 | 198 | 180 | 174 | 193 | 183 | 172 | 183 | |
| R2 | 0.999 | 0.978 | 0.989 | 0.998 | 0.984 | 0.996 | 0.996 | – | ||
| Starink | Ea (kJ/mol) | 170 | 172 | 175 | 177 | 176 | 175 | 176 | 174 | |
| R2 | 0.993 | 0.991 | 0.995 | 0.995 | 0.996 | 0.996 | 0.996 | – | ||
| Avrami | Reac. order | 0.42 | 0.41 | 0.41 | 0.41 | 0.43 | 0.46 | 0.50 | 0.43 | |
| R2 | 0.984 | 0.992 | 0.992 | 0.996 | 0.997 | 0.999 | ≈1 | – | ||
| AA-acetylated | F-W-O | Ea (kJ/mol) | 182 | 191 | 198 | 202 | 201 | 199 | 199 | 196 |
| R2 | 0.999 | ≈1 | 0.999 | 0.999 | 0.999 | ≈1 | ≈1 | – | ||
| Modified C-R | Ea (kJ/mol) | 181 | 190 | 197 | 202 | 200 | 198 | 199 | 195 | |
| R2 | 0.999 | ≈1 | 0.999 | 0.999 | 0.999 | ≈1 | 0.999 | – | ||
| Friedman | Ea (kJ/mol) | 201 | 232 | 205 | 229 | 191 | 192 | 206 | 208 | |
| R2 | 0.980 | 0.980 | 0.997 | 0.992 | 0.998 | 0.999 | 0.997 | – | ||
| Starink | Ea (kJ/mol) | 182 | 191 | 198 | 202 | 201 | 198 | 199 | 196 | |
| R2 | 0.999 | ≈1 | 0.999 | 0.999 | 0.999 | ≈1 | 0.999 | – | ||
| Avrami | Reac. order | 0.57 | 0.54 | 0.50 | 0.49 | 0.52 | 0.56 | 0.61 | 0.54 | |
| R2 | 0.997 | 0.998 | 0.998 | 0.999 | ≈1 | 0.997 | 0.994 | – | ||
| VA-acetylated | F-W-O | Ea (kJ/mol) | 175 | 186 | 194 | 201 | 202 | 201 | 203 | 195 |
| (PC) | R2 | 0.988 | 0.998 | 0.999 | ≈1 | ≈1 | ≈1 | ≈1 | – | |
| Modified C-R | Ea (kJ/mol) | 174 | 186 | 194 | 201 | 202 | 201 | 203 | 194 | |
| R2 | 0.987 | 0.997 | 0.999 | 0.999 | ≈1 | ≈1 | ≈1 | – | ||
| Friedman | Ea (kJ/mol) | 172 | 206 | 202 | 196 | 205 | 206 | 213 | 200 | |
| R2 | 0.980 | 0.995 | 0.996 | 0.999 | 0.999 | 0.996 | 0.999 | – | ||
| Starink | Ea (kJ/mol) | 175 | 186 | 194 | 201 | 202 | 201 | 203 | 195 | |
| R2 | 0.987 | 0.997 | 0.999 | 0.999 | ≈1 | ≈1 | ≈1 | – | ||
| Avrami | Reac. order | 0.51 | 0.51 | 0.48 | 0.52 | 0.57 | 0.62 | 0.65 | 0.55 | |
| R2 | 0.926 | 0.989 | 0.996 | 0.998 | 0.998 | 0.997 | 0.996 | – | ||
| VA-acetylated | F-W-O | Ea (kJ/mol) | 163 | 173 | 183 | 189 | 194 | 193 | 194 | 184 |
| (PA) | R2 | 0.995 | 0.995 | 0.995 | 0.995 | 0.996 | 0.996 | 0.994 | – | |
| Modified C-R | Ea (kJ/mol) | 161 | 172 | 182 | 189 | 194 | 193 | 193 | 183 | |
| R2 | 0.995 | 0.994 | 0.995 | 0.995 | 0.995 | 0.995 | 0.993 | – | ||
| Friedman | Ea (kJ/mol) | 157 | 170 | 200 | 193 | 195 | 186 | 201 | 186 | |
| R2 | 0.987 | 0.989 | 0.997 | 0.995 | 0.995 | 0.991 | 0.991 | – | ||
| Starink | Ea (kJ/mol) | 162 | 172 | 182 | 189 | 194 | 193 | 193 | 184 | |
| R2 | 0.995 | 0.994 | 0.995 | 0.995 | 0.995 | 0.995 | 0.994 | – | ||
| Avrami | Reac. order | 0.59 | 0.58 | 0.55 | 0.53 | 0.55 | 0.59 | 0.63 | 0.57 | |
| R2 | 0.995 | 0.995 | 0.996 | 0.996 | 0.996 | 0.995 | 0.992 | – | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, K.-C.; Yang, C.-N.; Yang, T.-C.; Wu, T.-L.; Chen, Y.-L.; Wu, J.-H. Characterization and Thermal Stability of Acetylated Slicewood Production by Alkali-Catalyzed Esterification. Materials 2017, 10, 393. https://doi.org/10.3390/ma10040393
Hung K-C, Yang C-N, Yang T-C, Wu T-L, Chen Y-L, Wu J-H. Characterization and Thermal Stability of Acetylated Slicewood Production by Alkali-Catalyzed Esterification. Materials. 2017; 10(4):393. https://doi.org/10.3390/ma10040393
Chicago/Turabian StyleHung, Ke-Chang, Chen-Ning Yang, Teng-Chun Yang, Tung-Lin Wu, Yong-Long Chen, and Jyh-Horng Wu. 2017. "Characterization and Thermal Stability of Acetylated Slicewood Production by Alkali-Catalyzed Esterification" Materials 10, no. 4: 393. https://doi.org/10.3390/ma10040393
APA StyleHung, K.-C., Yang, C.-N., Yang, T.-C., Wu, T.-L., Chen, Y.-L., & Wu, J.-H. (2017). Characterization and Thermal Stability of Acetylated Slicewood Production by Alkali-Catalyzed Esterification. Materials, 10(4), 393. https://doi.org/10.3390/ma10040393








