Enhanced Unipolar Resistive Switching Characteristics of Hf0.5Zr0.5O2 Thin Films with High ON/OFF Ratio
Abstract
:1. Introduction
2. Materials and Methods
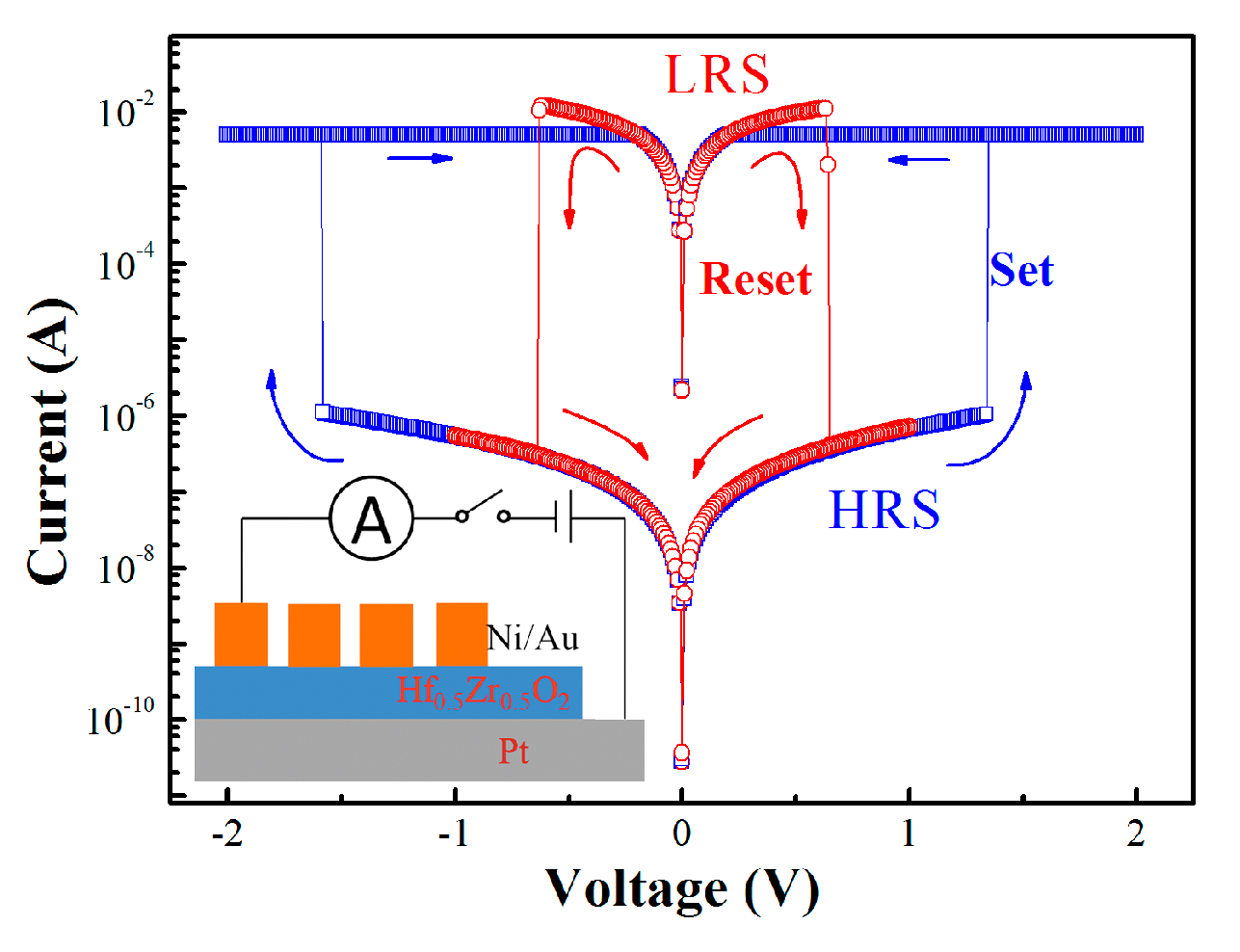
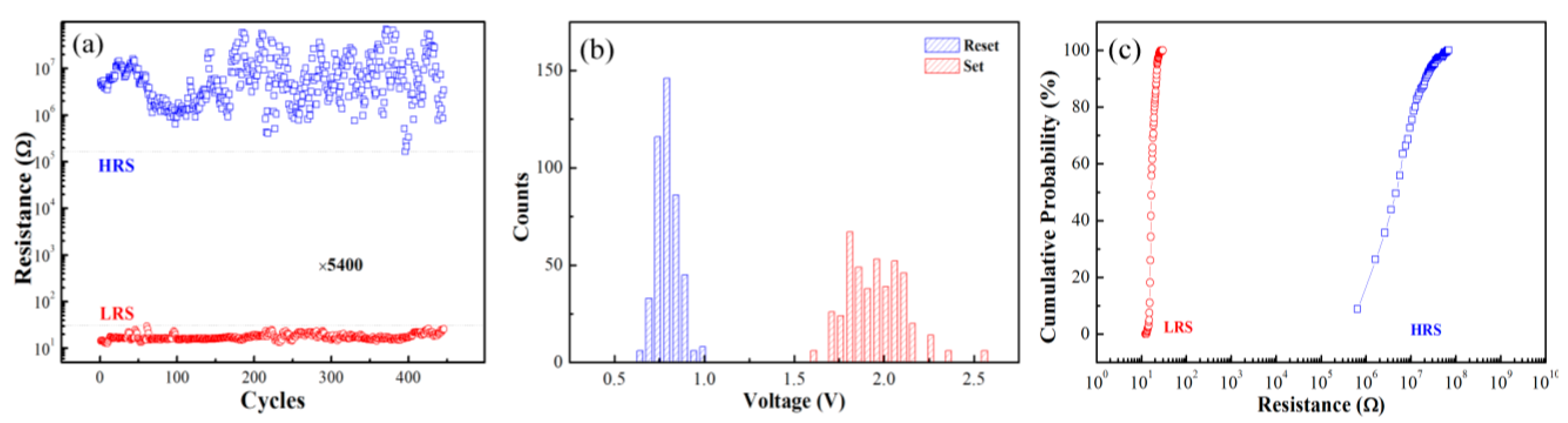
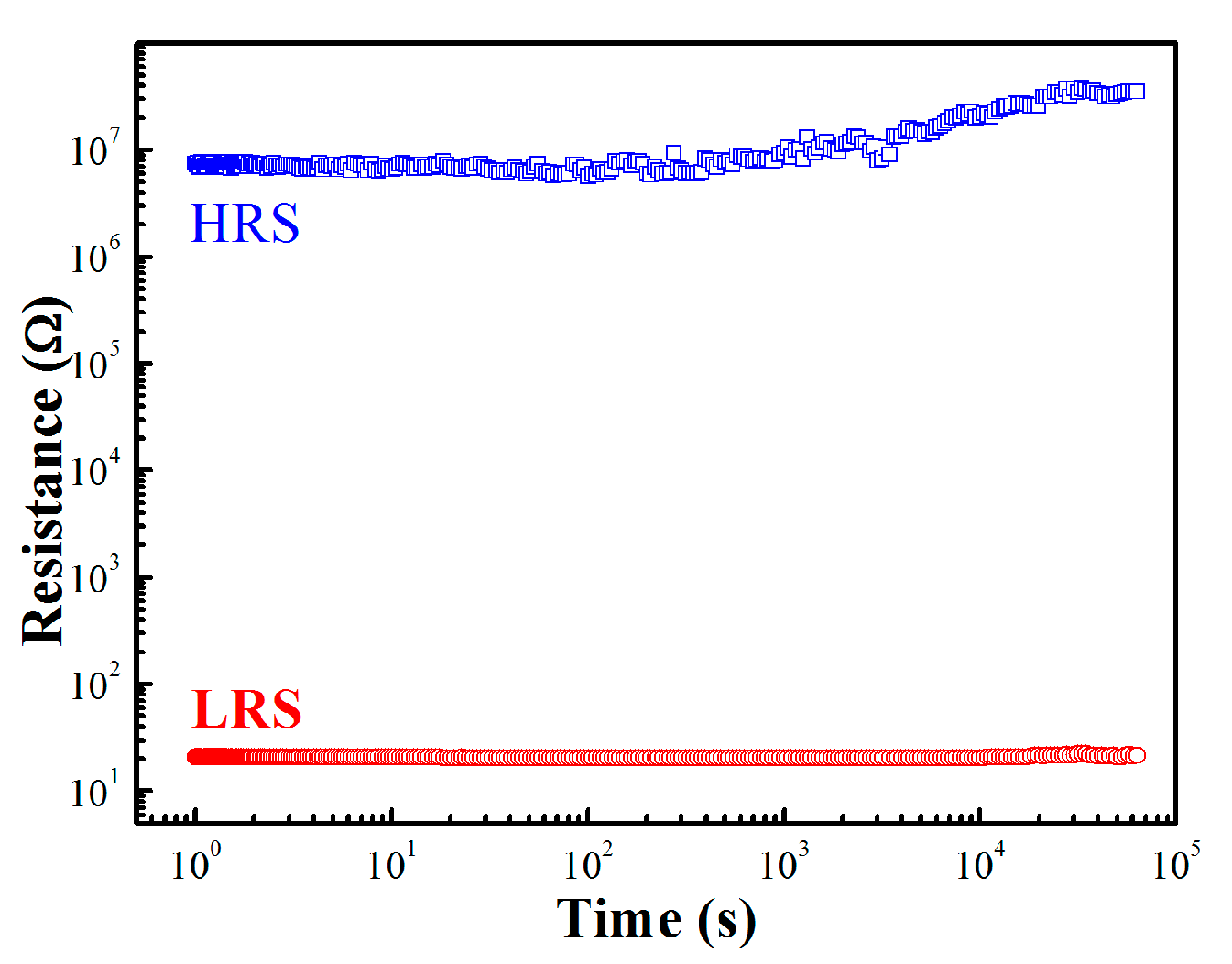
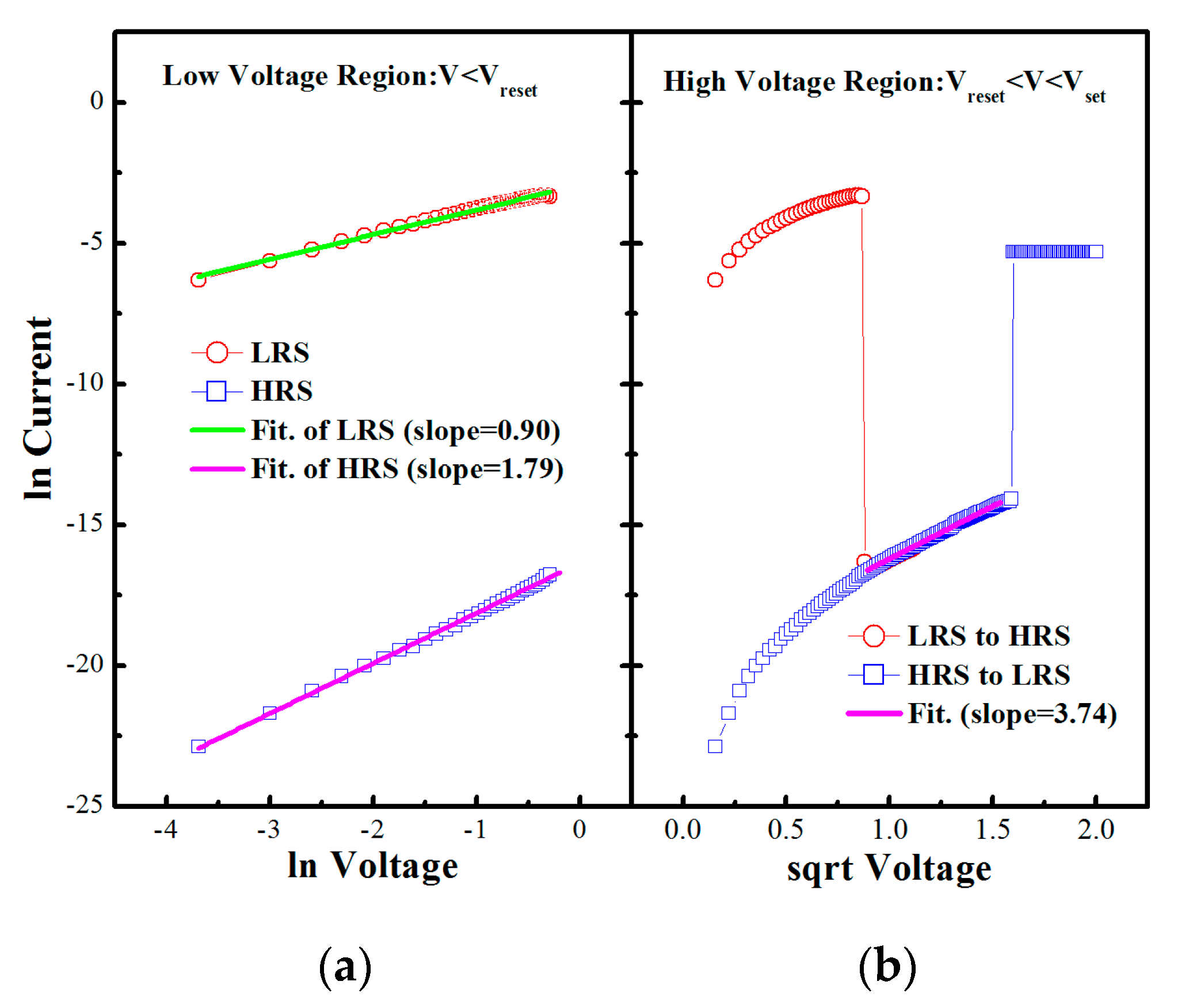
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verrelli, E.; Tsoukalas, D.; Normand, P.; Kean, A.H.; Boukos, N. Forming-free resistive switching memories based on titanium-oxide nanoparticles fabricated at room temperature. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Noman, M.; Sharma, A.A.; Lu, Y.M.; Skowronski, M.; Paul, A.; Salvador, P.A.; Bain, J.A. Transient characterization of the electroforming process in TiO2 based resistive switching devices. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Zou, L.; Hu, W.; Xie, W.; Chen, R.Q.; Qin, N.; Li, B.J.; Bao, D.H. Excellent resistive switching property and physical mechanism of amorphous TiO2 thin films fabricated by a low-temperature photochemical solution deposition method. Appl. Surf. Sci. 2014, 311, 697–702. [Google Scholar] [CrossRef]
- Kim, D.C.; Seo, S.S.; Ahn, E.; Suh, D.S.; Lee, M.J. Electrical observations of filamentary conductions for the resistive memory switching in NiO films. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Yu, Q.; Lim, W.M.; Hu, S.G.; Chen, T.P.; Deng, L.J.; Liu, Y. Flexible Nanoscale Memory Device Based on Resistive Switching in Nickel Oxide Thin Film. Nanosci. Nanotechnol. Lett. 2012, 4, 940–943. [Google Scholar] [CrossRef]
- Wang, X.L.; Shao, Q.; Leung, C.W.; Lortz, R.; Ruotolo, A. Non-volatile, electric control of magnetism in Mn-substituted ZnO. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Zhang, Q.L.; Shurong, D.; Luo, J.K. Bipolar resistive switching characteristics of low temperature grown ZnO thin films by plasma-enhanced atomic layer deposition. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Wu, Y.L.; Liao, C.W.; Ling, J.J. Effect of current compliance and voltage sweep rate on the resistive switching of HfO2/ITO/Invar structure as measured by conductive atomic force microscopy. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Sharath, S.U.; Bertaud, T.; Kurian, J.; Hildebrandt, E.; Walczyk, C.; Calka, P.; Zaumseil, P.; Sowinska, M.; Walczyk, D.; Gloskovskii, A.; et al. Towards forming-free resistive switching in oxygen engineered HfO2-x. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Haemori, M.; Nagata, T.; Chikyow, T. Impact of Cu Electrode on Switching Behavior in a Cu/HfO2/Pt Structure and Resultant Cu Ion Diffusion. Appl. Phys. Express 2009, 2. [Google Scholar] [CrossRef]
- Ramadoss, A.; Krishnamoorthy, K.; Kim, S.J. Resistive Switching Behaviors of HfO2 Thin Films by Sol–Gel Spin Coating for Nonvolatile Memory Applications. Appl. Phys. Express 2012, 5. [Google Scholar] [CrossRef]
- Ye, C.; Zhan, C.; Tsai, T.M.; Chang, K.C.; Chen, M.C.; Chang, T.C.; Deng, T.F.; Wang, H. Low-power bipolar resistive switching TiN/HfO2/ITO memory with self-compliance current phenomenon. Appl. Phys. Express 2014, 7. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, B.; Sun, B.; Chen, G.P.; Zeng, L.; Liu, L.F.; Liu, X.Y.; Lu, J.; Han, R.Q.; Kang, J.F.; et al. Ionic doping effect in ZrO2 resistive switching memory. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, P.; Li, J.; Chen, L.Y.; Lv, H.B.; Lin, Y.Y.; Tang, T.A. Reproducible unipolar resistance switching in stoichiometric ZrO2 films. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, L.; Xu, X.G.; Miao, J.; Jiang, Y. Effect of oxide/oxide interface on polarity dependent resistive switching behavior in ZnO/ZrO2 heterostructures. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Lee, D.Y.; Yao, I.C.; Hung, C.J.; Wang, S.Y.; Huang, T.Y.; Wu, J.W.; Tseng, T.Y. Unipolar Resistive Switching in ZrO2 Thin Films. Jpn. J. Appl. Phys. 2013, 52. [Google Scholar] [CrossRef]
- Waser, R.; Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 2007, 6, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-Based Resistive Switching Memories—Nanoionic Mechanisms, Prospects, and Challenges. Adv. Mater. 2009, 21, 2632–2663. [Google Scholar] [CrossRef]
- Muller, J.; Boscke, T.S.; Schröder, U.; Mueller, S.; Bräuhaus, D.; Böttger, U.; Frey, L.; Mikolajick, T. Ferroelectricity in Simple Binary ZrO2 and HfO2. Nano Lett. 2012, 12, 4318–4323. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Tsai, T.L.; Tseng, T.Y. Unipolar resistive switching behavior in Pt/HfO2/TiN device with inserting ZrO2 layer and its 1 diode–1 resistor characteristics. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Lee, J.; Bourim, E.M.; Lee, W.; Park, J.; Jo, M.; Jung, S.; Shin, J.; Hwang, H. Effect of ZrOx/HfOx bilayer structure on switching uniformity and reliability in nonvolatile memory applications. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, C.Y.; Tsai, T.L.; Lin, C.A.; Tseng, T.Y. Switching mechanism of double forming process phenomenon in ZrOx/HfOy bilayer resistive switching memory structure with large endurance. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Tulu, B.; Chang, W.Z.; Chu, J.P.; Wang, S.F. Forming-free resistive switching characteristics of 15 nm-thick multicomponent oxide. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Choi, B.J.; Jeong, D.S.; Kim, S.K.; Rohde, C.; Choi, S.; Oh, J.H.; Kim, H.J.; Hwang, C.S.; Szot, K.; Waser, R.; et al. Resistive switching mechanism of TiO2 thin films grown by atomic-layer deposition. J. Appl. Phys. 2005, 98. [Google Scholar] [CrossRef]
- Chudnovskii, F.A.; Odynets, L.L.; Pergament, A.L.; Stefanovich, G.B. Electroforming and switching in oxides of transition metals: The role of metal–insulator transition in the switching mechanism. J. Solid State Chem. 1996, 122, 95–99. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Zhu, J. Enhanced Unipolar Resistive Switching Characteristics of Hf0.5Zr0.5O2 Thin Films with High ON/OFF Ratio. Materials 2017, 10, 322. https://doi.org/10.3390/ma10030322
Wu Z, Zhu J. Enhanced Unipolar Resistive Switching Characteristics of Hf0.5Zr0.5O2 Thin Films with High ON/OFF Ratio. Materials. 2017; 10(3):322. https://doi.org/10.3390/ma10030322
Chicago/Turabian StyleWu, Zhipeng, and Jun Zhu. 2017. "Enhanced Unipolar Resistive Switching Characteristics of Hf0.5Zr0.5O2 Thin Films with High ON/OFF Ratio" Materials 10, no. 3: 322. https://doi.org/10.3390/ma10030322
APA StyleWu, Z., & Zhu, J. (2017). Enhanced Unipolar Resistive Switching Characteristics of Hf0.5Zr0.5O2 Thin Films with High ON/OFF Ratio. Materials, 10(3), 322. https://doi.org/10.3390/ma10030322




