Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting
Abstract
:1. Introduction
2. Results
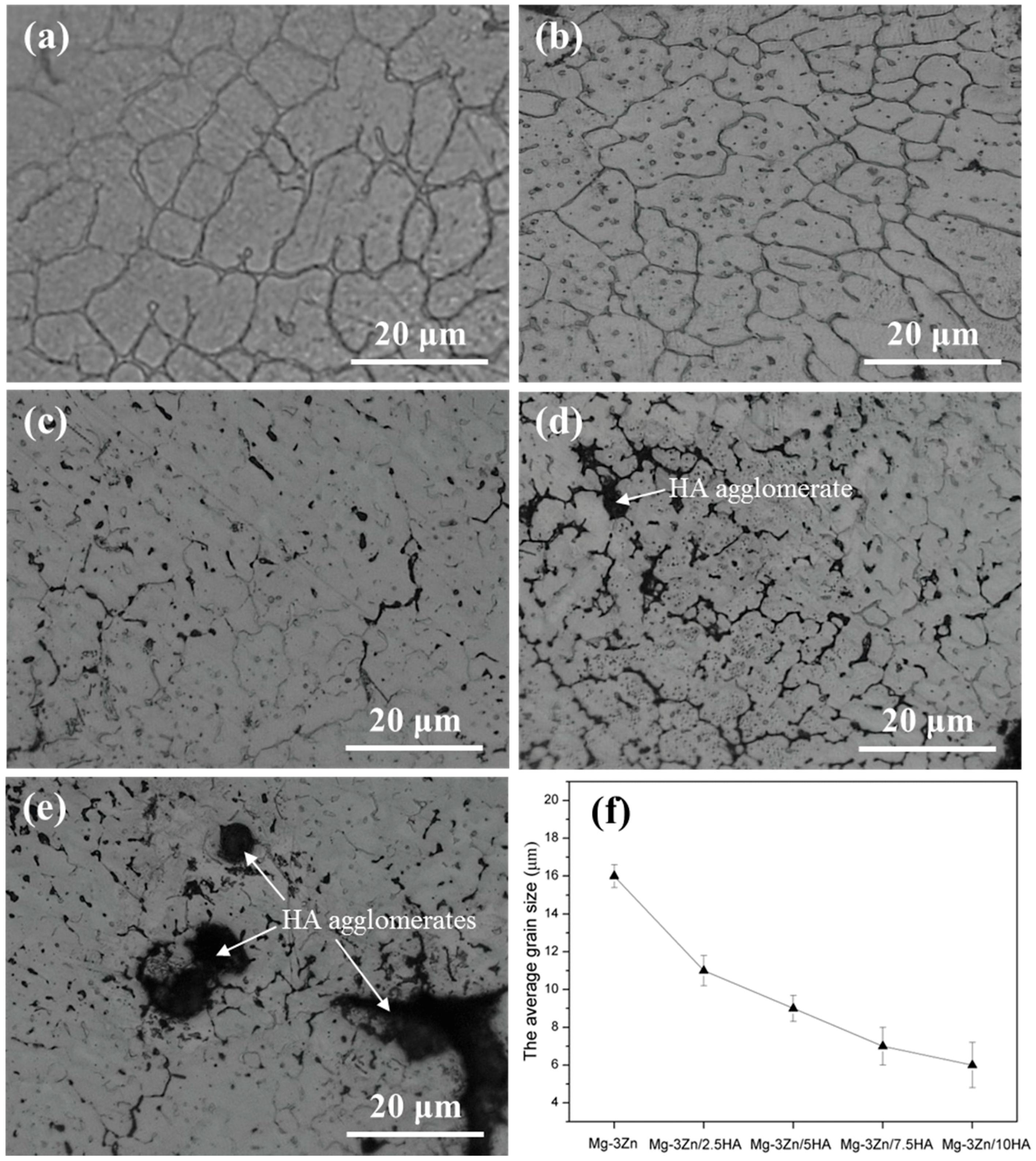
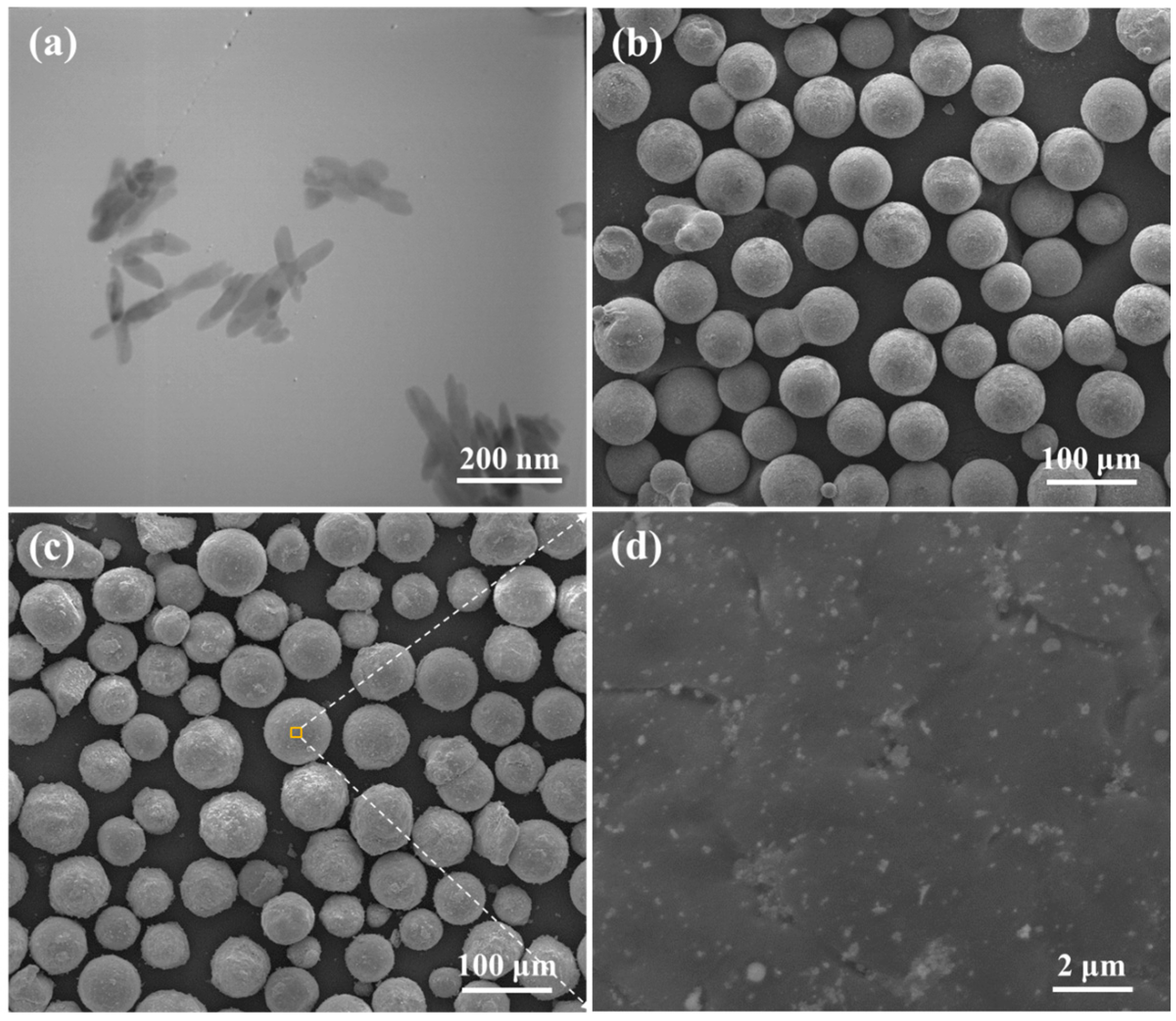
2.1. Microstructure and Composition
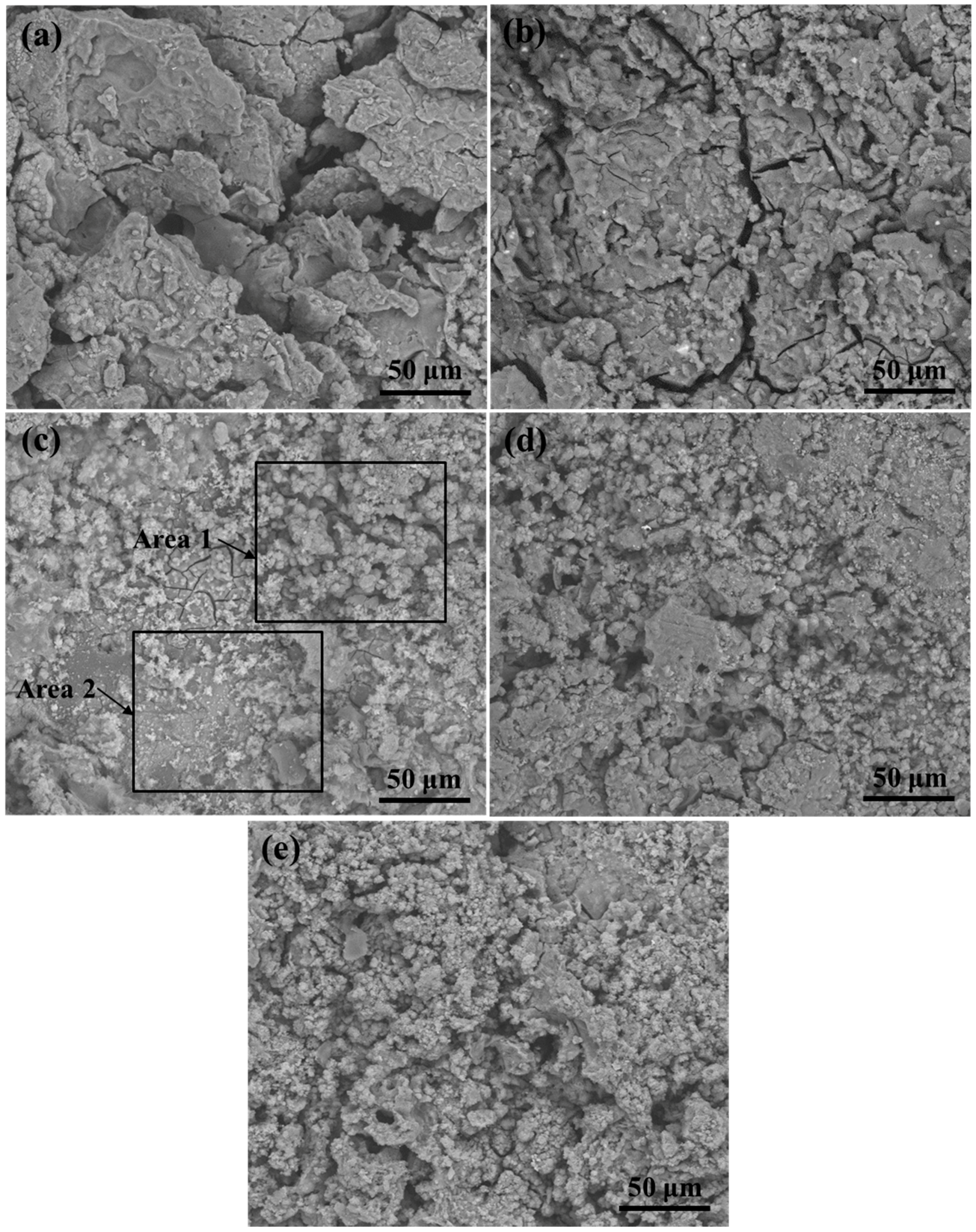
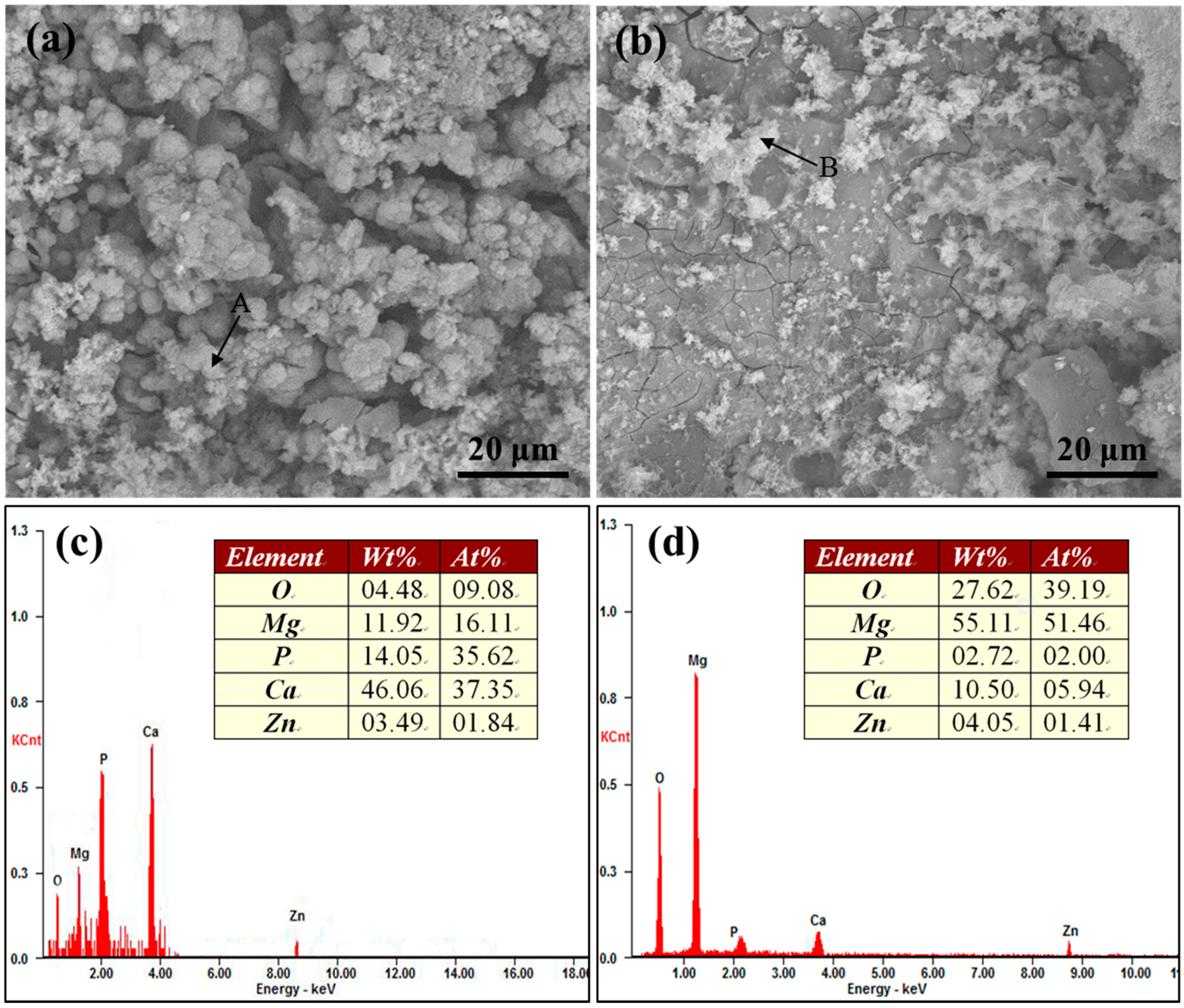
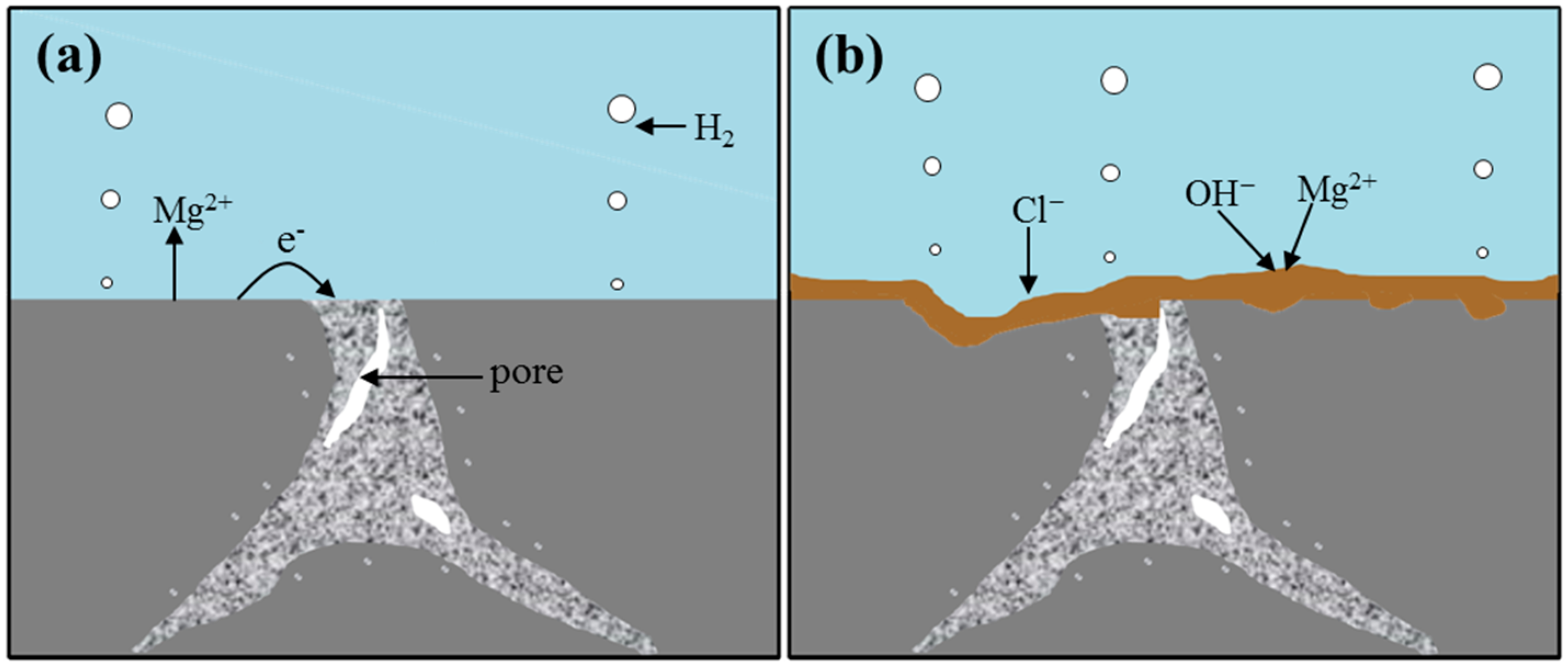
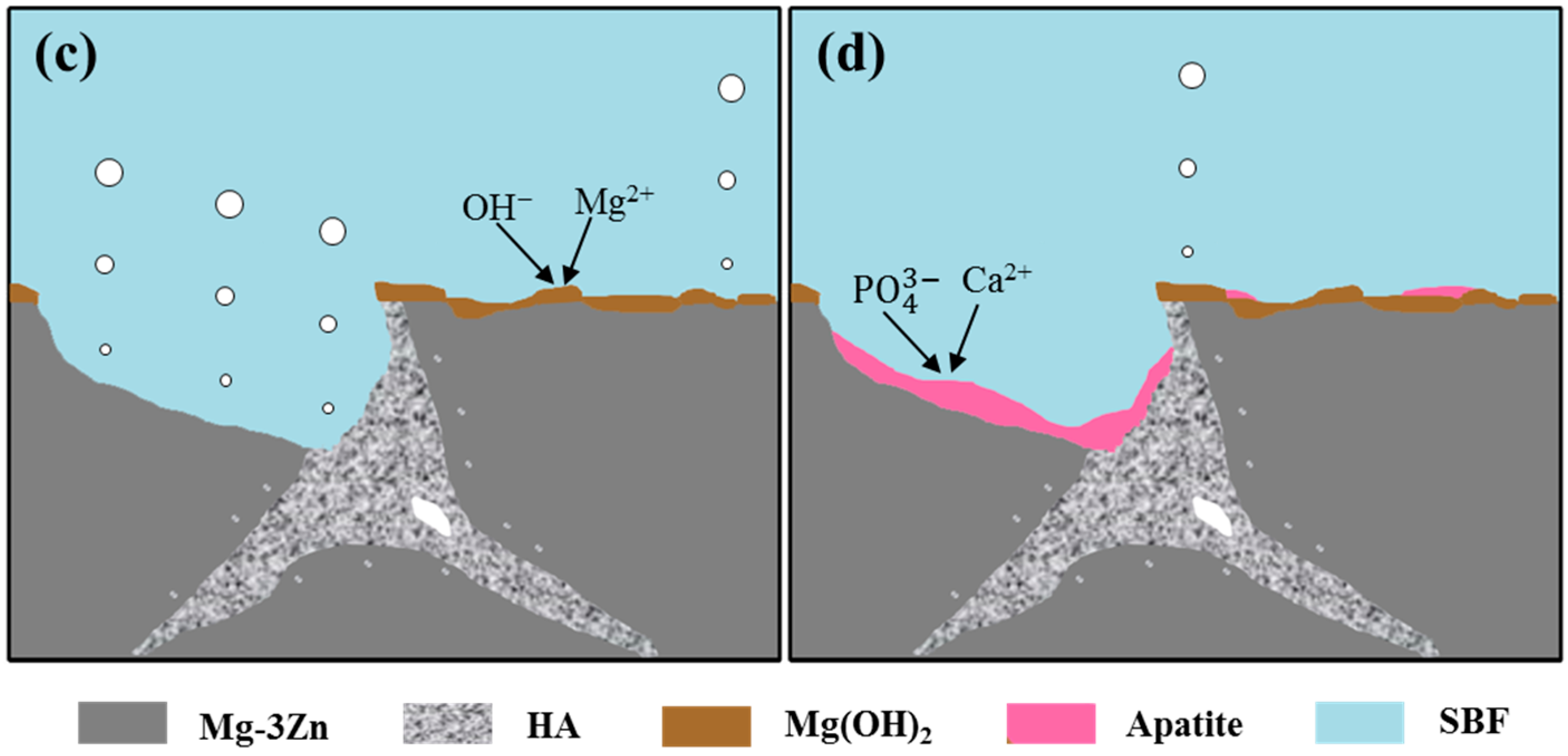
2.2. Immersion Tests
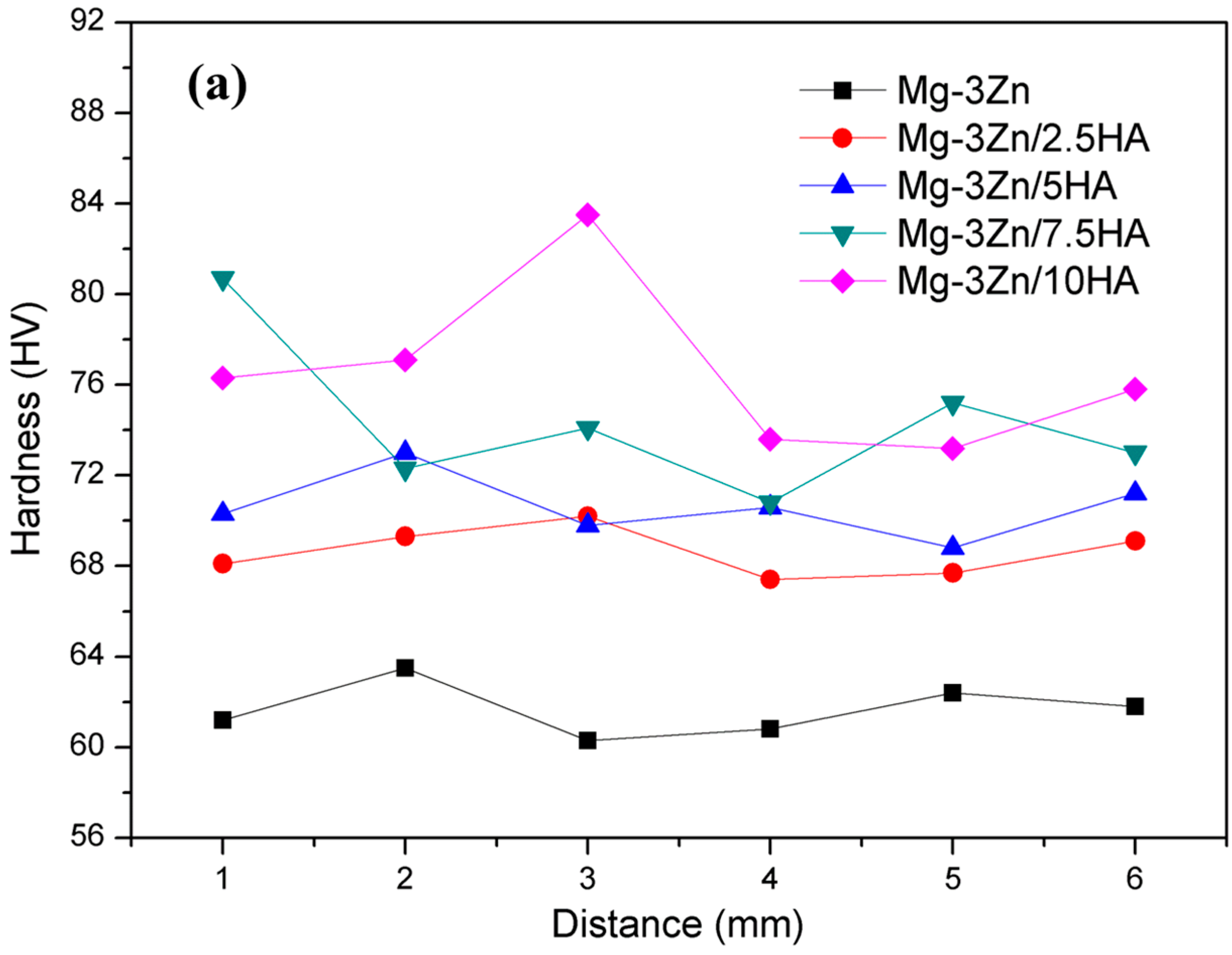
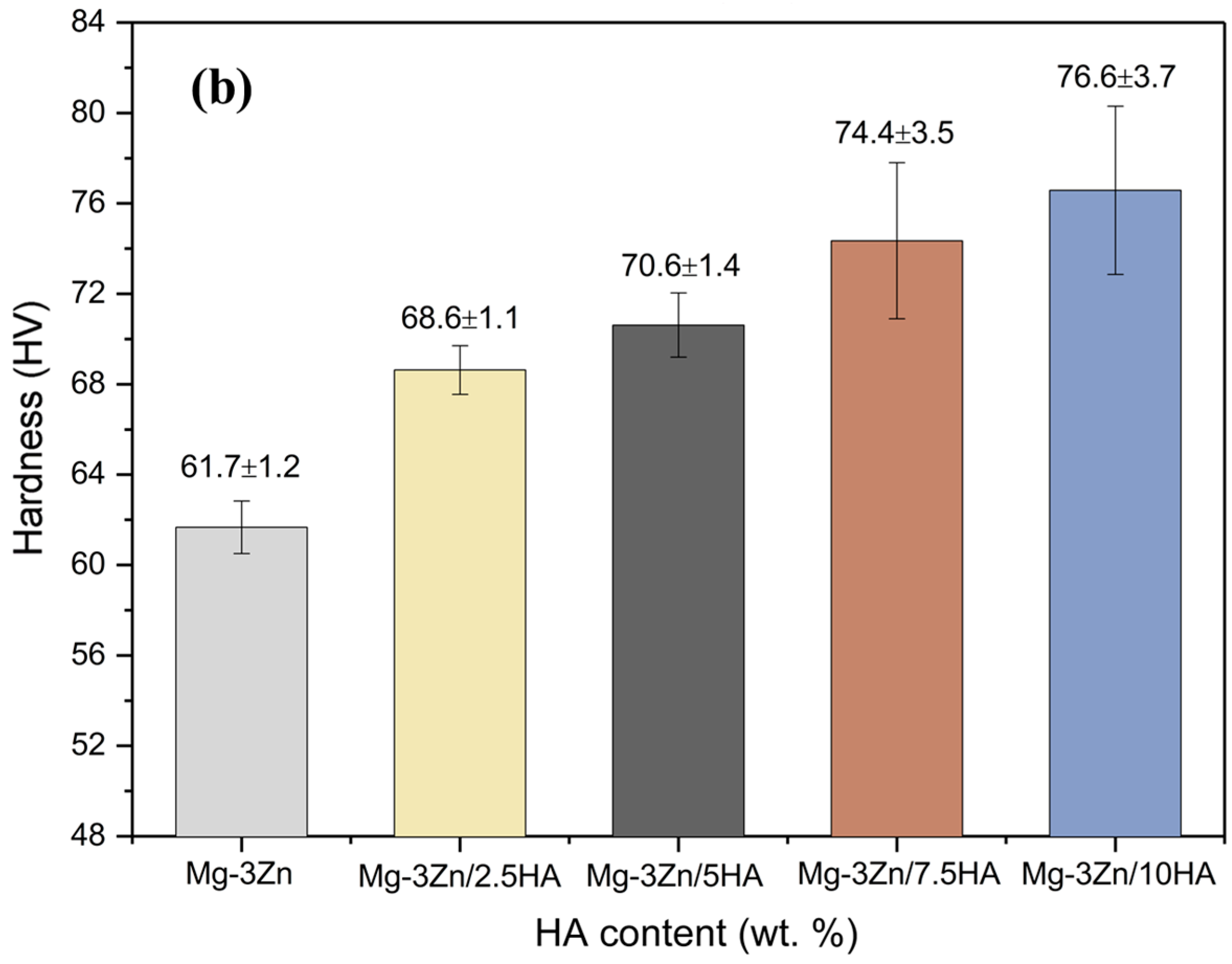
2.3. Mechanical Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Processing
4.3. Microstructure
4.4. Immersion Tests
4.5. Mechanical Properties
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Hu, T.; Chu, P. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.; Idris, M.; Kadir, M.; Farahany, S. Microstructure analysis and corrosion behavior of biodegradable Mg–Ca implant alloys. Mater. Des. 2011, 33, 88–97. [Google Scholar] [CrossRef]
- Li, H.; Peng, Q.; Li, X.; Li, K.; Han, Z.; Fang, D. Microstructures, mechanical and cytocompatibility of degradable Mg-Zn based orthopedic biomaterials. Mater. Des. 2014, 58, 43–51. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg-Zn alloys. Mater. Sci. Eng. A Struct. 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Li, J.; Cao, P.; Zhang, X.; Zhang, S.; He, Y. In vitro degradation and cell attachment of a PLGA coated biodegradable Mg–6Zn based alloy. J. Mater. Sci. 2010, 45, 6038–6045. [Google Scholar] [CrossRef]
- Luo, X.; Barbieri, D.; Davison, N.; Yan, Y.; Bruijn, J.; Yuan, H. Zinc in calcium phosphate mediates bone induction: In vitro and in vivo model. Acta Biomater. 2014, 10, 477–485. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Pellicer, E.; Fornell, J.; Blanquer, A.; Barrios, L.; Ibáñez, E.; Solsona, P.; Suriñach, S.; Baró, M.; Nogués, C. Improved mechanical performance and delayed corrosion phenomena in biodegradable Mg-Zn-Ca alloys through Pd-alloying. J. Mech. Behav. Biomed. 2012, 6, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Chen, L.; Zhao, J.; Li, S.; Dai, Y.; Huang, Q.; Yu, Z. In vitro corrosion behavior and in vivo biodegradation of biomedical β-Ca3(PO4)2/Mg-Zn composites. Acta Biomater. 2012, 8, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, C.; Wei, J.; Wen, Z. Study on the bonding strength between calcium phosphate/chitosan composite coatings and a Mg alloy substrate. Appl. Surf. Sci. 2012, 261, 276–286. [Google Scholar] [CrossRef]
- Chen, X.; Birbilis, N.; Abbott, T. A simple route towards a hydroxyapatite–Mg(OH) conversion coating for magnesium. Corros. Sci. 2011, 53, 2263–2268. [Google Scholar] [CrossRef]
- Mróz, W.; Bombalska, A.; Burdyńska, S.; Jedyński, M.; Prokopiuk, A.; Budner, B.; Ślósarczyk, A.; Zima, A.; Menaszek, E.; Ścisłowska-Czarnecka, A.; et al. Structural studies of magnesium doped hydroxyapatite coatings after osteoblast culture. J. Mol. Struct. 2010, 977, 145–152. [Google Scholar] [CrossRef]
- Ratna Sunil, B.; Sampath Kumar, T.; Chakkingal, U.; Nandakumar, V.; Doble, M. Friction stir processing of magnesium-nanohydroxyapatite composites with controlled in vitro degradation behavior. Mat. Sci. Eng. C Mater. 2014, 39, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Campo, R.; Savoini, B.; Muñoz, A.; Monge, M.; Garcés, G. Mechanical properties and corrosion behavior of Mg–HAP composites. J. Mech. Behav. Biomed. 2014, 39, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Contuzzi, N.; Campanelli, S.; Ludovico, A. 3D Finite Element Analysis in the selective laser melting process. Int. J. Simul. Mod. 2011, 10, 113–121. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Liu, J.; Xie, Z.; Wang, Z. Selective laser melting W–10 wt.% Cu composite powders. Int. J. Adv. Manuf. Tech. 2010, 48, 597–605. [Google Scholar] [CrossRef]
- Mróz, W.; Budner, B.; Syroka, R.; Niedzielski, K.; Golański, G.; Slósarczyk, A.; Schwarze, D.; Douglas, T. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser depostion. J. Biomed. Mater. Res. B 2015, 103, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, S.; Han, C.; Li, W.; Cheng, L.; Hao, L.; Shi, Y. Selective laser melting of stainless-steel/nano-hydroxyapatite composites for medical applications: Microstructure, element distribution, crack and mechanical properties. J. Mater. Process. Technol. 2015, 222, 444–453. [Google Scholar] [CrossRef]
- Hao, L.; Dadbakhsh, S.; Seaman, O.; Felstead, M. Selective laser melting of a stainless steel and hydroxyapatite composite for load-bearing implant development. J. Mater. Process. Technol. 2009, 17, 5793–5801. [Google Scholar] [CrossRef]
- Ng, C.; Savalani, M.; Man, H. Fabrication of magnesium using selective laser melting technique. Rapid Prototyp. J. 2011, 17, 479–490. [Google Scholar]
- Ng, C.; Savalani, M.; Lau, M.; Man, H. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Pawlak, A.; Rosienkiewicz, M.; Chlebus, E. Design of experiments approach in AZ31 powder selective laser melting process optimization. Arch. Civ. Mech. Eng. 2017, 17, 9–18. [Google Scholar] [CrossRef]
- Vandenbroucke, B.; Kruth, J. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Schubert, T.; Trindade, B.; Weißgärber, T.; Kieback, B. Interfacial design of Cu-based composites prepared by powder metallurgy for heat sink applications. Mater. Sci. Eng. A 2008, 475, 39–44. [Google Scholar] [CrossRef]
- Dini, G.; Najafizadeh, A.; Ueji, R.; Monir-Vaghefi, S. Tensile deformation behavior of high manganese austenitic steel: The role of grain size. Mater. Des. 2010, 31, 3395–3402. [Google Scholar] [CrossRef]
- Fadli, A.; Sopyan, I.; Singh, R. Porous Alumina from Protein Foaming-Consolidation Method Containing Hydrothermal Derived Hydroxyapatite Powder. Appl. Mech. Mater. 2012, 117, 782–785. [Google Scholar] [CrossRef]
- Laurencin, D.; Almora-Barrios, N.; Leeuw, N.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.; Newport, R.; Wong, A. Magnesium incorporation into hydroxyapatite. Biomaterial 2011, 32, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Maeng, D.; Kim, T.; Lee, J.; Hong, S.; Seo, S.; Chun, B. Microstructure and strength of rapidly solidified and extruded Mg-Zn alloys. Scripta Mater. 2000, 43, 385–389. [Google Scholar] [CrossRef]
- Kim, H.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterial 2005, 26, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Bian, H.; Zhou, Y.; Gao, C.; Shuai, C. System development, formability quality and microstructure evolution of selective laser-melted magnesium. Virtual Phys. Prototyp. 2016, 11, 173–181. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Ren, W.; Zhang, S.; Liu, F. Microstructure evolution and corrosion mechanism of dicalcium phosphate dihydrate coating on magnesium alloy in simulated body fluid. Mater. Chem. Phys. 2010, 119, 294–298. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Zhou, Y.; Yang, Y.; Feng, P.; Liu, L.; He, C.; Zhao, M.; Yang, S.; Gao, C.; Wu, P. Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting. Materials 2017, 10, 307. https://doi.org/10.3390/ma10030307
Shuai C, Zhou Y, Yang Y, Feng P, Liu L, He C, Zhao M, Yang S, Gao C, Wu P. Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting. Materials. 2017; 10(3):307. https://doi.org/10.3390/ma10030307
Chicago/Turabian StyleShuai, Cijun, Yuanzhuo Zhou, Youwen Yang, Pei Feng, Long Liu, Chongxian He, Mingchun Zhao, Sheng Yang, Chengde Gao, and Ping Wu. 2017. "Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting" Materials 10, no. 3: 307. https://doi.org/10.3390/ma10030307
APA StyleShuai, C., Zhou, Y., Yang, Y., Feng, P., Liu, L., He, C., Zhao, M., Yang, S., Gao, C., & Wu, P. (2017). Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting. Materials, 10(3), 307. https://doi.org/10.3390/ma10030307








