Separation of Lead with a Novel Ion Separating Agent Prepared by Clothing a Chitin Whisker on a Potassium Tetratitanate Whisker
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of ChW-PTW
2.2.1. Preparation of ChW
2.2.2. Preparation of ChW-PTW
2.3. Characterization
2.4. Difference of Adsorption Property between ChW and PTW
2.4.1. Adsorption Isotherm
2.4.2. Adsorption Kinetics
2.5. Separation of Double-Ions-Mixed Solution
2.5.1. Ionic Adsorption
2.5.2. Determination of Separation Capability
3. Results and Discussions
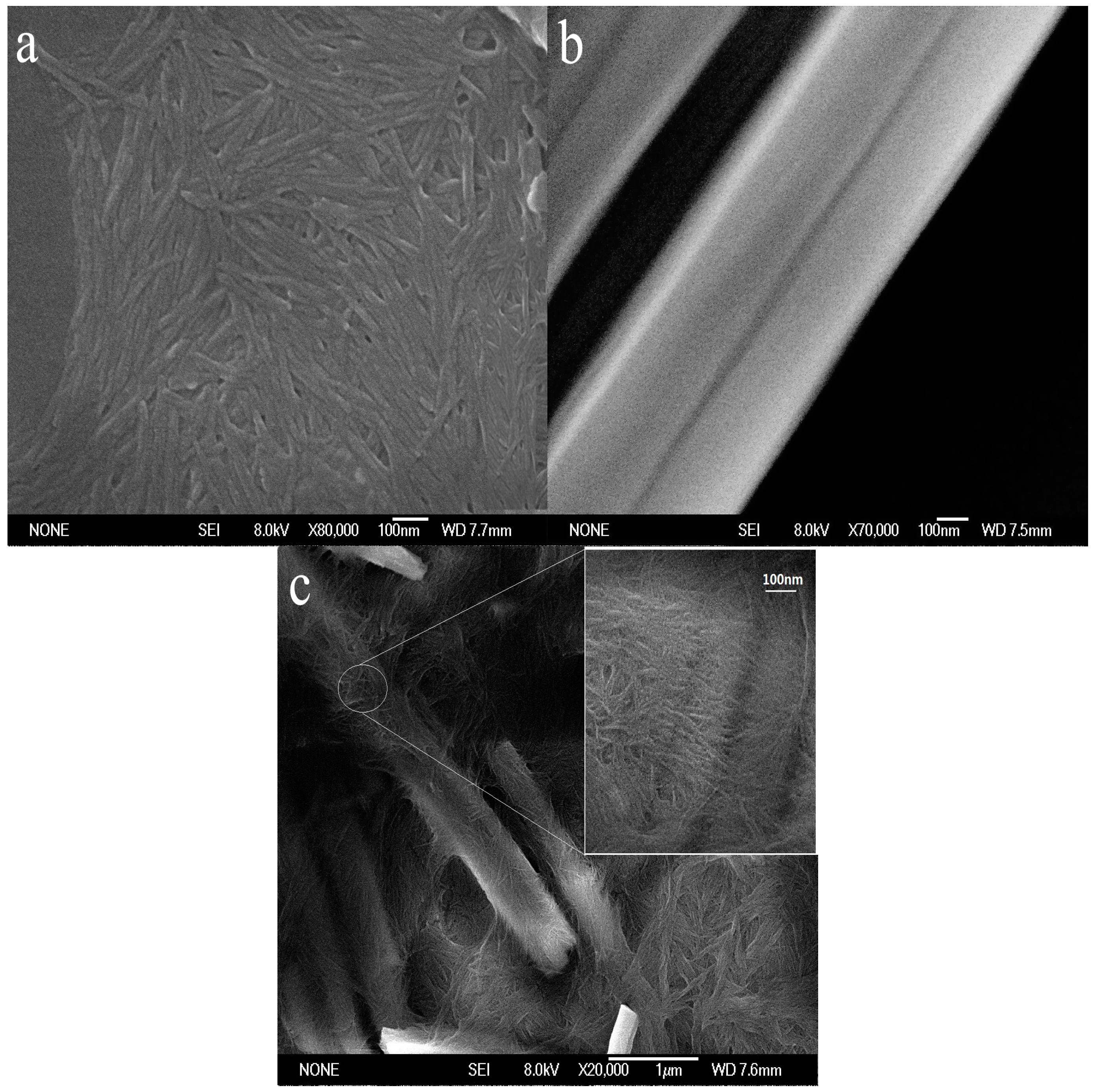
3.1. Morphological and Structural Characteristics
3.2. The Adsorption Property of ChW and PTW
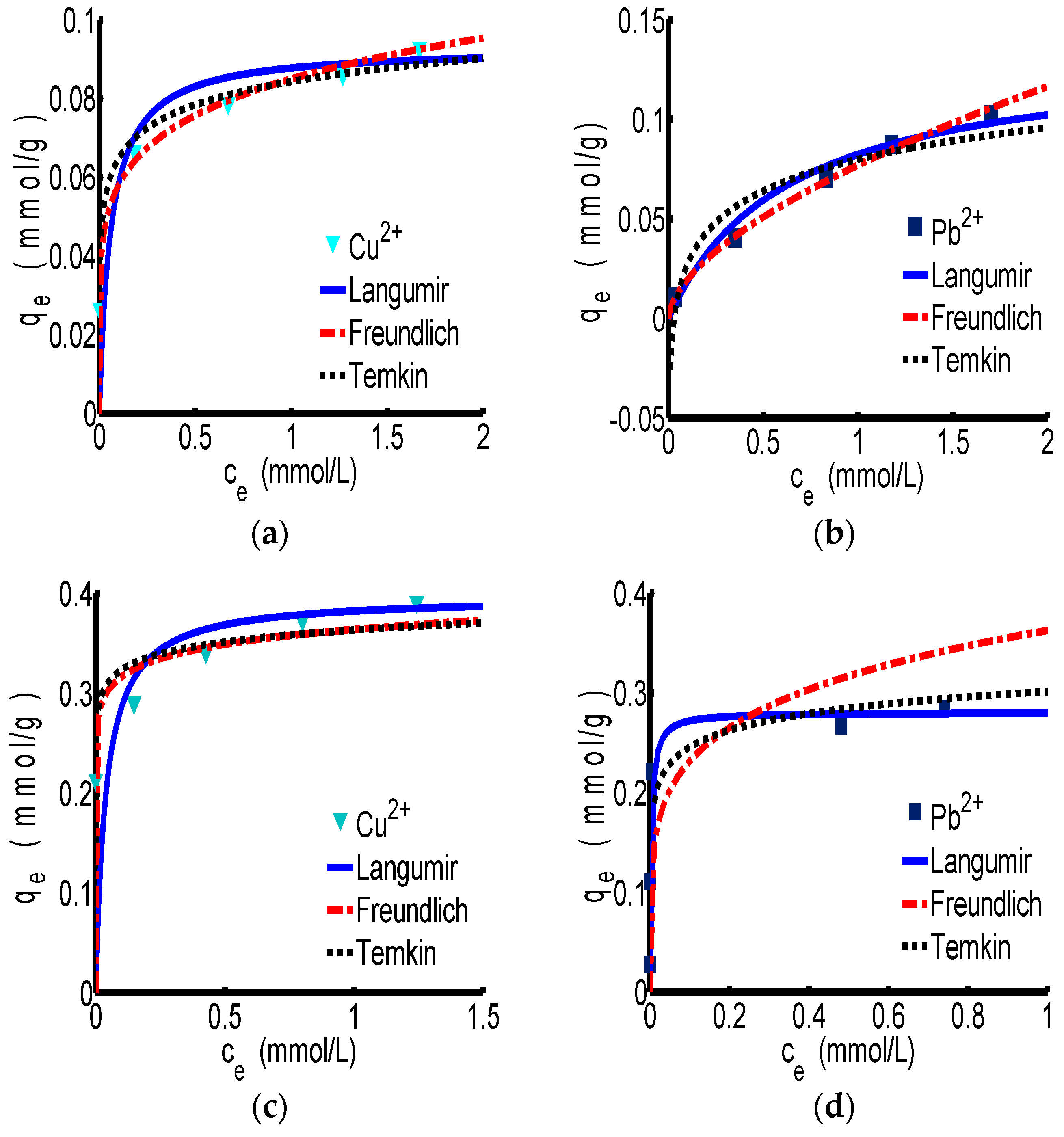
3.2.1. Adsorption Isotherm
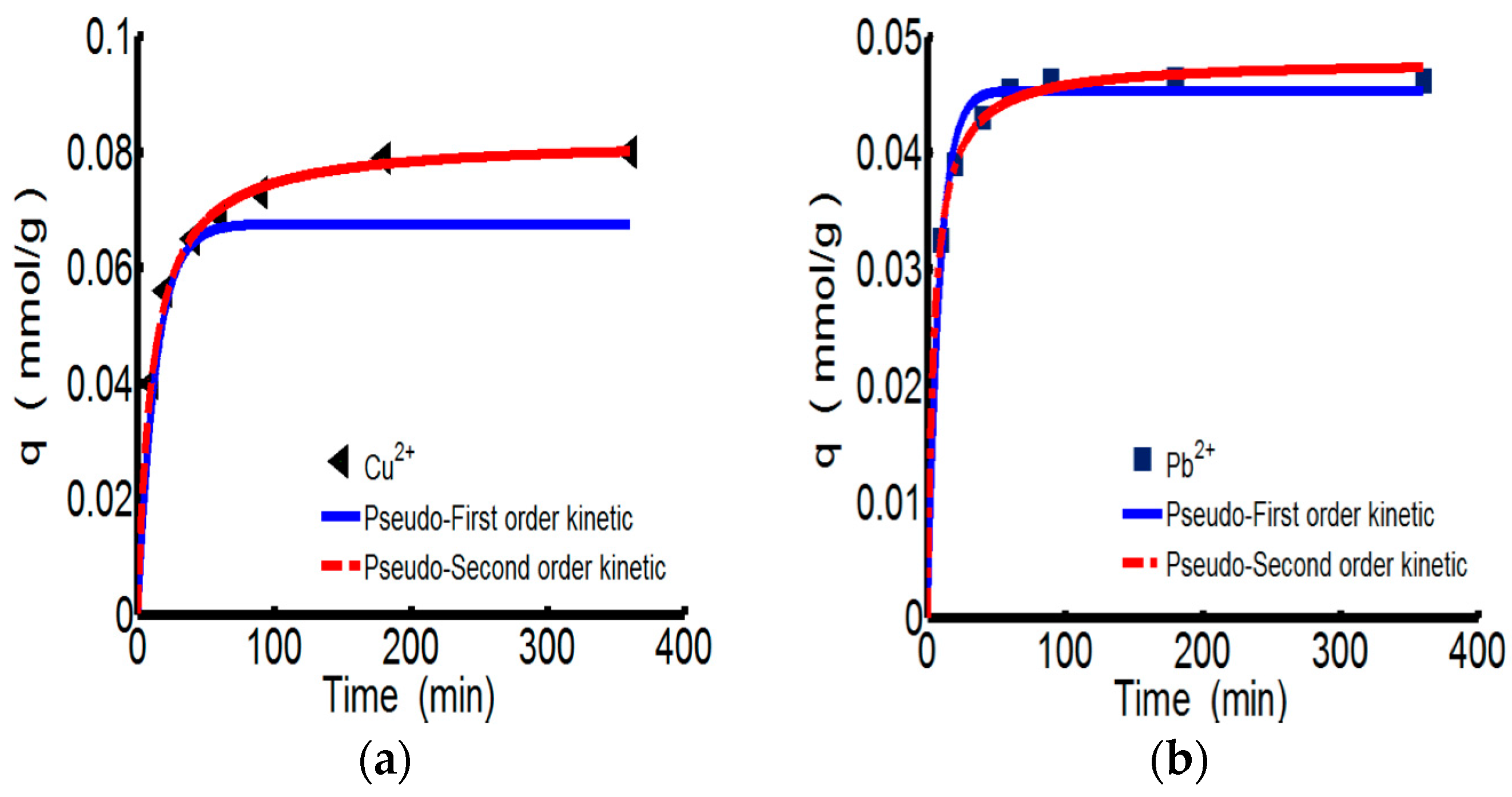
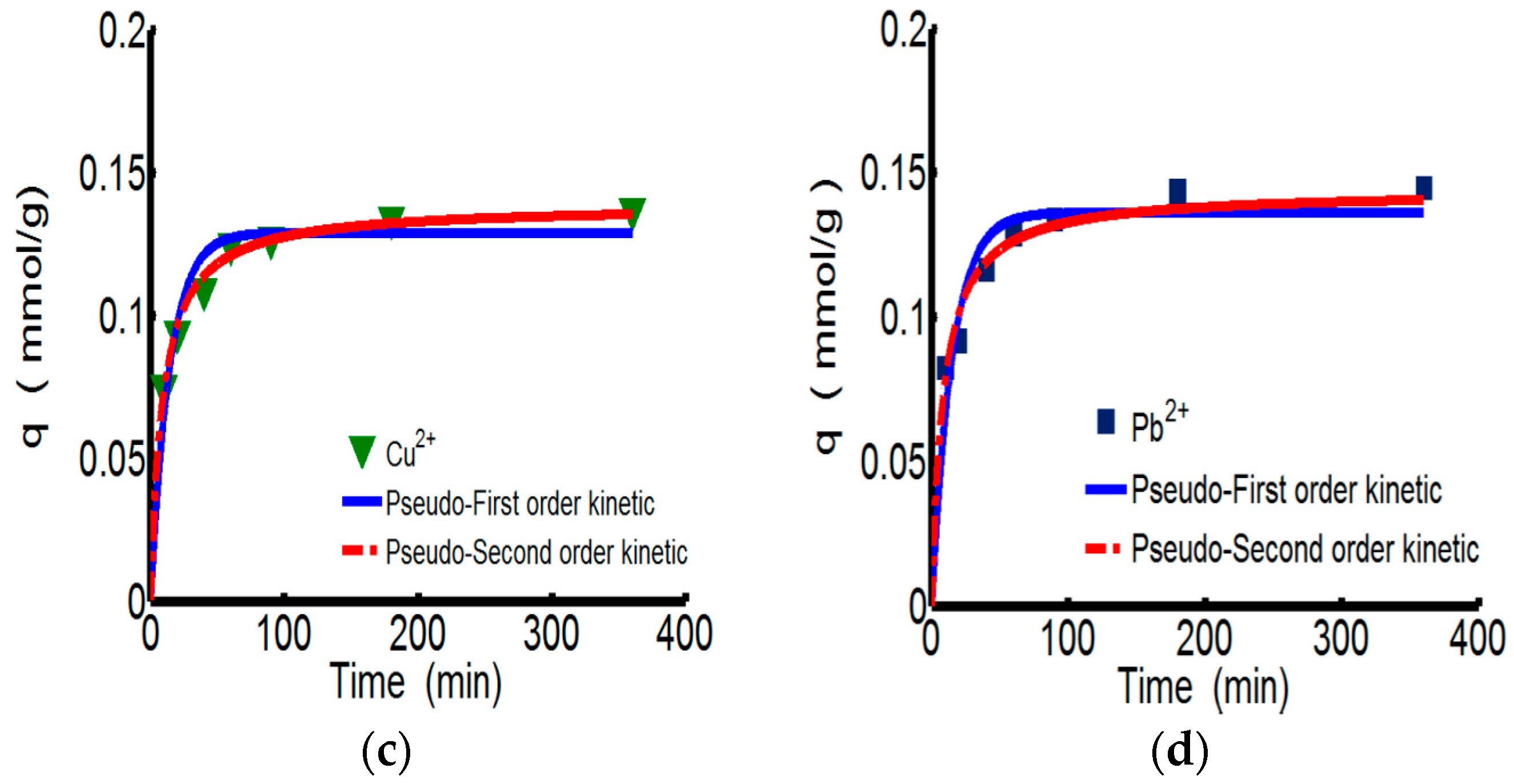
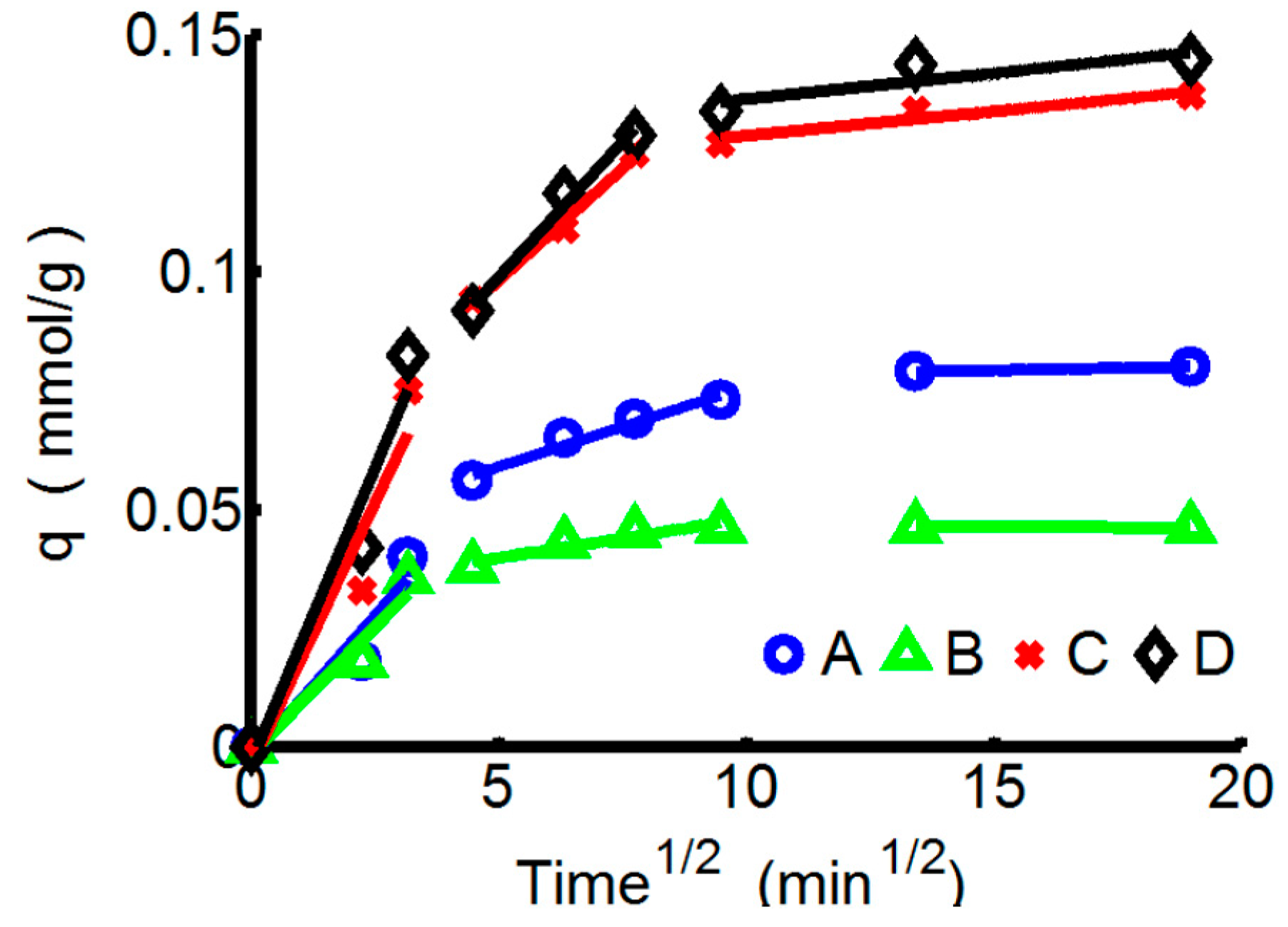
3.2.2. Adsorption Kinetics
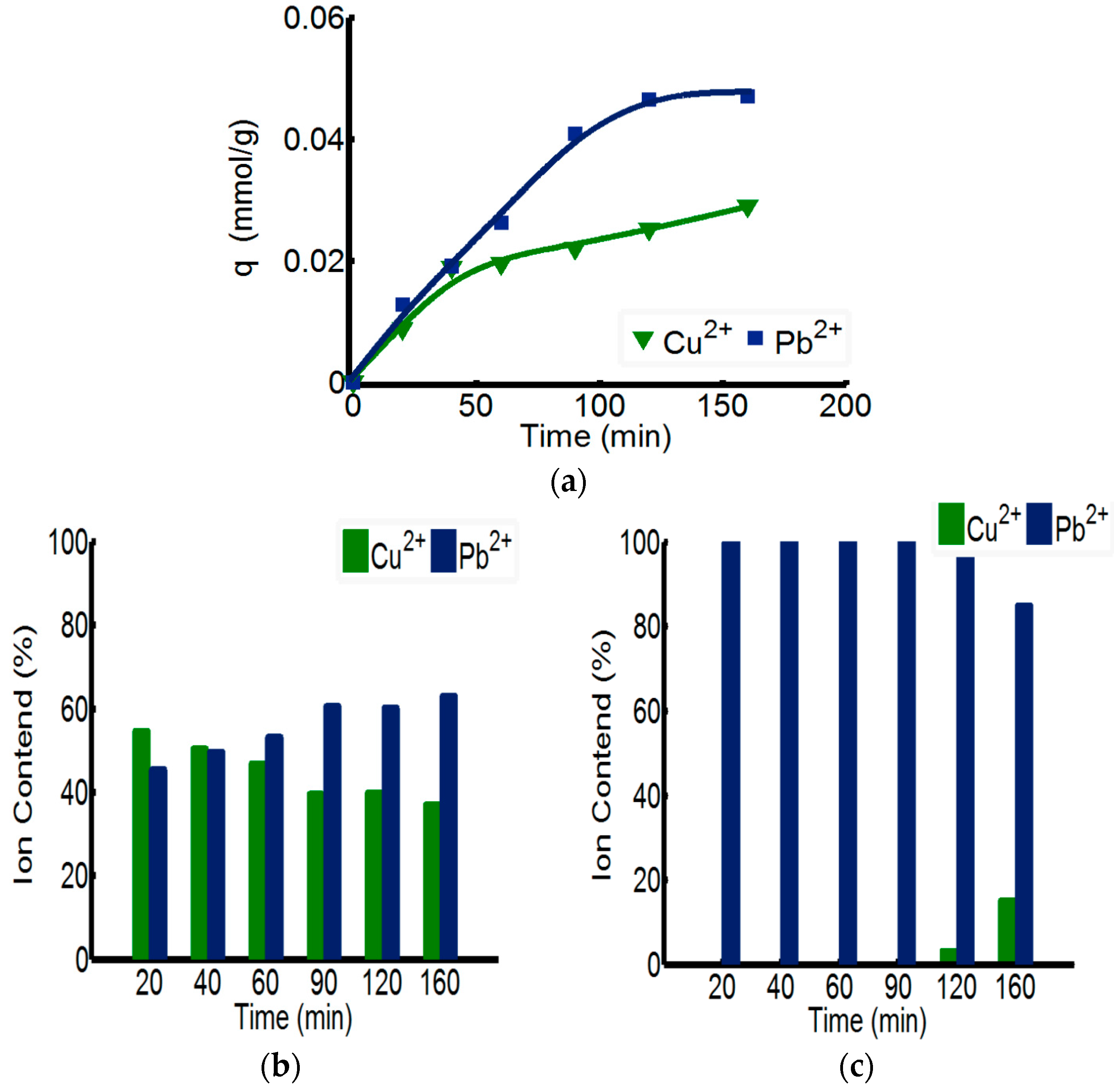
3.3. The Separation Effect of Double-Ion-Mixed Solution
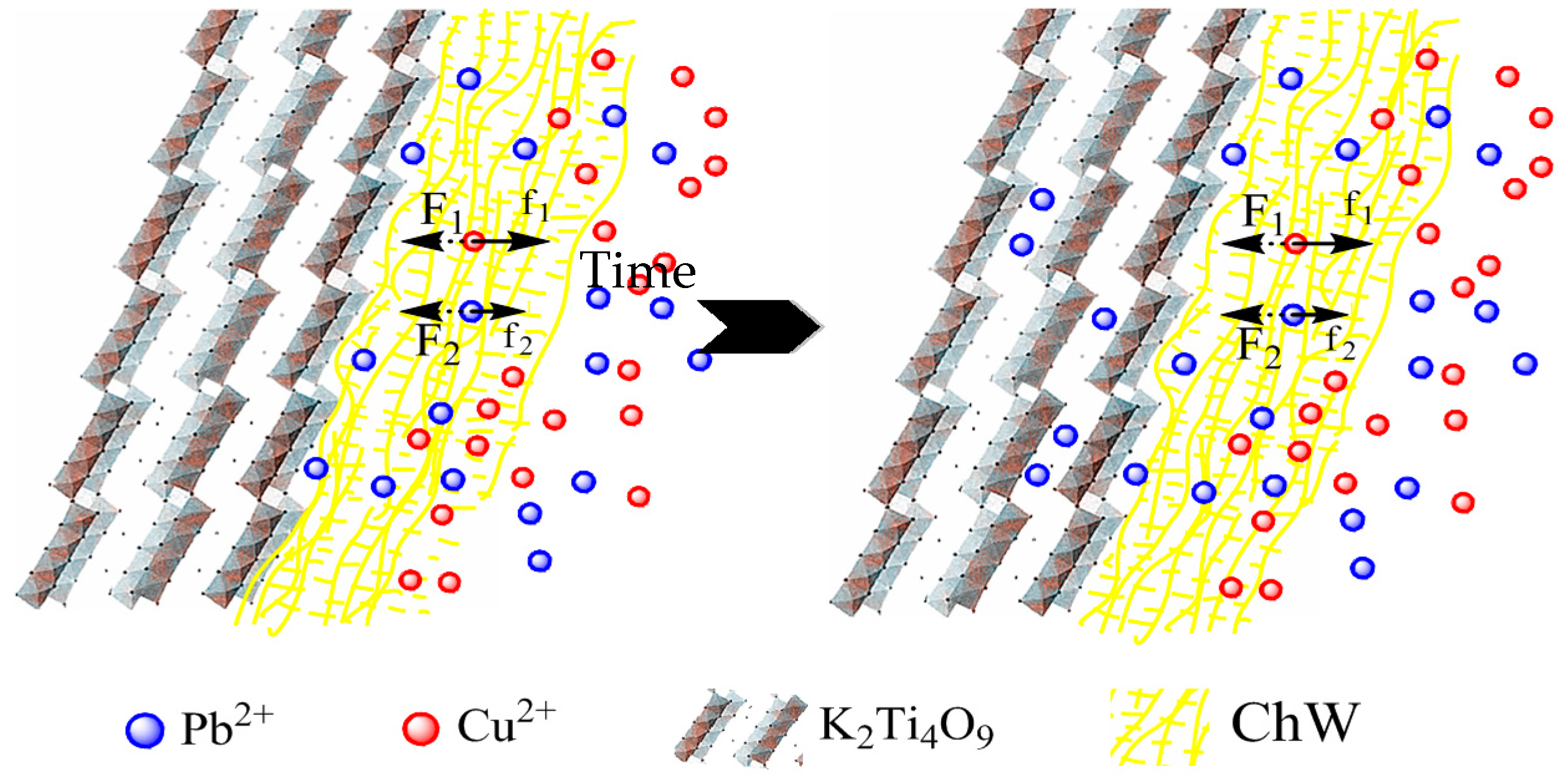
3.4. The Possible Separation Mechanism of ChW-PTW
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, R.; Tanaka, H.; Kawamoto, T.; Wang, J.; Zhang, Y. Battery-type column for caesium ions separation using electroactive film of copper hexacyanoferrate nanoparticles. Sep. Purif. Technol. 2017, 173, 44–48. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.G.; Li, M.Y.A. Synthesis and characterization of a surface-grafted Cd(II) ion-imprinted polymer for selective separation of Cd(II) ion from aqueous solution. Appl. Surf. Sci. 2015, 332, 463–472. [Google Scholar] [CrossRef]
- Zaremba, T.; Hadryś, A. Synthesis of K2Ti4O9 whiskers. J. Mater. Sci. 2004, 39, 4561–4568. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Y.; Gong, H. Investigation on K2Ti4O9 Whisker Absorbent and Applications in Heavy Metal Ions Removal. J. Water Environ. Technol. 2007, 5, 13–18. [Google Scholar] [CrossRef]
- Wang, S.M.; Liu, L.K.; Xu, W.Z. Study on the Adsorption Behavior of Chrome and Manganese on Potassium Tetratitanate Whisker. Adv. Mater. Res. 2012, 534, 126–130. [Google Scholar] [CrossRef]
- Xu, W.Z.; Yan, Y.S.; Yang, M.H.; Jing, J.J. Study on the adsorption behavior of nickel on potassium tetratitanate whisker by flame atomic absorption spectrometry. Spectrosc. Spect. Anal. 2009, 29, 1698–1701. [Google Scholar]
- Zhang, X.; Li, C.; Yan, Y.; Pan, J.; Xu, P.; Zhao, X. A Ce3+-imprinted functionalized potassium tetratitanate whisker sorbent prepared by surface molecularly imprinting technique for selective separation and determination of Ce3+. Microchim. Acta 2010, 169, 289–296. [Google Scholar] [CrossRef]
- Xu, W.Z.; Zhou, W.; Bian, L.H.; Huang, W.H.; Wu, X.Y. Preparation of molecularly imprinted polymer by surface imprinting technique and its performance for adsorption of dibenzothiophene. J. Sep. Sci. 2011, 34, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Takizawa, Y. Intercalation of Alkylammonium Cations into a Layered Titanate in the Presence of Macrocyclic Compounds. Chem. Mater. 1999, 11, 30–32. [Google Scholar] [CrossRef]
- Ogawa, M.; Takizawa, Y. One Pot Synthesis of Layered Tetratitanate-Organic Intercalation Compounds with the Aid of Macrocyclic Compounds. Mol. Cryst. Liq. Cryst. 2000, 341, 357–362. [Google Scholar] [CrossRef]
- Izawa, H.; Kikkawa, S.; Koizumi, M. Effect of intercalated alkylammonium on cation exchange properties of H2Ti3O7. J. Solid State Chem. 1987, 69, 336–342. [Google Scholar] [CrossRef]
- Airoldi, C.; Nunes, L.M.; Farias, R.F.D. The intercalation of n-alkyldiamines into crystalline layered titanate. Mater. Res. Bull. 2000, 35, 2081–2090. [Google Scholar] [CrossRef]
- Klapiszewski, A.; Wysokowski, M.; Majchrzak, I.; Szatkowski, T. Preparation and Characterization of Multifunctional Chitin/Lignin Materials. J. Nanomater. 2013, 20, 12–25. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin Adsorbents for Toxic Metals: A Review. Int. J. Mol. Sci. 2017, 18, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Klapiszewski, Ł.; Moszyński, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwińska-Stefańska, K.; BazhenovVasilii, V.; Jesionowski, T. Modification of Chitin with Kraft Lignin and Development of New Biosorbents for Removal of Cadmium(II) and Nickel(II) Ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater-A short review. Adv. Coll. Interfac. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.R.B.; Jorge, M.; Gomes, P. Interaction of chitosan and chitin with Ni, Cu and Zn ions: A computational study. J. Chem. Thermodyn. 2014, 73, 121–129. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromolecules 2011, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Weng, L.; Zhang, L. Morphology and Properties of Soy Protein Isolate Thermoplastics Reinforced with Chitin Whiskers. Biomacromolecules 2004, 5, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Q.G.; Zhang, C.P. The preparation of Chitin Whisker by two-steps of acid-alkali. In Proceedings of the 2016 International Workshop on Material Science and Environmental Engineering, Wuhan, China, 24–26 January 2016.
- Wu, T.; Wang, G.; Gao, C.; Chen, Z.; Feng, L.; Wang, P. Phosphoric acid-based preparing of chitin nanofibers and nanospheres. Cellulose 2016, 23, 477–491. [Google Scholar] [CrossRef]
- And, K.G.N.; Dufresne, A. Crab Shell Chitin Whisker Reinforced Natural Rubber Nanocomposites. 2. Mechanical Behavior. Biomacromolecules 2003, 4, 666–674. [Google Scholar]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as alpha-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Karaarslan, M. A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Nat. Prod. Res. 2015, 29, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, A.; Kahu, S.S.; Saravanan, D.; Jugade, R.M. Assimilation of chitin with tin for defluoridation of water. Rsc. Adv. 2016, 6, 18936–18945. [Google Scholar] [CrossRef]
- Nair, K.G.; Dufresne, A.; Gandini, A.; Belgacem, M.N. Crab Shell Chitin Whiskers Reinforced Natural Rubber Nanocomposites. 3. Effect of Chemical Modification of Chitin Whiskers. Biomacromolecules 2003, 4, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Pius, A.; Thomas, S. Enhanced adsorption of crystal violet by synthesized and characterized chitin nano whiskers from shrimp shell. J. Water Proc. Eng. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Ramos, M.L.P.; González, J.A.; Albornoz, S.G.; Pérez, C.J.; Villanueva, M.E.; Giorgieri, S.A.; Copello, G.J. Chitin hydrogel reinforced with TiO2 nanoparticles as an arsenic sorbent. Chem. Eng. J. 2016, 285, 581–587. [Google Scholar] [CrossRef]
- Huang, G.; Wanga, D.; Mab, S.; Chen, J.; Jiang, L.; Wang, P. A new, low-cost adsorbent: Preparation, characterization, and adsorption behavior of Pb(II) and Cu(II). J. Coll. Interfac. 2015, 445, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, P.; Norman, M.; Klapiszewski, Ł.; Karwanska, N.; Kawalec, M.; Baczynska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2015, 7, 18–31. [Google Scholar] [CrossRef]
- Ciesielczyk, F.; Bartczak, P.; Klapiszewski, Ł.; Poznan, T.J. Treatment of model and galvanic waste solutions of copper(II) ionsusing a lignin/inorganic oxide hybrid as an effective sorbent. J. Hazard. Mater. 2017, 328, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Szlachta, M.; Chubar, N. The application of Fe-Mn hydrous oxides based adsorbent for removing selenium species from water. Chem. Eng. J. 2013, 217, 159–168. [Google Scholar] [CrossRef]
| Samples | Adsorption Capacities mmol/g | Reference |
|---|---|---|
| ChW-Cu | 0.080 | - |
| ChW-Pb | 0.075 | - |
| PTW-Cu | 0.362 | - |
| PTW-Pb | 0.296 | - |
| Mg2Al–LS–LDH composite-Cu | 1.000 | [33] |
| Mg2Al–LS–LDH composite-Pb | 0.594 | [33] |
| Peat-Pb | 0.398 | [34] |
| Lignin/inorganic oxide system-Cu | 1.312 | [35] |
| Symbol | Description | Unit |
|---|---|---|
| kL | The adsorption affinity | L·mmol−1 |
| kF | Characterization of adsorption capacity | g−1 |
| At | The maximum adsorption equilibrium constant | L·mmol−1 |
| qe | Adsorption capacity | mmol·g−1 |
| k1 | Reaction rate constant for pseudo-first-order kinetic | min−1 |
| k2 | Reaction rate constant for pseudo-second-order kinetic | g·min·mmol−1 |
| R2 | Correlation coefficient transformation | - |
| E2 | The estimate of the error variance | - |
| Isotherm Type | Parameter | ChW | PTW | ||
|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Cu2+ | Pb2+ | ||
| Langmuir | kL | 16.725 | 1.574 | 25.851 | 310.59 |
| R2 | 0.994 | 0.943 | 0.997 | 0.999 | |
| E2 | 4.65 × 10−4 | 1.93 | 6.90 × 10−3 | 3.00 × 10−3 | |
| Freundlich | kF | 0.085 | 0.077 | 0.364 | 0.363 |
| n | 6.011 | 1.676 | 16.484 | 5.141 | |
| R2 | 0.998 | 0.999 | 0.926 | 0.744 | |
| E2 | 2.00 × 10−4 | 2.00 × 10−4 | 1.10 × 10−3 | 0.61 | |
| Temkin | BT | 2.91 × 105 | 1.08 × 105 | 1.43 × 105 | 1.03 × 105 |
| R2 | 0.988 | 0.92 | 0.878 | 0.907 | |
| E2 | 1.00 × 10−4 | 1.00 × 10−4 | 8.00 × 10−4 | 1.50 × 10−3 | |
| At | 1.99 × 104 | 32.73 | 1.0 × 109 | 2.64 × 105 | |
| Kinetics Type | Parameter | ChW | PTW | |||
|---|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Cu2+ | Pb2+ | |||
| qe, exp | 0.080 | 0.046 | 0.137 | 0.145 | ||
| Pseudo-first-order | qe, cal | 0.068 | 0.1016 | 0.068 | 0.067 | |
| k1 | 0.075 | 0.046 | 0.075 | 0.136 | ||
| R2 | 0.878 | 0.927 | 0.878 | 0.877 | ||
| Pseudo-second-order | Type 1 | qe, cal | 0.083 | 0.047 | 0.141 | 0.149 |
| k2 | 1.152 | 6.518 | 0.750 | 0.772 | ||
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| h | 0.008 | 0.014 | 0.015 | 0.015 | ||
| Type 2 | qe, cal | 0.083 | 0.049 | 0.139 | 0.144 | |
| k2 | 1.162 | 3.553 | 0.825 | 0.846 | ||
| R2 | 0.995 | 0.988 | 0.988 | 0.934 | ||
| h | 0.008 | 0.008 | 0.016 | 0.018 | ||
| Type 3 | qe, cal | 10.500 | 5.878 | 8.873 | 8.957 | |
| k2 | 1.153 | 3.495 | 0.810 | 0.763 | ||
| R2 | 0.995 | 0.988 | 0.988 | 0.934 | ||
| h | 127.127 | 120.729 | 63.778 | 61.195 | ||
| Type 4 | qe, cal | −0.028 | −0.039 | −0.036 | 3.602 | |
| k2 | 5.351 | 7.569 | 3.740 | 3.602 | ||
| R2 | 0.982 | 0.979 | 0.954 | 0.890 | ||
| E2 | 0.004 | 0.011 | 0.015 | 0.005 | ||
| Type of Kinetics Pseudo-Second-Order | Linear Form | Plots |
|---|---|---|
| Type 1 | ||
| Type 2 | ||
| Type 3 | ||
| Type 4 |
| Samples | kind1 | C1 | R12 | kind2 | C1 | R22 | kind3 | C3 | R32 |
|---|---|---|---|---|---|---|---|---|---|
| ChW-Cu2+ | 0.012 | −0.002 | 0.9169 | 0.003 | 0.042 | 0.9643 | 0 | 0.077 | 1 |
| ChW-Pb2+ | 0.011 | −0.001 | 0.9495 | 0.002 | 0.032 | 0.9013 | 0 | 0.047 | 1 |
| PTW-Cu2+ | 0.022 | −0.004 | 0.9104 | 0.009 | 0.051 | 0.9911 | 0.001 | 0.118 | 0.8976 |
| PTW-Pb2+ | 0.025 | −0.003 | 0.9509 | 0.011 | 0.042 | 0.9863 | 0.001 | 0.126 | 0.7363 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, Q.-g.; Xue, W.-j. Separation of Lead with a Novel Ion Separating Agent Prepared by Clothing a Chitin Whisker on a Potassium Tetratitanate Whisker. Materials 2017, 10, 262. https://doi.org/10.3390/ma10030262
Liu J, Li Q-g, Xue W-j. Separation of Lead with a Novel Ion Separating Agent Prepared by Clothing a Chitin Whisker on a Potassium Tetratitanate Whisker. Materials. 2017; 10(3):262. https://doi.org/10.3390/ma10030262
Chicago/Turabian StyleLiu, Juan, Qin-guo Li, and Wen-jing Xue. 2017. "Separation of Lead with a Novel Ion Separating Agent Prepared by Clothing a Chitin Whisker on a Potassium Tetratitanate Whisker" Materials 10, no. 3: 262. https://doi.org/10.3390/ma10030262
APA StyleLiu, J., Li, Q.-g., & Xue, W.-j. (2017). Separation of Lead with a Novel Ion Separating Agent Prepared by Clothing a Chitin Whisker on a Potassium Tetratitanate Whisker. Materials, 10(3), 262. https://doi.org/10.3390/ma10030262





