LiV3O8/Polytriphenylamine Composites with Enhanced Electrochemical Performances as Cathode Materials for Rechargeable Lithium Batteries
Abstract
:1. Introduction
2. Experimental
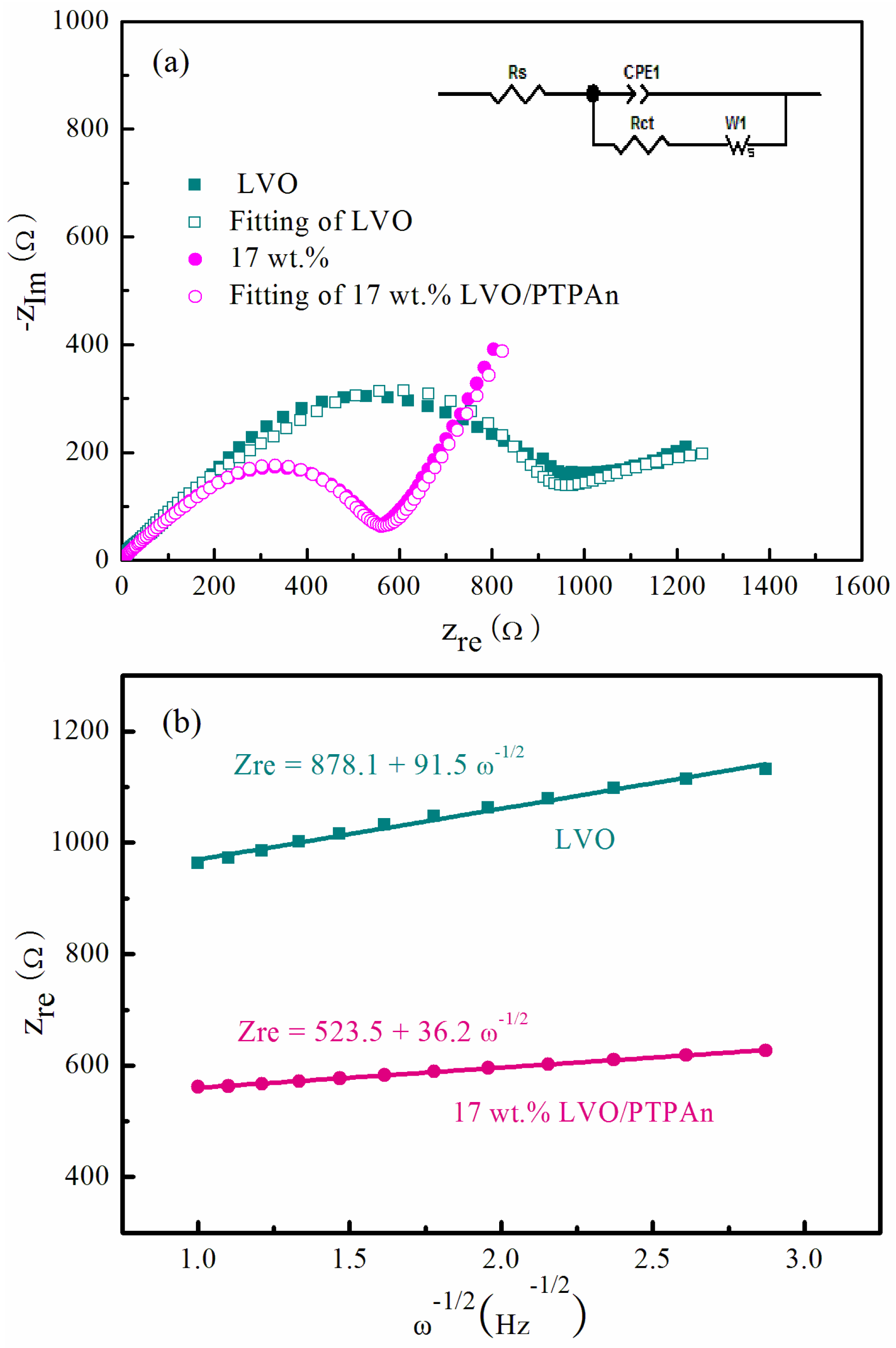
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pistoia, G.; Di Vona, M.L.; Tagliatesta, P. Transport and equilibrium characteristics of γ-lithium vanadium bronze. Solid State Ion. 1987, 24, 103–109. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wang, H.; Song, Z.Q.; Wang, Y.W.; Yan, H.; Yoshimura, M. Novel chemical method for synthesis of LiV3O8 nanorods as cathode materials for lithium ion batteries. Electrochim. Acta 2004, 49, 349–353. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, K.X.; Wei, X.; Chen, J.S. Carbon-coated V2O5 nanocrystals as high performance cathode material for lithium ion batteries. Chem. Mater. 2011, 23, 5290–5292. [Google Scholar] [CrossRef]
- Mo, R.; Du, Y.; Rooney, D.; Ding, G.; Sun, K. Ultradispersed nanoarchitecture of LiV3O8 nanoparticle/reduced graphene oxide with high capacity and long-life lithium-ion battery cathodes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, F.; Cao, S.; Li, Z.; Yang, H.; Ai, X.; Cao, Y. High rate, long lifespan LiV3O8 nanorods as a cathode material for lithium-ion batteries. Small 2017. [Google Scholar] [CrossRef] [PubMed]
- Manev, V.; Momchilov, A.; Nassalevska, A.; Pistoia, G.; Pasquali, M. A new approach to the improvement of Li1+xV3O8 performance in rechargeable lithium batteries. J. Power Sources 1995, 4, 501–507. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Zhou, X.; Cong, C.; Zhang, K. A soft chemistry synthesis and electrochemical properties of LiV3O8 as cathode material for lithium secondary batteries. Solid State Ion. 2005, 176, 1549–1554. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Schöllhorn, R. The discharge reaction mechanism of the MoO3 electrode in organic electrolytes. J. Power Sources 1976, 1, 267–276. [Google Scholar] [CrossRef]
- Nassau, K.D.; Murphy, W. The quenching and electrochemical behavior of Li2O V2O5 glasses. J. Non-Cryst. Solids 1981, 44, 297–304. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, L.; Zhang, Y.; Sun, J.; Yang, L.; Miao, Y.; Yuan, H.; Wang, Y. Synthesis of LiV3O8 by an improved citric acid assisted sol–gel method at low temperature. Mater. Chem. Phys. 2008, 111, 565–569. [Google Scholar] [CrossRef]
- Dubarry, M.; Gaubicher, J.; Guyomard, D.; Dupré, N.; Grey, C. Ultrafast synthesis of Li1+αV3O8 gel precursors for lithium battery applications. Solid State Ion. 2009, 180, 1511–1516. [Google Scholar] [CrossRef]
- Jouanneau, S.; Verbaere, A.; Guyomard, D. A combined X-ray and neutron Rietveld study of the chemically lithiated electrode materials Li2.7V3O8 and Li4.8V3O8. J. Solid State Chem. 2005, 178, 22–27. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Zhang, X.; Jin, Y. Synthesis of LiV3O8 nanocrystallites as cathode materials for lithium ion batteries. J. Mater. Process. Technol. 2008, 207, 265–270. [Google Scholar] [CrossRef]
- Zhou, Y.; Yue, H.F.; Zhang, X.Y.; Deng, X.Y. Preparation and characterization of LiV3O8 cathode material for lithium secondary batteries through an EDTA-sol-gel method. Solid State Ion. 2008, 179, 1763–1767. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, Y.C. Morphological and electrochemical properties of LiV3O8 cathode powders prepared by spray pyrolysis. Electrochim. Acta 2010, 55, 6088–6092. [Google Scholar] [CrossRef]
- Tran, N.; Bramnik, K.G.; Hibst, H.; Proelss, J.; Mronga, N.; Holzapfel, M.; Scheifele, W.; Novak, P. Spray-drying synthesis and electrochemical performance of lithium vanadates as positive electrode materials for lithium batteries. J. Electrochem. Soc. 2008, 155, A384–A389. [Google Scholar] [CrossRef]
- Liu, H.; Yang, H.; Huang, T. Synthesis, structure and electrochemical properties of one-dimensional nanometer materials LiV3O8. Mater. Sci. Eng. B 2007, 143, 60–63. [Google Scholar] [CrossRef]
- Jouanneau, S.; Le Gal La Salle, A.; Verbaere, A.; Deschamps, M.; Lascaud, S.; Guyomard, D. Influence of the morphology on the Li insertion properties of Li1.1V3O8. J. Mater. Chem. 2003, 13, 921–927. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Guo, Y. Structure and electrochemical performance of LiV3O8 synthesized by solid-state routine with quenching in freezing atmosphere. Mater. Chem. Phys. 2009, 114, 915–919. [Google Scholar] [CrossRef]
- Gao, X.W.; Wang, J.Z.; Chou, S.L.; Liu, H.K. Synthesis and electrochemical performance of LiV3O8/polyaniline as cathode material for the lithium battery. J. Power Sources 2012, 220, 47–53. [Google Scholar] [CrossRef]
- Benedek, R.; Thackeray, M.M.; Yang, L.H. First-principles calculation of atomic structure and electrochemical potential of Li1+xV3O8. J. Power Sources 1999, 81–82, 487–490. [Google Scholar] [CrossRef]
- Jouanneau, S.; Le Gal La Salle, A.; Verbaere, A.; Guyomard, D. The origin of capacity fading upon lithium cycling in Li1.1V3O8. J. Electrochem. Soc. 2005, 152, A1660–A1667. [Google Scholar] [CrossRef]
- Arnanchulvu, C.V.; Gauthier, M.; Batcho, T.P.; Symister, C.; Shao-Horn, Y.; D’Arcy, J.M.; Hammond, P.T. Evaluation and stability of PEDOT polymer electrodes for Li-O2 batteries. J. Phys. Chem. Lett. 2016, 7, 3770–3775. [Google Scholar]
- Ali, G.; Lee, J.H.; Susanto, D.; Choi, S.W.; Cho, B.W.; Nam, K.W.; Chung, K.Y. Polythiophene-wrapped olivine NaFePO4 as a cathode for Na-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 15422–15429. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.Q.; Chew, S.Y.; Guo, Z.P.; Wang, J.Z.; Liu, H.K. An investigation of polypyrrole-LiV3O8 composite cathode materials for lithium-ion batteries. J. Power Sources 2007, 174, 1095–1099. [Google Scholar] [CrossRef]
- Xie, L.L.; Cao, X.Y.; Zhang, L.X.; Dai, Z.X.; Qu, L.B. Synthesis and electrochemical properties of LiV3O8/PAn composite as a cathode material for lithium secondary batteries. Electron. Mater. Lett. 2013, 9, 183–186. [Google Scholar]
- Cao, X.; Zhu, L.; Wu, H. Preparation and electrochemical performances of rod-like LiV3O8/carbon composites using polyaniline as carbon source. Electron. Mater. Lett. 2015, 11, 650–657. [Google Scholar]
- Liu, L.L.; Wang, X.J.; Zhu, Y.S.; Hu, C.L.; Wu, Y.P.; Holze, R. Polypyrrole-coated LiV3O8-nanocomposites with good electrochemical performance as anode material for aqueous rechargeable lithium batteries. J. Power Sources 2013, 224, 290–294. [Google Scholar] [CrossRef]
- Tian, F.; Liu, L.; Yang, Z.; Wang, X.; Chen, Q.; Wang, X. Electrochemical characterization of a LiV3O8-polypyrrole composite as a cathode material for lithium ion batteries. Mater. Chem. Phys. 2011, 127, 151–155. [Google Scholar] [CrossRef]
- Novak, P.; Haas, O.; Santhanam, K.; Mueller, K. Electrochemically active polymers for rechargeable batteries. Chem. Rev. 1997, 97, 207–281. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Feng, C.Q.; Ng, S.H.; Wang, J.Z.; Guo, Z.P.; Liu, H.K. Low-temperature synthesis of polypyrrole-coated LiV3O8 composite with enhanced electrochemical properties. J. Electrochem. Soc. 2007, 154, A633–A637. [Google Scholar] [CrossRef]
- Feng, J.; Cao, Y.; Ai, X.; Yang, H. Polytriphenylamine: A high power and high capacity cathode material for rechargeable lithium batteries. J. Power Sources 2008, 177, 199–204. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, J. Rheological phase synthesis and characterization of Li3V2(PO4)3/C composites as cathode materials for lithium ion batteries. Electrochim. Acta 2014, 129, 305–311. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Z.; Luo, Y.; Chen, W.; Niu, C.; Zhao, K.; Yan, M.; Zhang, L.; Meng, J.; Mai, L. An electrospun hierarchical LiV3O8 nanowire-in-network for high-rate and long-life lithium batteries. J. Mater. Chem. A 2015, 3, 19850–19856. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Zhang, C.; Liu, C.; Cao, G. Mo-doped LiV3O8 nanorod-assembled nanosheets as a high performance cathode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 3547–3558. [Google Scholar] [CrossRef]
- Pan, A.; Liu, J.; Zhang, J.G.; Cao, G.; Xu, W.; Nie, Z.; Jie, X.; Choi, D.; Arey, B.W.; Wang, C.; Liang, S. Template free synthesis of LiV3O8 nanorods as a cathode material for high-rate secondary lithium batteries. J. Mater. Chem. 2011, 21, 1153–1161. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Tu, J.P.; Wang, X.L.; Zhang, J.; Yu, Y.X.; Gu, C.D. Self-assembled synthesis of hierarchical wafer like porous Li-V-O composites as cathode materials for lithium ion batteries. J. Phys. Chem. C 2011, 115, 25508–25518. [Google Scholar] [CrossRef]
- Huang, S.; Lu, Y.; Wang, T.Q.; Gu, C.D.; Wang, X.L.; Tu, J.P. Polyacrylamide-assisted freeze drying synthesis of hierarchical plate-arrayed LiV3O8 for high-rate lithium-ion batteries. J. Power Sources 2013, 235, 256–264. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Y.; Wang, Y.; Wang, W.; Liu, S. Synthesis of LiV3O8 nanosheets as a high-rate cathode material for rechargeable lithium batteries. CrystEngComm 2012, 14, 2831–2836. [Google Scholar] [CrossRef]
- Murugan, A.V.; Kale, B.B.; Kwon, C.W.; Campet, G.; Vijayamohanan, K. Synthesis and characterization of a new organo-inorganic poly(3,4-ethylene dioxythiophene) PEDOT/V2O5 nanocomposite by intercalation. J. Mater. Chem. 2001, 11, 2470–2475. [Google Scholar] [CrossRef]
- Murugan, A.V.; Kwon, C.W.; Campet, G.; Kale, B.B.; Maddanimath, T.; Vijayamohanan, K. Electrochemical lithium insertion into a poly(3,4-ethylenedioxythiophene) PEDOT/V2O5 nanocomposite. J. Power Sources 2002, 105, 1–5. [Google Scholar] [CrossRef]
- Yang, G.; Hou, W.; Sun, Z.; Yan, Q. A novel inorganic-organic polymer electrolyte with a high conductivity: Insertion of poly(ethylene) oxide into LiV3O8 in one step. J. Mater. Chem. 2005, 15, 1369–1374. [Google Scholar] [CrossRef]
- Koval’chuk, E.P.; Reshetnyak, O.V.; Kovalyshyn, Y.S.; Blażejowski, J. Structure and properties of lithium trivanadate-a potential electroactive material for a positive electrode of secondary storage. J. Power Sources 2002, 107, 61–66. [Google Scholar] [CrossRef]
- Kosova, N.V.; Anufrienko, V.F.; Vasenin, N.T.; Vosel, S.V.; Devyatkina, E.T. Electronic state of vanadium ions in Li1+xV3O8 according to EPR spectroscopy. J. Solid State Chem. 2002, 163, 421–426. [Google Scholar] [CrossRef]
- Dubarry, M.; Gaubicher, J.; Guyomard, D.; Steunou, N.; Livage, J.; Dupré, N.; Grey, C.P. Synthesis of Li1+αV3O8 via a gel precursor: Part II, from xerogel to the anhydrous material. Chem. Mater. 2006, 18, 629–636. [Google Scholar] [CrossRef]
- De Picciotto, L.A.; Adendorff, K.T.; Liles, D.C.; Thackeray, M.M. Structural characterization of Li1+xV3O8 insertion electrodes by single-crystal X-ray diffraction. Solid State Ion. 1993, 62, 297–307. [Google Scholar] [CrossRef]
- Kawakita, J.; Majima, M.; Miura, T.; Kishi, T. Preparation and lithium insertion behaviour of oxygen-deficient Li1+xV3O8−δ. J. Power Sources 1997, 66, 135–139. [Google Scholar] [CrossRef]
- Sakunthala, A.; Reddy, M.V.; Selvasekarapandian, S.; Chowdari, B.V.R.; Selvin, P.C. Preparation, characterization, and electrochemical performance of lithium trivanadate rods by a surfactant-assisted polymer precursor method for lithium batteries. J. Phys. Chem. C 2010, 114, 8099–8107. [Google Scholar] [CrossRef]
- Song, H.; Luo, M.; Wang, A. High rate and stable Li-ion insertion in oxygen-deficient LiV3O8 nanosheets as a cathode material for lithium-ion battery. ACS Appl. Mater. Interfaces 2017, 9, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
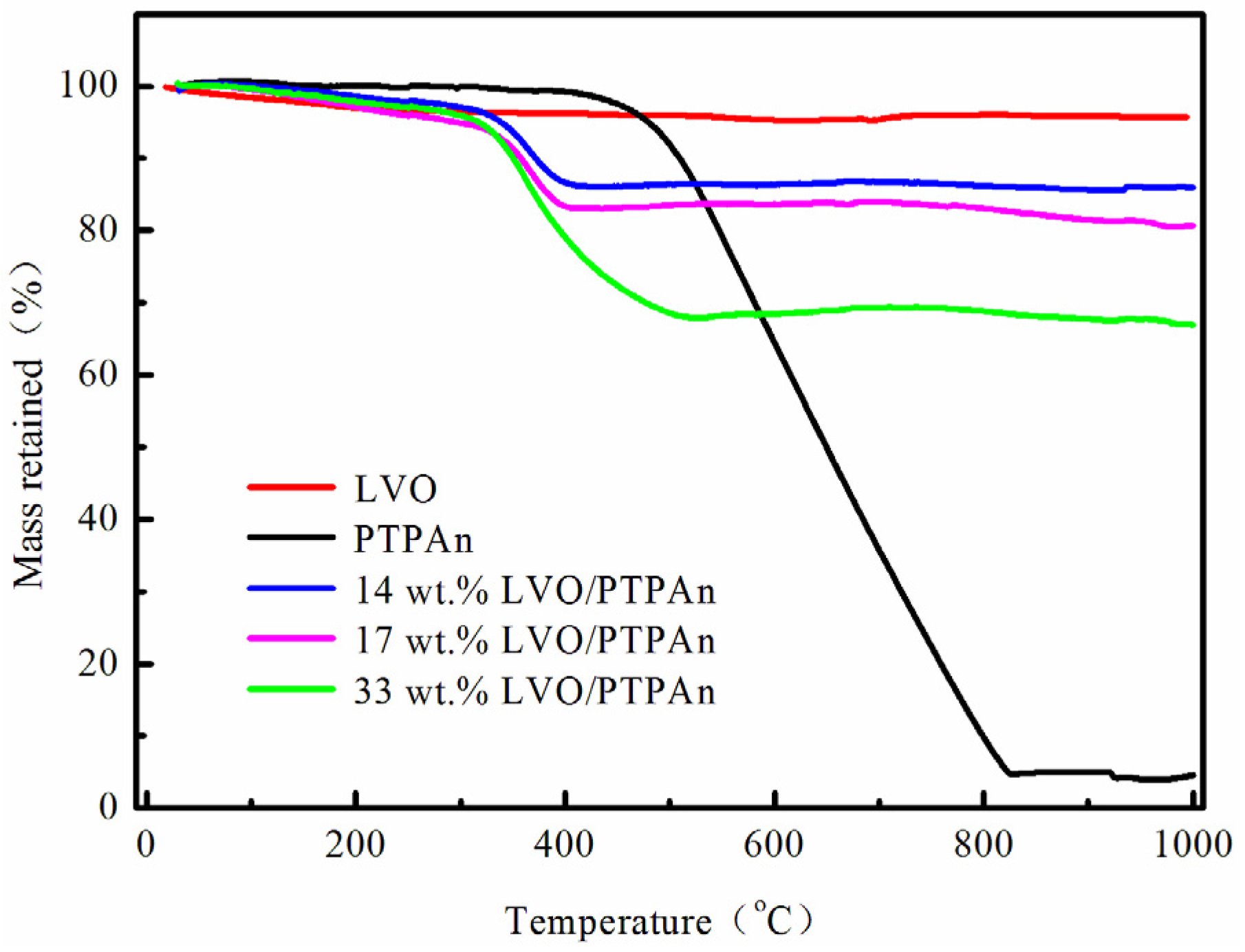
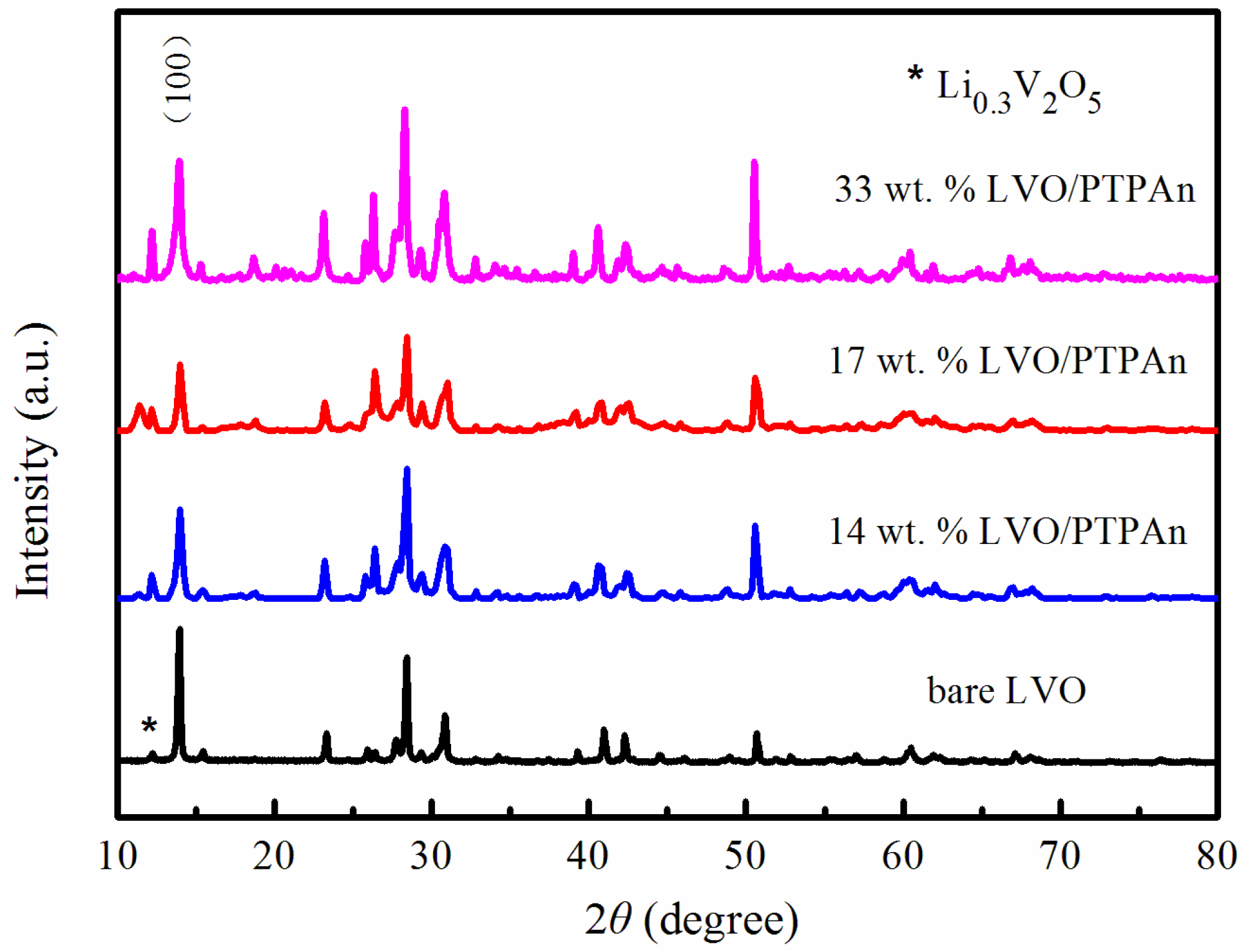

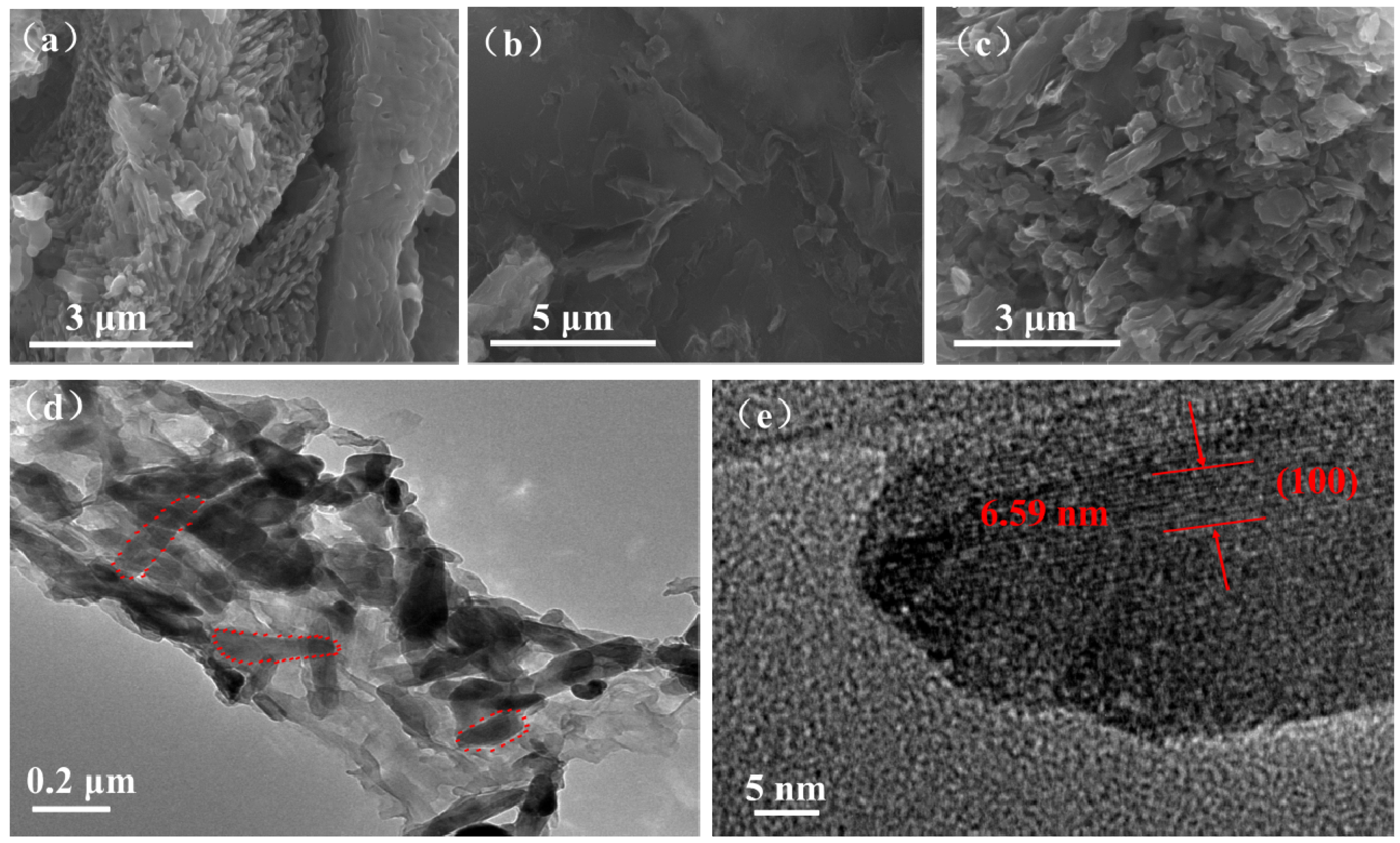
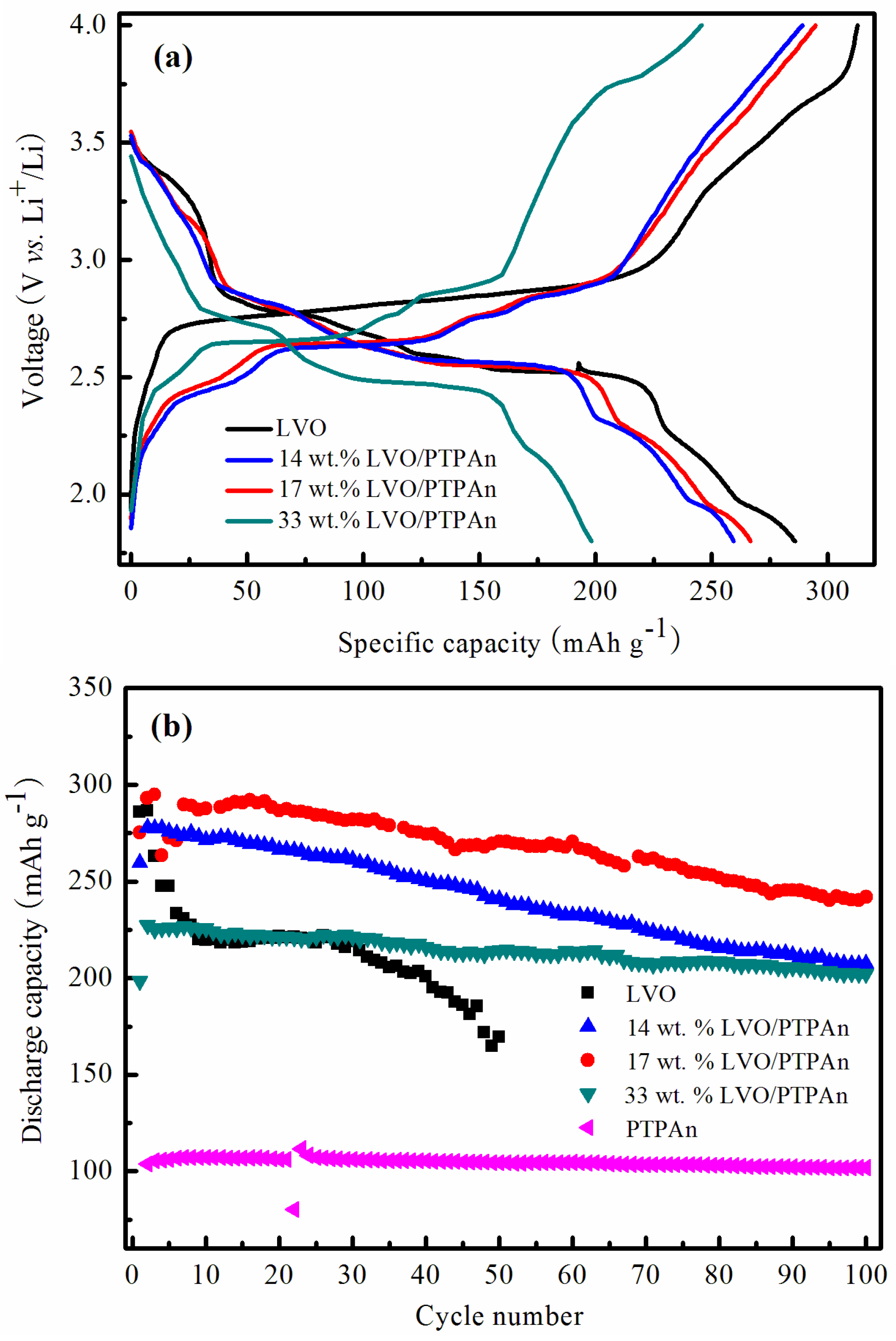
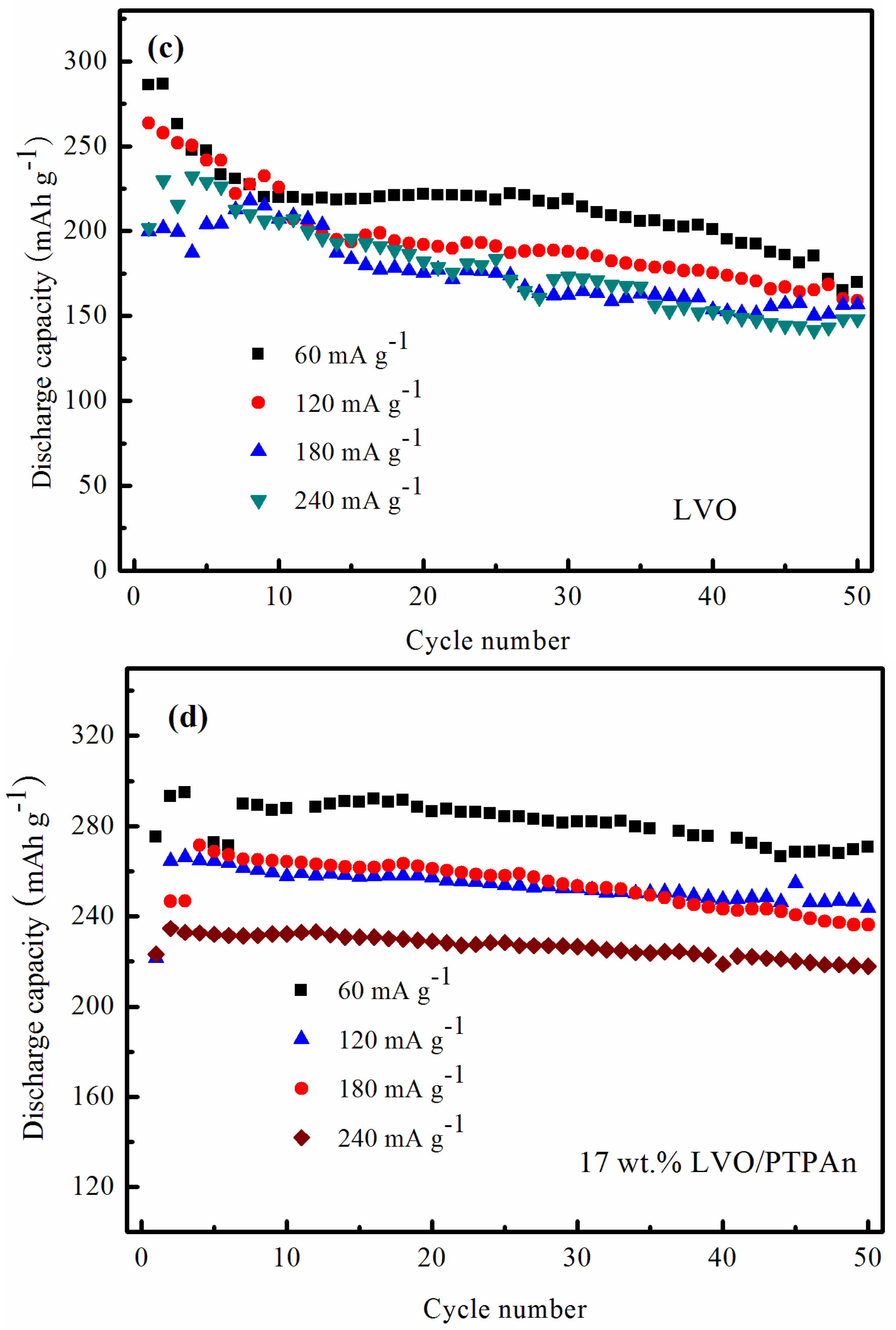
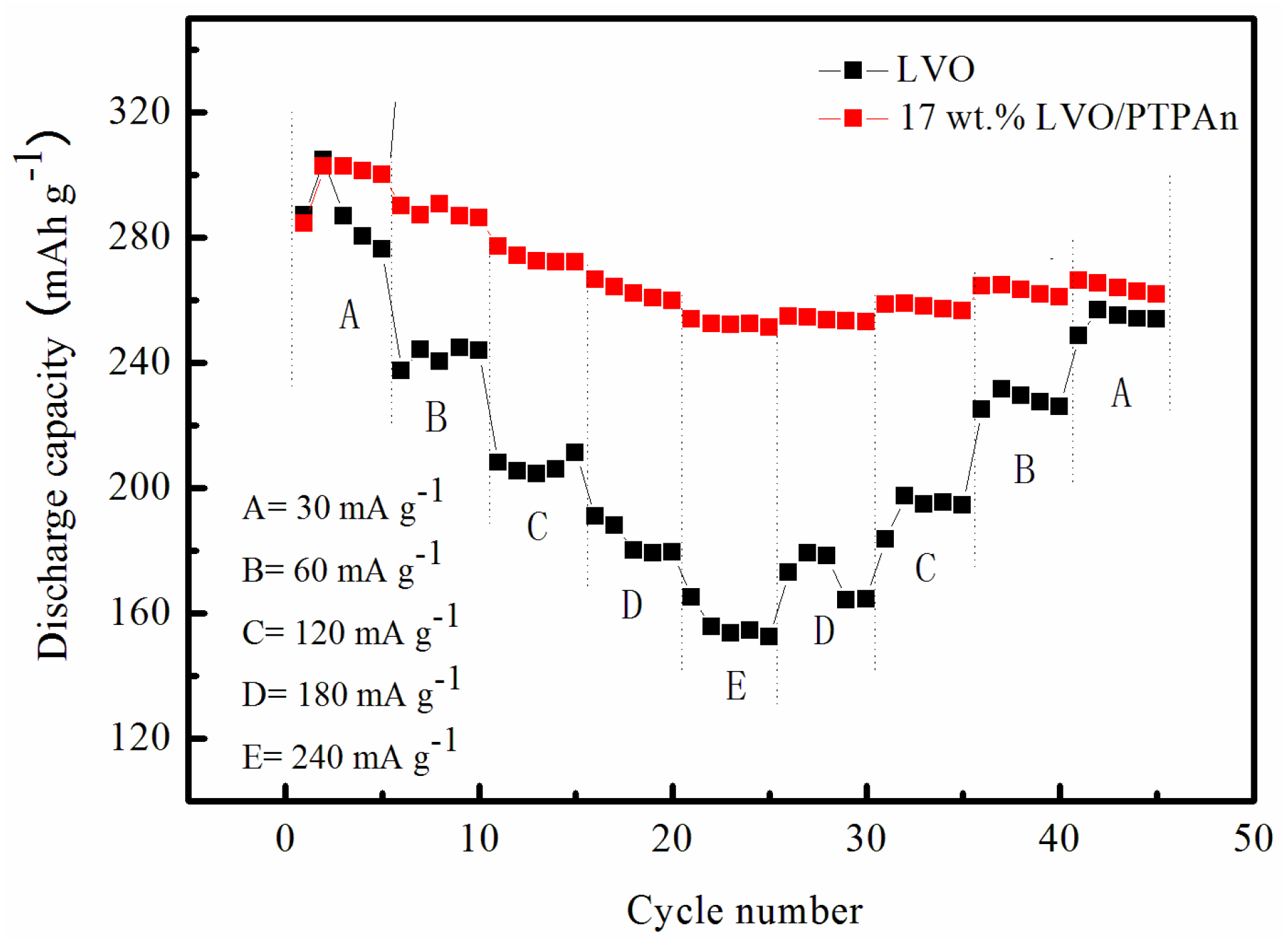
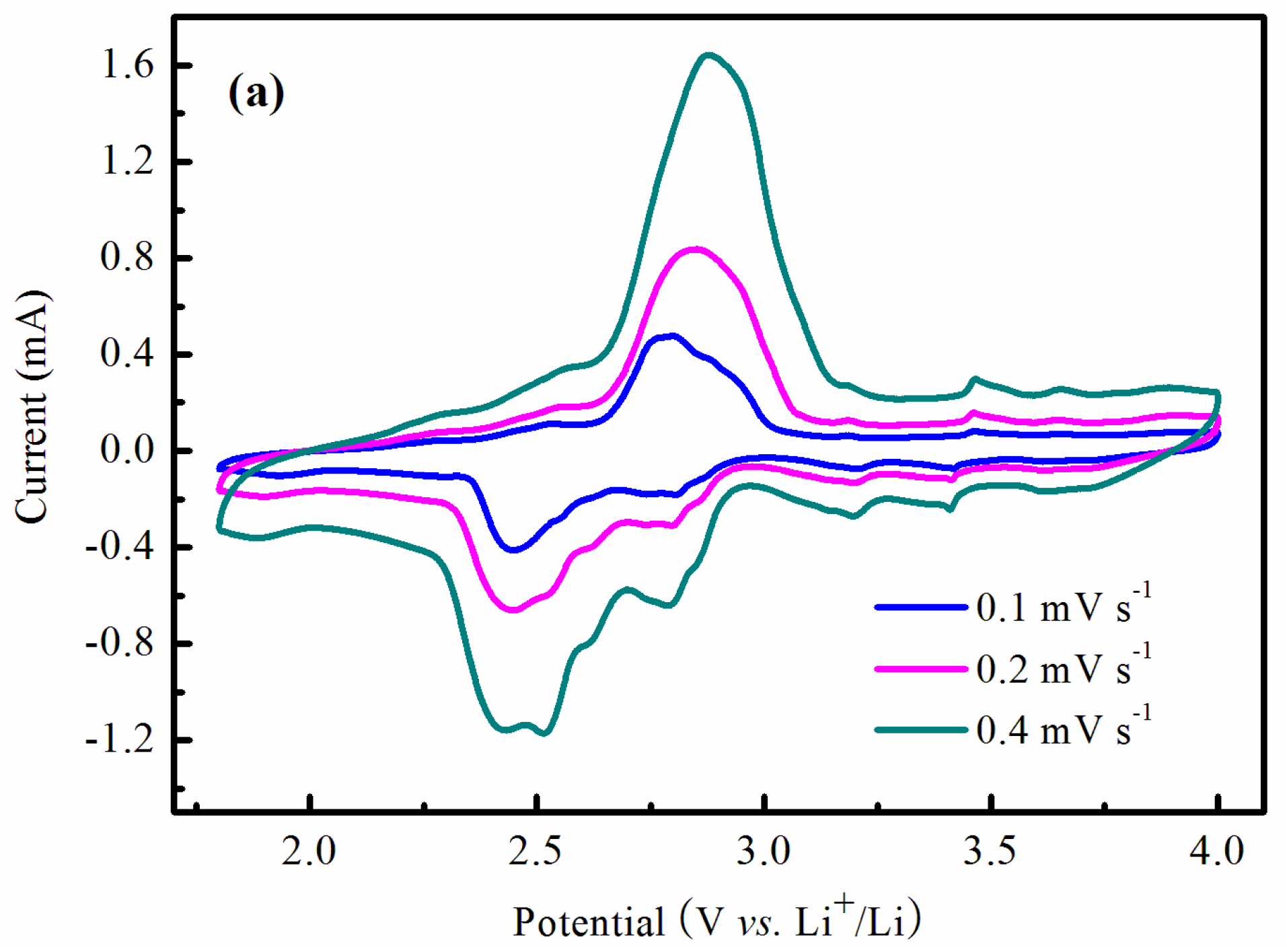
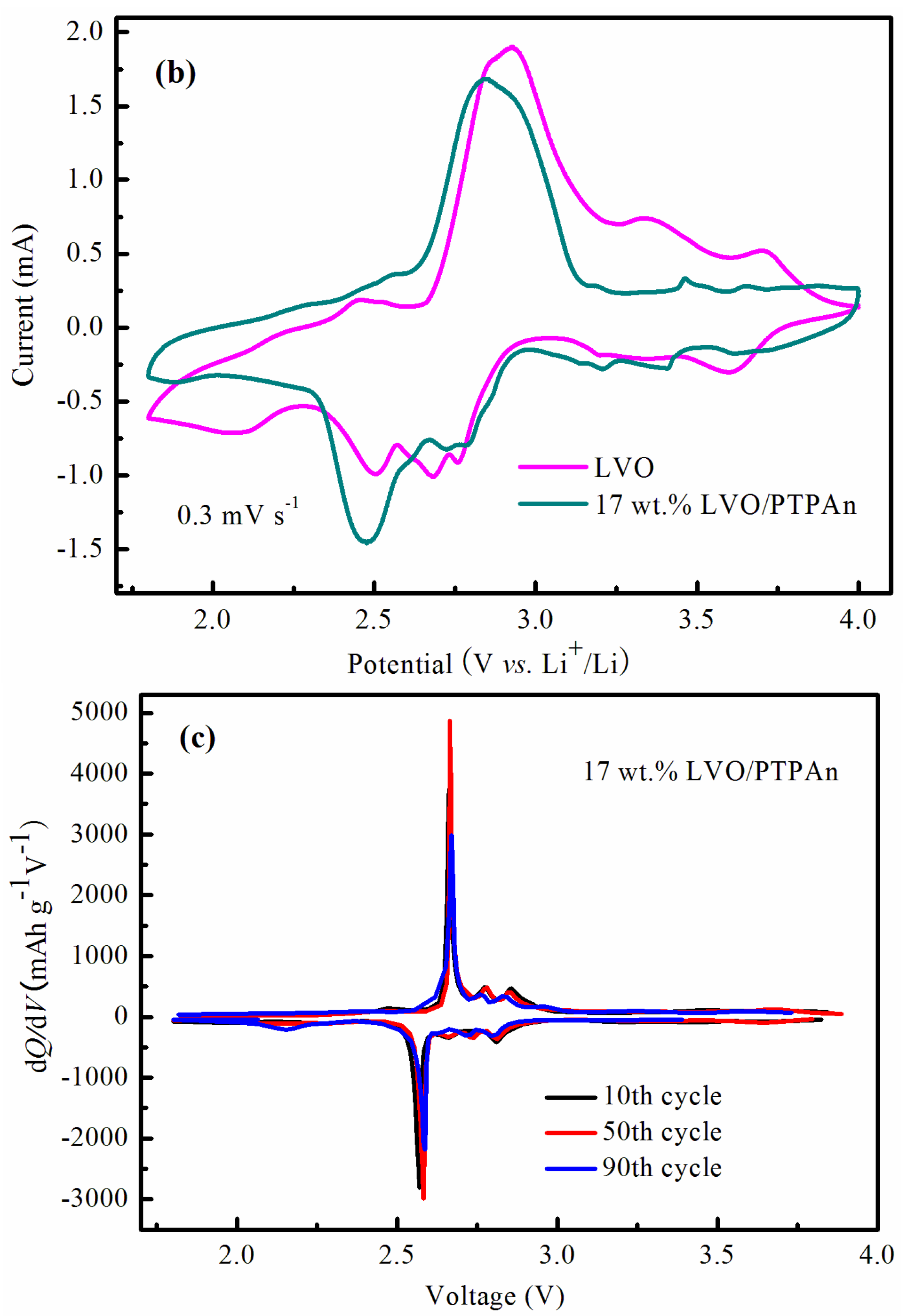
| Samples | Bare LVO | 14 wt % LVO/PTPAn | 17 wt % LVO/PTPAn | 33 wt % LVO/PTPAn |
| d100-spacing (Å) | 6.4361 | 6.5284 | 6.5727 | 6.3449 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhu, L.; Yu, Z.; Xie, L.; Cao, X. LiV3O8/Polytriphenylamine Composites with Enhanced Electrochemical Performances as Cathode Materials for Rechargeable Lithium Batteries. Materials 2017, 10, 344. https://doi.org/10.3390/ma10040344
Li W, Zhu L, Yu Z, Xie L, Cao X. LiV3O8/Polytriphenylamine Composites with Enhanced Electrochemical Performances as Cathode Materials for Rechargeable Lithium Batteries. Materials. 2017; 10(4):344. https://doi.org/10.3390/ma10040344
Chicago/Turabian StyleLi, Wenjuan, Limin Zhu, Ziheng Yu, Lingling Xie, and Xiaoyu Cao. 2017. "LiV3O8/Polytriphenylamine Composites with Enhanced Electrochemical Performances as Cathode Materials for Rechargeable Lithium Batteries" Materials 10, no. 4: 344. https://doi.org/10.3390/ma10040344





