Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks
Abstract
:1. Introduction
2. Data and Methods
2.1. Data
2.2. Methods
3. Results
3.1. Number of Publications on Zeolites and MOFs
3.2. Populations and Representative Researchers in Research Countries
3.3. Research Area Population
3.3.1. Research Area Population for Zeolites
3.3.2. Research Area Population for MOFs
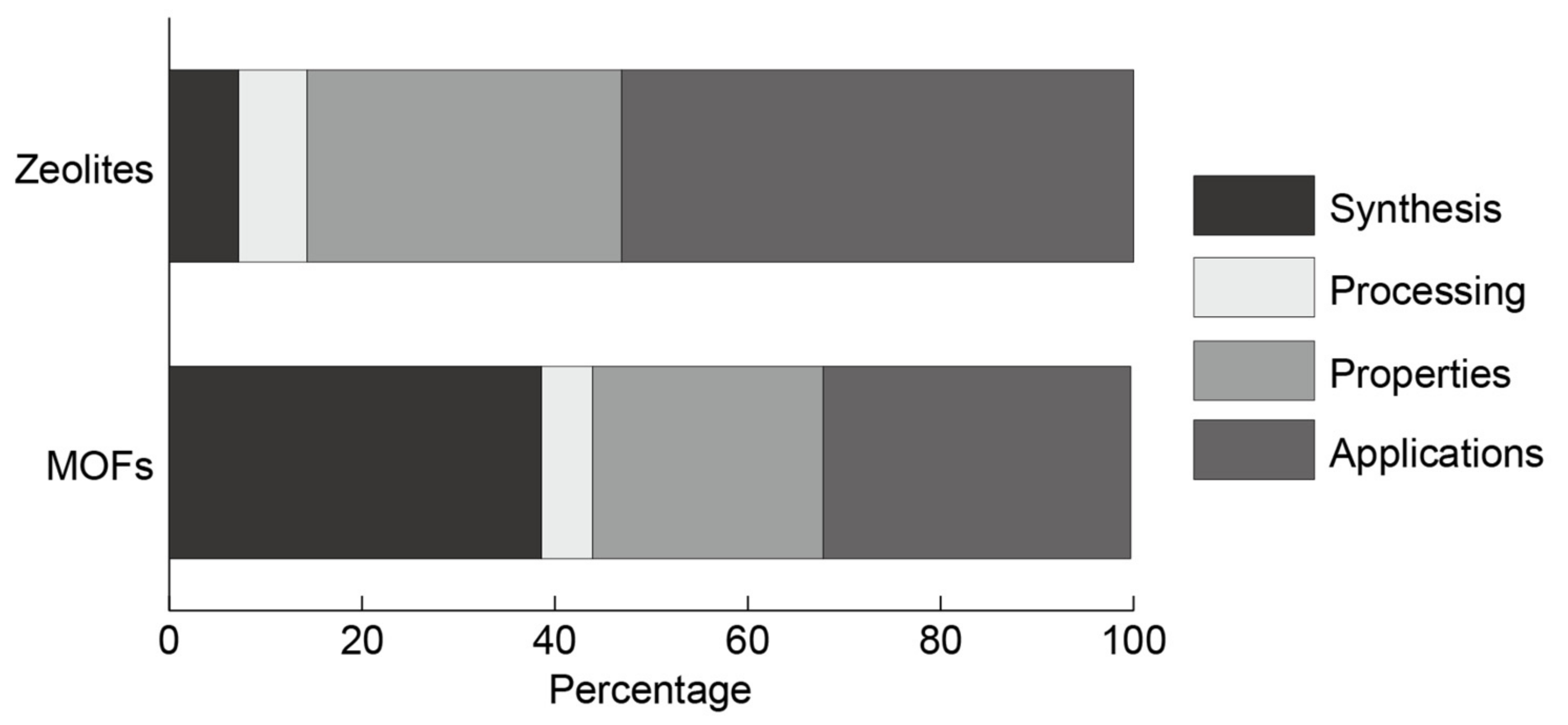
3.4. Classification of Current Research Domain for Zeolites and MOFs
3.4.1. Synthesis
Synthesis of Zeolites
Synthesis of MOFs
3.4.2. Process
Process of Zeolites
Process of MOFs
3.4.3. Properties
Properties of Zeolites
Properties of MOFs
3.4.4. Applications
Applications of Zeolites
Applications of MOFs
4. Discussion
4.1. “Well-Cited” Research Areas from Citation Network Analysis
4.2. Research Area Preferences by Country
4.3. Why Are These Materials Suitable for the Current Research Area?
4.4. Research Domain Having Potentials to Be Developed in the Future
4.4.1. From Zeolites to MOFs
4.4.2. From MOFs to Zeolites
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Barrer, R.M. 33. Synthesis of a zeolitic mineral with chabazite-like sorptive properties. J. Chem. Soc. 1948, 2, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Ohrstrom, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal-organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef]
- Keggin, J.F.; Miles, F.D. Structures and formulæ of the prussian blues andrelated compounds. Nature 1936, 137, 577–578. [Google Scholar] [CrossRef]
- Kondo, M.; Yoshitomi, T.; Matsuzaka, H.; Kitagawa, S.; Seki, K. Three-dimensional framework with channeling cavities for small molecules: {[M2(4, 4′-bpy)3(NO3)4]·xH2O}n (M = Co, Ni, Zn). Angew. Chem. Int. Ed. 1997, 36, 1725–1727. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, Y.; Abe, K.; Noda, S. Filling the gap between researchers studying different materials and different methods: A proposal for structured keywords. J. Inf. Sci. 2006, 32, 511–524. [Google Scholar] [CrossRef]
- Bar-Ilan, J. Informetrics at the beginning of the 21st century—A review. J. Informetr. 2008, 2, 1–52. [Google Scholar] [CrossRef]
- Rotolo, D.; Hicks, D.; Martin, B.R. What is an emerging technology? Res. Policy 2015, 44, 1827–1843. [Google Scholar] [CrossRef]
- Shibata, N.; Kajikawa, A.; Sakata, I. Measuring relatedness between communities in a citation network. J. Am. Soc. Inf. Sci. Technol. 2011, 62, 1360–1369. [Google Scholar] [CrossRef]
- Fujita, K.; Kajikawa, Y.; Mori, J.; Sakata, I. Detecting research fronts using different types of weighted citation networks. J. Eng. Technol. Manag. 2014, 32, 129–146. [Google Scholar] [CrossRef]
- Shibata, N.; Kajikawa, Y.; Takeda, Y.; Matsushima, K. Comparative study on methods of detecting research fronts using different types of citation. J. Am. Soc. Inf. Sci. Technol. 2009, 60, 571–580. [Google Scholar] [CrossRef]
- Ho, J.C.; Saw, E.C.; Lu, L.Y.Y.; Liu, J.S. Technological barriers and research trends in fuel cell technologies: A citation network analysis. Technol. Forecast. Soc. Chang. 2014, 82, 66–79. [Google Scholar] [CrossRef]
- Kajikawa, Y.; Takeda, Y. Citation network analysis of organic LEDs. Technol. Forecast. Soc. Chang. 2009, 76, 1115–1123. [Google Scholar] [CrossRef]
- Shibata, N.; Kajikawa, Y.; Takeda, Y.; Matsushima, K. Detecting emerging research fronts based on topological measures in citation networks of scientific publications. Technovation 2008, 28, 758–775. [Google Scholar] [CrossRef]
- Takeda, Y.; Kajikawa, Y. Optics: A bibliometric approach to detect emerging research domains and intellectual bases. Scientometrics 2009, 78, 543–558. [Google Scholar] [CrossRef]
- Glänzel, W.; Thijs, B. Using ‘core documents’ for detecting and labelling new emerging topics. Scientometrics 2012, 91, 399–416. [Google Scholar] [CrossRef]
- Small, H.; Boyack, K.W.; Klavans, R. Identifying emerging topics in science and technology. Res. Policy 2014, 43, 1450–1467. [Google Scholar] [CrossRef]
- Kajikawa, Y.; Takeda, Y. Structure of research on biomass and bio-fuels: A citation-based approach. Technol. Forecast. Soc. Chang. 2008, 75, 1349–1359. [Google Scholar] [CrossRef]
- Ogawa, T.; Kajikawa, Y. Assessing the industrial opportunity of academic research with patent relatedness: A case study on polymer electrolyte fuel cells. Technol. Forecast. Soc. Chang. 2015, 90, 469–475. [Google Scholar] [CrossRef]
- Nakamura, H.; Ii, S.; Chida, H.; Friedl, K.; Suzuki, S.; Mori, J.; Kajikawa, Y. Shedding light on a neglected area: A new approach to knowledge creation. Sustain. Sci. 2014, 9, 193–204. [Google Scholar] [CrossRef]
- Ittipanuvat, V.; Fujita, K.; Sakata, I.; Kajikawa, Y. Finding linkage between technology and social issue: A literature based discovery approach. J. Eng. Technol. Manag. 2014, 32, 160–184. [Google Scholar] [CrossRef]
- Shibata, N.; Kajikawa, Y.; Sakata, I. Extracting the commercialization gap between science and technology case study of a solar cell. Technol. Forecast. Soc. Chang. 2010, 77, 1147–1155. [Google Scholar] [CrossRef]
- Ogawa, T.; Kajikawa, Y. Generating novel research ideas using computational intelligence: A case study involving fuel cells and ammonia synthesis. Technol. Forecast. Soc. Chang. 2017, 120, 41–47. [Google Scholar] [CrossRef]
- Olson, G.B. Computational design of hierarchically structured materials. Science 1997, 277, 1237–1242. [Google Scholar] [CrossRef]
- Olson, G.B. Designing a new material world. Science 2000, 288, 993–998. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Komiyama, H. Structuring knowledge project in nanotechnology materials program launched in japan. J. Nanopart. Res. 2001, 3, 105–110. [Google Scholar] [CrossRef]
- Newman, M.E.J.; Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E 2004, 69, 026113. [Google Scholar] [CrossRef] [PubMed]
- Newman, M. Fast algorithm for detecting community structure in networks. Phys. Rev. E 2004, 69, 066133. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, Y.; Ohno, J.; Takeda, Y.; Matsushima, K.; Komiyama, H. Creating an academic landscape of sustainability science: An analysis of the citation netwsork. Sustain. Sci. 2007, 2, 221–231. [Google Scholar] [CrossRef]
- Takeda, Y.; Kajikawa, Y. Tracking modularity in citation networks. Scientometrics 2010, 83, 783–792. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Čejka, J.; Centi, G.; Perez-Pariente, J.; Roth, W.J. Zeolite-based materials for novel catalytic applications: Opportunities, perspectives and open problems. Catal. Today 2012, 179, 2–15. [Google Scholar] [CrossRef]
- Bordiga, S.; Lamberti, C.; Bonino, F.; Travert, A.; Thibault-Starzyk, F. Probing zeolites by vibrational spectroscopies. Chem. Soc. Rev. 2015, 44, 7262–7341. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramı́rez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Towards a molecular understanding of shape selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Ghoshal, A.K. Removal of volatile organic compounds from polluted air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Hedström, A. Ion exchange of ammonium in zeolites: A literature review. J. Environ. Eng. 2001, 127, 673–681. [Google Scholar] [CrossRef]
- Simon, U.; Franke, M.E. Electrical properties of nanoscaled host/guest compounds. Microporous Mesoporous Mater. 2000, 41, 1–36. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Zeolites from a materials chemistry perspective. Chem. Mater. 2014, 26, 239–245. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes—Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with mofs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.C. Supported metal clusters: Synthesis, structure, and catalysis. Chem. Rev. 1995, 95, 511–522. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Reticular chemistry: Occurrence and taxonomy of nets and grammar for the design of frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal–organic frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071–2100. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. Ionothermal synthesis of zeolites, metal–organic frameworks, and inorganic–organic hybrids. Acc. Chem. Res. 2007, 40, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal-organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Report, I. IZA Structure Database. Available online: http://www.iza-structure.org/databases/ (accessed on 31 August 2017).
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite mfi as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Norbert Stock, S.B. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Robson, R. Interpenetrating nets: Ordered, periodic entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Lee, H.; Zones, S.I.; Davis, M.E. A combustion-free methodology for synthesizing zeolites and zeolite-like materials. Nature 2003, 425, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Iyoki, K.; Itabashi, K.; Okubo, T. Progress in seed-assisted synthesis of zeolites without using organic structure-directing agents. Microporous Mesoporous Mater. 2014, 189, 22–30. [Google Scholar] [CrossRef]
- Valtchev, V.; Tosheva, L. Porous nanosized particles: Preparation, properties, and applications. Chem. Rev. 2013, 113, 6734–6760. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Metal-organic frameworks—Prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Deleu, W.P.R.; Stassen, I.; Jonckheere, D.; Ameloot, R.; De Vos, D.E. Waste pet (bottles) as a resource or substrate for mof synthesis. J. Mater. Chem. A 2016, 4, 9519–9525. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Niwa, M.; Katada, N. New method for the temperature- programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: A review. Chem. Rec. 2013, 13, 432–455. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.D.; Zheng, A.M.; Huang, S.J.; Zhang, H.L.; Yu, N.Y.; Yang, C.Y.; Liu, S.B.; Deng, F. Combined solid-state NMR and theoretical calculation studies of bronsted acid properties in anhydrous 12-molybdophosphoric acid. J. Phys. Chem. C 2010, 114, 15464–15472. [Google Scholar] [CrossRef]
- Gunther, W.R.; Michaelis, V.K.; Griffin, R.G.; Roman-Leshkov, Y. Interrogating the lewis acidity of metal sites in beta zeolites with 15N pyridine adsorption coupled with MAS NMR spectroscopy. J. Phys. Chem. C 2016, 120, 28533–28544. [Google Scholar] [CrossRef] [PubMed]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Southon, P.D.; Liu, L.; Fellows, E.A.; Price, D.J.; Halder, G.J.; Chapman, K.W.; Moubaraki, B.; Murray, K.S.; Letard, J.F.; Kepert, C.J. Dynamic interplay between spin-crossover and host-guest function in a nanoporous metal-organic framework material. J. Am. Chem. Soc. 2009, 131, 10998–11009. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Ramachandran, V.; Clark, R.J.; Zhou, H.D.; Toby, B.H.; Dalal, N.S.; Kroto, H.W.; Cheetham, A.K. Multiferroic behavior associated with an order-disorder hydrogen bonding transition in metal-organic frameworks (MOFs) with the perovskite ABX(3) architecture. J. Am. Chem. Soc. 2009, 131, 13625–13627. [Google Scholar] [CrossRef] [PubMed]
- Bureekaew, S.; Horike, S.; Higuchi, M.; Mizuno, M.; Kawamura, T.; Tanaka, D.; Yanai, N.; Kitagawa, S. One-dimensional imidazole aggregate in aluminium porous coordination polymers with high proton conductivity. Nat. Mater. 2009, 8, 831–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurd, J.A.; Vaidhyanathan, R.; Thangadurai, V.; Ratcliffe, C.I.; Moudrakovski, I.L.; Shimizu, G.K.H. Anhydrous proton conduction at 150 °C in a crystalline metal–organic framework. Nat. Chem. 2009, 1, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Tiana, D.; Walsh, A. Conductive metal-organic frameworks and networks: Fact or fantasy? Phys. Chem. Chem. Phys. 2012, 14, 13120–13132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Reboul, J.; Furukawa, S.; Torad, N.L.; Ji, Q.; Srinivasu, P.; Ariga, K.; Kitagawa, S.; Yamauchi, Y. Direct carbonization of al-based porous coordination polymer for synthesis of nanoporous carbon. J. Am. Chem. Soc. 2012, 134, 2864–2867. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Muller, U. Catalytic applications of zeolites in chemical industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for baeyer-villiger oxidations. Nature 2001, 412, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Yang, R.T. Hydrogen storage in metal-organic frameworks by bridged hydrogen spillover. J. Am. Chem. Soc. 2006, 128, 8136–8137. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Brozek, C.K.; Dinca, M. Cation exchange at the secondary building units of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5456–5467. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metal-organic frameworks from edible natural products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef] [PubMed]










| Cluster | Publication Number | Average Year | HIJ Index | Keywords |
|---|---|---|---|---|
| A-1 | 13732 | 2003.5 | 0.71 | Cation exchange |
| A-2 | 7000 | 2003.7 | 0.69 | Film membrane |
| A-3 | 632 | 2006.7 | 1.05 | Hierarchical pore, VOC removal |
| B-1 | 23381 | 2000.6 | 0.35 | Characterization for acid sites |
| B-2 | 6789 | 2000.1 | 0.33 | Reactions using extra-framework cations |
| B-3 | 6466 | 2000.1 | 0.23 | Reactions using zeolite-supported metal clusters |
| B-4 | 1558 | 1992 | 0.17 | Other reactions |
| C-1 | 3548 | 2008.4 | 0.88 | Nanosheets, catalysis |
| C-2 | 3445 | 2002.7 | 1.12 | Electron transfer, photo catalyst |
| C-3 | 2311 | 2004.2 | 0.18 | Acid catalysis |
| C-4 | 1517 | 2005.9 | 0.56 | TiO2-based photo catalysis |
| D | 1250 | 1988.8 | 0.049 | Physical properties |
| E | 1096 | 2004.8 | 0.12 | Methane aromatization |
| Cluster | Publication Number | Average Year | HIJ Index | Keywords |
|---|---|---|---|---|
| A-1 | 4156 | 2011.4 | 0.42 | Synthesis and new structure |
| A-2 | 3509 | 2012.3 | 1.17 | Synthesis and new structure |
| A-3 | 2999 | 2009.6 | 1.07 | Synthesis and new structure |
| B-1 | 4439 | 2013.2 | 2.36 | Catalysis, semiconductor, film |
| B-2 | 4398 | 2012.3 | 2.12 | Gas adsorption |
| B-3 | 419 | 2012.4 | 2.41 | Energy storage related gas adsorption |
| C-1 | 698 | 2012.9 | 3.66 | COF |
| C-2 | 694 | 2013.5 | 3.64 | Battery, proton conductivity |
| C-3 | 685 | 2013.7 | 2.09 | Thermolysis, magnetism |
| Zeolites to MOFs | MOFs to Zeolites |
|---|---|
| Membrane reactor | Basic physicochemical properties |
| Cation exchange (radioactive decontamination, soil improvement) | Flexible structure |
| Edible materials | |
| Nanosheets, topotactic conversion |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, T.; Iyoki, K.; Fukushima, T.; Kajikawa, Y. Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks. Materials 2017, 10, 1428. https://doi.org/10.3390/ma10121428
Ogawa T, Iyoki K, Fukushima T, Kajikawa Y. Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks. Materials. 2017; 10(12):1428. https://doi.org/10.3390/ma10121428
Chicago/Turabian StyleOgawa, Takaya, Kenta Iyoki, Tomohiro Fukushima, and Yuya Kajikawa. 2017. "Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks" Materials 10, no. 12: 1428. https://doi.org/10.3390/ma10121428