Preparation and Characterization of Extruded Composites Based on Polypropylene and Chitosan Compatibilized with Polypropylene-Graft-Maleic Anhydride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extruded Composites of Polypropylene and Chitosan
2.2. Extruded Composites of Polypropylene and Chitosan with Compatibilizer
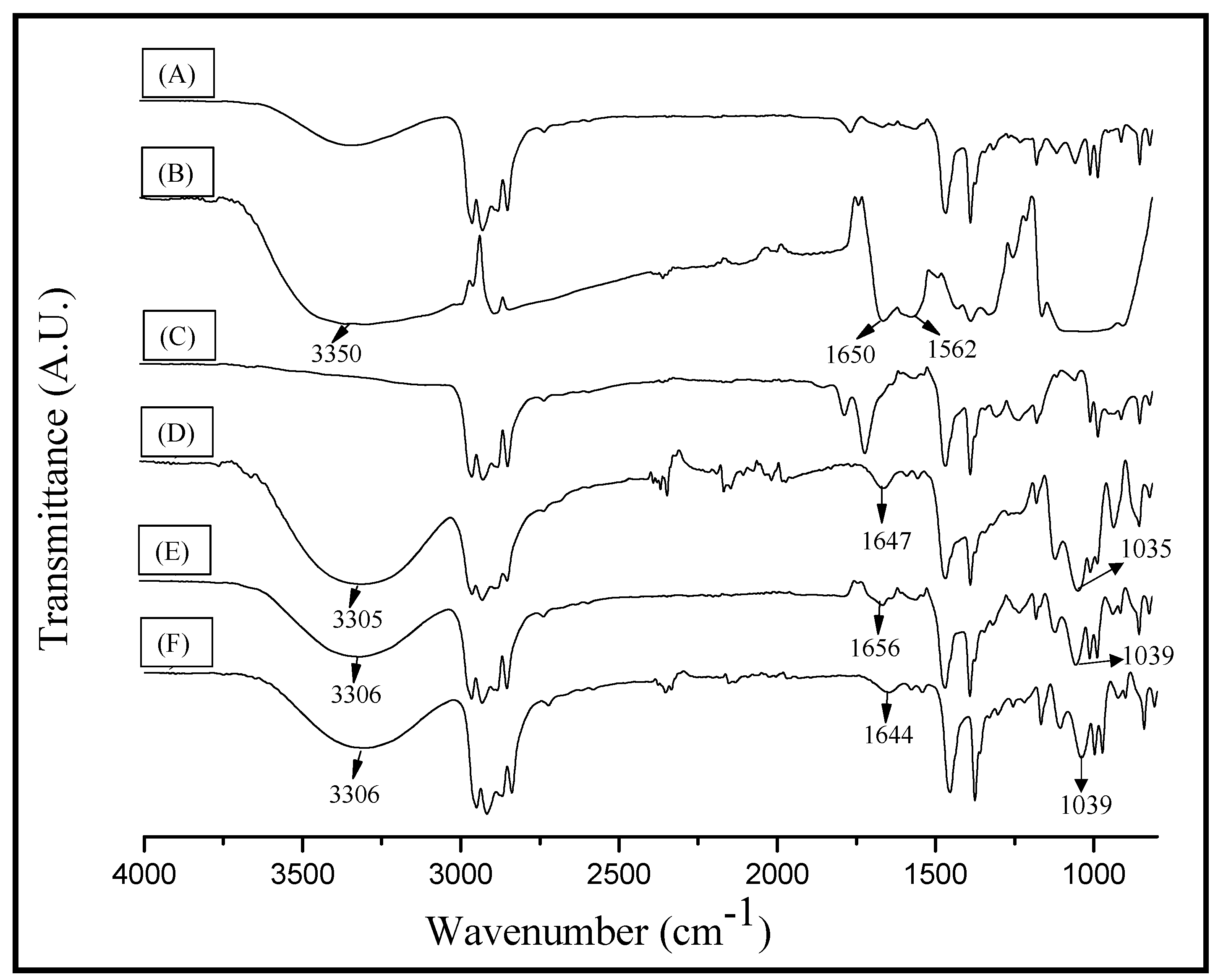
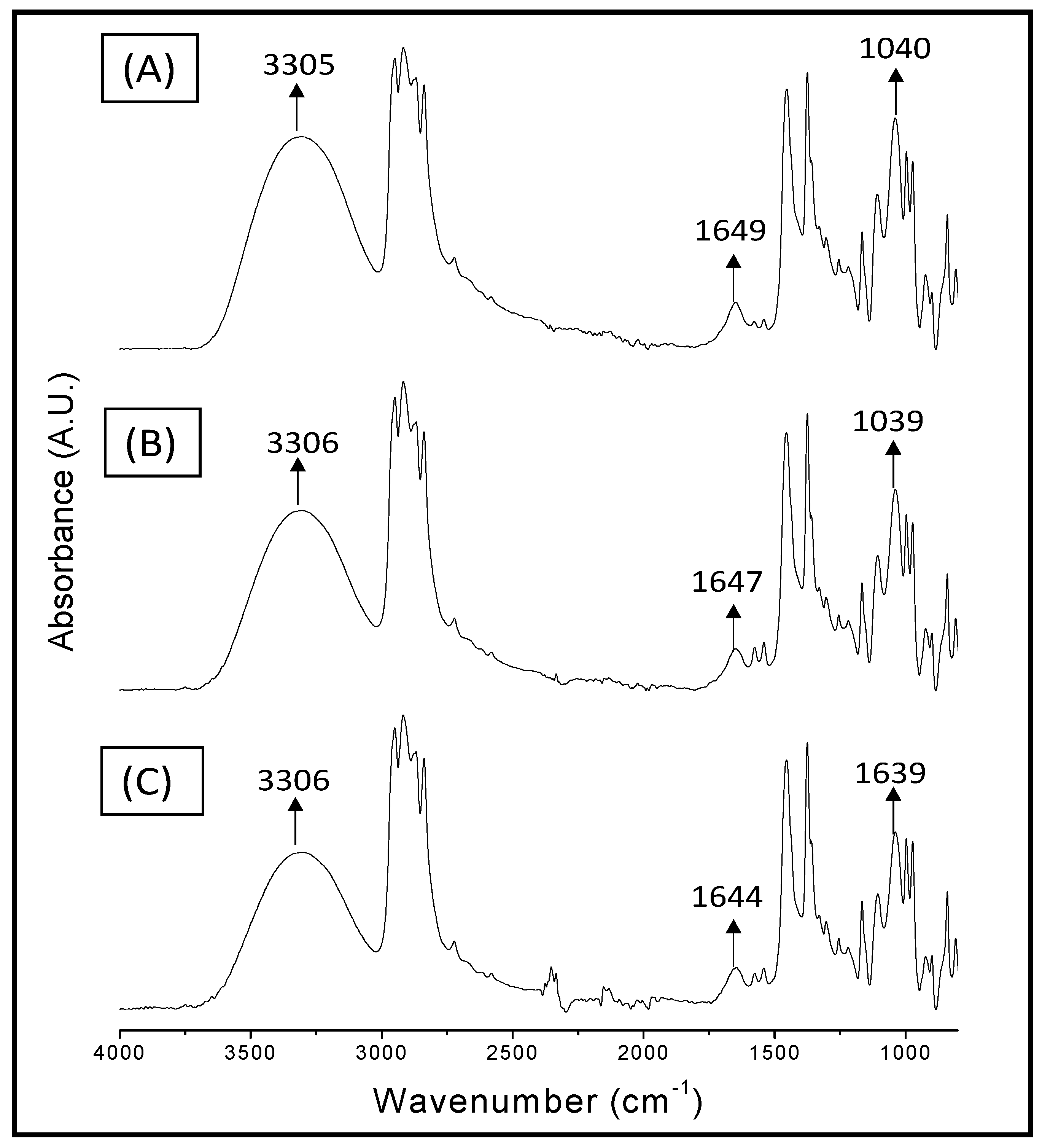
2.3. FT-IR Spectroscopy Analysis
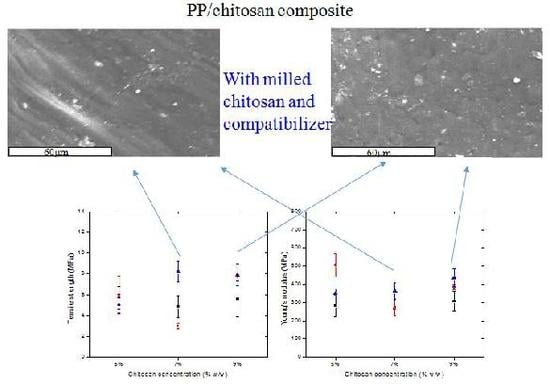
2.4. Scanning Electronic Microscopy
2.5. Contact Angle
2.6. Thermal Properties
2.7. Mechanical Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Composites
3.3. Characterization
3.3.1. FT-IR Spectroscopy
3.3.2. Scanning Electronic Microscopy
3.3.3. Contact Angle Determination
3.3.4. Thermal Analysis
3.3.5. Tensile Strength Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Maddah, H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Husseinsyah, S.; Amri, F.; Husin, K.; Ismail, H. Mechanical and thermal properties of chitosan-filled polypropylene composites: The effect of acrylic Acid. J. Vinyl. Addit. Technol. 2011, 17, 125–131. [Google Scholar] [CrossRef]
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.; Doble, M.; Bhaduri, S.; Veera Uppara, P. Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 2008, 7, 9–22. [Google Scholar]
- Quiroz-Castillo, J.M.; Rodríguez-Félix, D.E.; Grijalva-Monteverde, H.; Del Castillo-Castro, T.; Plascencia-Jatomea, M.; Rodríguez-Félix, F.; Herrera-Franco, P.J. Preparation of extruded polyethylene/chitosan blends compatibilized with polyethylene-graft-maleic anhydride. Carbohydr. Polym. 2014, 101, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Bonhommea, S.; Cuer, A.; Delort, A.M.; Lemaire, J.; Sancelme, M.; Scott, G. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Tanjung, F.A.; Husseinsyah, S.; Hussin, K.; Tahir, I. Chemically chitosan modified with methyl methacrylate and its effect on mechanical and thermal properties of polypropylene composites. Indones. J. Chem. 2013, 13, 114–121. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Robledo, D.; Azamar, J.A.; Ríos-Soberanis, C.R.; Freile-Pelegrín, Y. Preparation and Characterization of Low Density Polyethylene-Agar Biocomposites: Torque-Rheological, Mechanical, Thermal and Morphological Properties. Polym. Eng. Sci. 2010, 50, 585–591. [Google Scholar] [CrossRef]
- Cai, K.; Yao, K.; Cui, Y.; Lin, S.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Luo, J. Surface modification of poly (d,l-lactic acid) with chitosan and its effects on the culture of osteoblasts in vitro. J. Biomed. Mater. Res. 2002, 60, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prababaran, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan-present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Yazdani-Pedram, M.; Lagos, A.; Jaime Retuert, P. Study of the effect of reaction variables on grafting of polyacrylamide onto chitosan. Polym. Bull. 2002, 48, 93–98. [Google Scholar] [CrossRef]
- Quiroz-Castillo, J.M.; Rodríguez-Félix, D.E.; Grijalva-Monteverde, H.; Lizárraga-Laborín, L.L.; Castillo-Ortega, M.M.; Del Castillo-Castro, T.; Rodríguez-Félix, F.; Herrera-Franco, P.J. Preparation and characterization of films extruded of polyethylene/chitosan modified with poly(lactic acid). Materials 2015, 6, 137–148. [Google Scholar] [CrossRef]
- Del Castillo-Castro, T.; Castillo-Ortega, M.M.; Herrera-Franco, P.J.; Rodríguez-Félix, D.E. Compatibilization of polyethylene/polyaniline blends with polyethylene-graft-maleic anhydride. J. Appl. Polym. Sci. 2011, 119, 2895–2901. [Google Scholar] [CrossRef]
- Zelenetskii, A.N.; Volkov, V.P.; Artemeva, N.Y.; Sizova, M.D.; Nikolskaya, V.P.; Egorova, N.A. Functionalization of polyolefins by reactive processing. Cent. Eur. J. Chem. 2003, 1, 277–290. [Google Scholar] [CrossRef]
- Akopova, T.A.; Vladimirov, L.V.; Zhorin, V.A.; Zelenetskii, A.N. Solid-state synthesis of amphiphilic chitosan-polyethylene systems by the maleinization of both components. Polym. Sci. Ser. B 2009, 51, 124–134. [Google Scholar] [CrossRef]
- Rodríguez-Félix, D.E.; Quiroz-Castillo, J.M.; Grijalva-Monteverde, H.; Del Castillo-Castro, T.; Burruel-Ibarra, S.E.; Rodríguez-Félix, F.; Madera-Santana, T.; Cabanillas, R.E.; Herrera-Franco, P.J. Degradability of Extruded Polyethylene/Chitosan Blends Compatibilized with Polyethylene-Graft-Maleic Anhydride Under Natural Weathering. J. Appl. Polym. Sci. 2014, 131, 41045. [Google Scholar] [CrossRef]
- Lambertus, A.M.; Van den, B.; Rutger, J.I.K.; Frans, H.J.K.; Carmen, G.B. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Tanjung, F.A.; Husseinsyah, S.; Hussin, K. Chitosan-filled Polypropylene Composites: The Effect of Filler Loading and Organosolv Lignin on Mechanical, Morphological and Thermal Properties. Fibers Polym. 2014, 15, 800–808. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Mano, J.F.; Neves, N.M.; Reis, R.L. Properties of melt processed chitosan and aliphatic polyester blends. Mater. Sci. Eng. A 2005, 403, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Heuzey, M.C.; Ajji, A.; Sarazin, P. Plasticized chitosan/polyolefin films produced by extrusion. Carbohydr. Polym. 2015, 117, 177–184. [Google Scholar] [CrossRef]
- Lin, J.-H.; Pan, Y.-J.; Liu, C.-F.; Huang, C.-L.; Hsieh, C.-T.; Chen, C.-K.; Lin, Z.-I.; Lou, C.-W. Preparation and compatibility evaluation of polypropylene/High density polyethylene polyblends. Materials 2015, 8, 8850–8859. [Google Scholar] [CrossRef]
- Le-Tien, C.; Lacroix, M.; Ispas-Szabo, P.; Mateescu, M.A. N-acylated chitosan: Hydrophobic matrices for controlled drug release. J. Control. Release 2003, 93, 1–13. [Google Scholar] [CrossRef]
- Schönherr, H.; Hruska, Z.; Vancso, G.J. Surface characterization of oxyfluorinated isotactic polypropylene films: Scanning force microscopy with chemically modified probes and contact angle measurements. Macromolecules 1998, 31, 3679–3685. [Google Scholar] [CrossRef]
- Yildirim-Erbil, H.; Levent-Demirel, A.; Avci, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M.; Królikowski, Z. Application of dsc to study crystallization kinetics of polypropylene containing fillers. J. Thermal. Anal. Calorim. 2003, 74, 549–557. [Google Scholar] [CrossRef]
- Mihai, M.; Chapleau, N.; Denault, J. Processing-formulation-performance relationships of polypropylene/short flax fiber composites. J. Appl. Polym. Sci. 2015, 132, 41528. [Google Scholar] [CrossRef]
- Ermolovich, O.A.; Makarevich, A.V. Effect of compatibilizer additives on the technological and performance characteristics of biodegradable materials based on starch-filled polyethylene. Russ. J. Appl. Chem. 2006, 79, 1526–1531. [Google Scholar] [CrossRef]


| Film | Contact Angle (°) |
|---|---|
| PP | 104.7 ± 1.33 |
| A5 | 82.7 ± 0.70 |
| A7 | 79.8 ± 1.41 |
| A9 | 78.3 ± 0.97 |
| B5 | 78.8 ± 1.02 |
| B7 | 78.9 ± 1.85 |
| B9 | 78.4 ± 1.80 |
| C5 | 81.0 ± 2.04 |
| C7 | 76.8 ± 1.78 |
| C9 | 74.5 ± 1.59 |
| Film | Tm (°C) | ΔHf (J/g) | Xc (%) |
|---|---|---|---|
| PP | 165 | 96.69 | 46.26 |
| PPgMA | 157 | 77.16 | 36.92 |
| A5 | 166 | 76.26 | 36.49 |
| A7 | 165 | 61.07 | 29.22 |
| A9 | 167 | 68.83 | 32.93 |
| B5 | 166 | 70.13 | 33.55 |
| B7 | 165 | 68.84 | 32.94 |
| B9 | 167 | 66.89 | 32.01 |
| C5 | 166 | 81.03 | 38.77 |
| C9 | 166 | 66.48 | 31.81 |
| C9 | 167 | 63.69 | 30.48 |
| CODE | Tensile Strength (Mpa) | Young’s Modulus (Mpa) | Elongation at Break (%) |
|---|---|---|---|
| PP | 41.55 ± 1.14 | 1484.00 ± 37.85 | 396.79 ± 12.91 |
| A5 | 5.71 ± 0.44 | 282.33 ± 24.27 | 4.16 ± 0.23 |
| A7 | 4.86 ± 0.43 | 272.64 ± 17.71 | 3.58 ± 0.29 |
| A9 | 5.60 ± 0.68 | 306.44 ± 22.44 | 2.56 ± 0.26 |
| B5 | 5.97 ± 0.74 | 505.44 ± 26.15 | 4.58 ± 0.11 |
| B7 | 3.00 ± 0.10 | 266.82 ± 16.00 | 4.45 ± 0.82 |
| B9 | 7.71 ± 0.34 | 384.44 ± 6.52 | 3.49 ± 0.15 |
| C5 | 5.06 ± 0.33 | 350.70 ± 8.93 | 3.20 ± 0.20 |
| C7 | 8.22 ± 0.40 | 363.71 ± 17.69 | 10.47 ± 1.66 |
| C9 | 7.93 ± 0.43 | 435.28 ± 19.57 | 10.27 ± 0.48 |
| Composition of Films | |||
|---|---|---|---|
| CODE | PP (wt %) | Chitosan (wt %) | PPgMA (wt %) |
| PP | 100 | 0 | 0 |
| A5 | 95 | 5 | 0 |
| A7 | 93 | 7 | 0 |
| A9 | 91 | 9 | 0 |
| B5, C5 * | 90 | 5 | 5 |
| B7, C7 * | 88 | 7 | 5 |
| B9, C9 * | 86 | 9 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Guigón, F.J.; Rodríguez-Félix, D.E.; Castillo-Ortega, M.M.; Santacruz-Ortega, H.C.; Burruel-Ibarra, S.E.; Encinas-Encinas, J.C.; Plascencia-Jatomea, M.; Herrera-Franco, P.J.; Madera-Santana, T.J. Preparation and Characterization of Extruded Composites Based on Polypropylene and Chitosan Compatibilized with Polypropylene-Graft-Maleic Anhydride. Materials 2017, 10, 105. https://doi.org/10.3390/ma10020105
Carrasco-Guigón FJ, Rodríguez-Félix DE, Castillo-Ortega MM, Santacruz-Ortega HC, Burruel-Ibarra SE, Encinas-Encinas JC, Plascencia-Jatomea M, Herrera-Franco PJ, Madera-Santana TJ. Preparation and Characterization of Extruded Composites Based on Polypropylene and Chitosan Compatibilized with Polypropylene-Graft-Maleic Anhydride. Materials. 2017; 10(2):105. https://doi.org/10.3390/ma10020105
Chicago/Turabian StyleCarrasco-Guigón, Fernando Javier, Dora Evelia Rodríguez-Félix, María Mónica Castillo-Ortega, Hisila C. Santacruz-Ortega, Silvia E. Burruel-Ibarra, Jose Carmelo Encinas-Encinas, Maribel Plascencia-Jatomea, Pedro Jesus Herrera-Franco, and Tomas Jesus Madera-Santana. 2017. "Preparation and Characterization of Extruded Composites Based on Polypropylene and Chitosan Compatibilized with Polypropylene-Graft-Maleic Anhydride" Materials 10, no. 2: 105. https://doi.org/10.3390/ma10020105