Two-Dimensional Fluorescence Difference Spectroscopy of ZnO and Mg Composites in the Detection of Physiological Protein and RNA Interactions
Abstract
:1. Introduction
2. Results
2.1. Iteration of 2-D FDS in the Analysis of Nanoparticle Composition
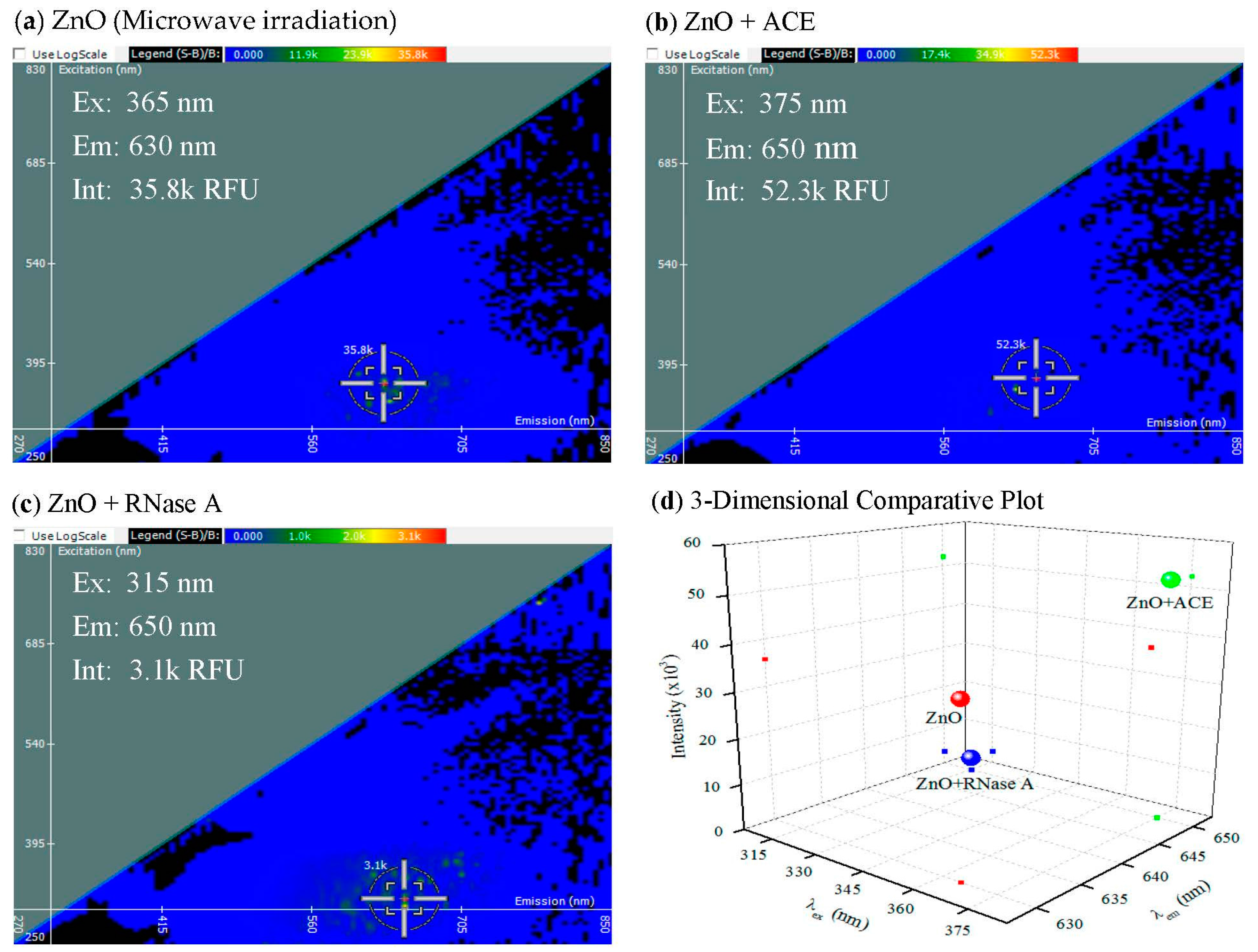
2.2. Iteration of 2-D FDS in the Analysis of Nanoparticle Interaction to Protein
2.3. Iteration of 2-D FDS in the Analysis of Protein–RNA Interaction to the Nanoparticle
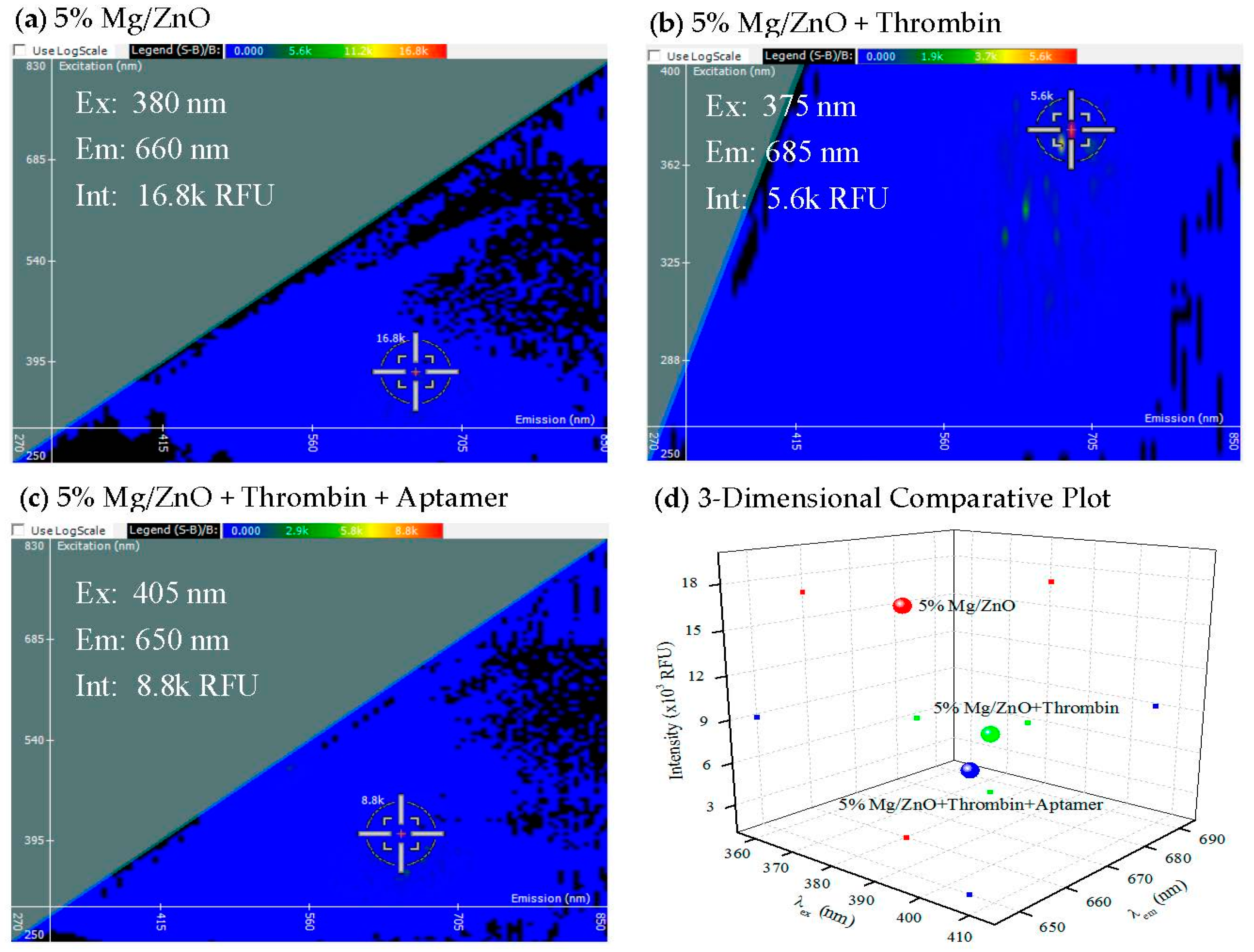
2.4. Iteration of 2-D FDS in the Detection of Specific Aptamer–Protein Interaction
2.5. NPs Do Not Affect Specific Aptamer–Protein Interaction by Gel Mobility Shift
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Stock Preparation
4.3. Nanomaterial Alone
4.4. Protein Interactions
4.5. Molecular Device Settings
4.6. RNA Aptamer-Thrombin and Gel Mobility Shift
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Linden, S.; Enkrich, C.; Wegener, M.; Zhou, J.; Koschny, T.; Soukoulis, C. Magnetic Response of Metamaterials at 100 Terahertz. Science 2004, 306, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Liu, X.; Hedrick, J.L.; Xie, Z.; Wang, S.; Lin, Q.Y.; Hersam, M.C.; Dravid, V.P.; Mirkin, C.A. Polyelemental nanoparticle libraries. Science 2016, 352, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Pendry, J.B.; Wiltshire, M.C. Metamaterials and Negative Refractive Index. Science 2004, 305, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, H.; Chen, L.; Yan, M.; Ge, L.; Ge, S.; Yu, J. A disoposable electrochemiluminescence device for ultrasensitive monitoring of K562 leukemia cells based on aptamers and ZnO@carbon quantum dots. Biosens. Bioelectron. 2013, 49, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.J.; Yang, Z.H.; Zhuo, Y.; Yuan, R.; Chai, Y.Q. A novel aptasensor for thrombin detection based on alkaline phosphatase decorated ZnO/Pt nanoflowers as signal amplifiers. Analyst 2015, 140, 8088–8091. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Triet, N.M.; Son, Y.; Lee, W.; Lee, N. Seesawed fluorescence nano-aptasensor based on highly vertical ZnO nanorods and three-dimensional quantitative fluorescence imaging for enhanced detection accuracy of ATP. Biosens. Bioelectron. 2017, 90, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, J.; Li, H.; Fang, H.; Fan, D.; Wang, W. A label-free photoelectrochemical aptasensor for bisphenol A based on surface plasmon resonance of gold nanoparticle-sensitized ZnO nanopencils. Biosens. Bioelectron. 2016, 86, 315–320. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, P.; Li, X.; Zhang, J.; Zhu, J. A Targeted DNAzyme-Nanocomposite Probe Equipped with Built-in Zn2+ Arsenal for Combined Treatment of Gene Regulation and Drug Delivery. Sci. Rep. 2016, 6, 22737. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiao, Y.; Li, J.; Fang, H.; Fan, D.; Weng, W. A sensitive and label-free photoelectrochemical aptasensor using Co-doped ZnO diluted magnetic semiconductor nanoparticles. Biosens. Bioelectron. 2016, 77, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, X.; Heng, C.; Han, Q.; Cai, S.; Li, J.; Qi, C.; Liang, W.; Yang, R.; Wang, C. Synergistically enhanced photocatalytic and chemotherapeutic effects of aptamer-functionalized ZnO nanoparticles towards cancer cells. Phys. Chem. Chem. Phys. 2015, 17, 21576–21582. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhuo, Y.; Yuan, R.; Chai, Y. Amplified Thrombin Aptasensor Based on Alkaline Phosphatase and Hemin/G-Quadruplex-Catalyzed Oxidation of 1-Naphthol. ACS Appl. Mater. Interfaces 2015, 7, 10308–10315. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.; Yu, J.; Wang, S.; Ge, S.; Song, X. Application of ZnO/graphene and S6 aptamers for sensitive photoelectrochemical detection of SK-BR-3 breast cancer cells based on a disposable indium tin oxide device. Biosens. Bioelectron. 2014, 51, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.A.; Kim, S.N.; Bayraktaroglu, B.; Leedy, K.; Chavez, J.L.; Kelley-Loughnane, N.; Naik, R.R.; Stone, M.O. Biofunctionalized Zinc Oxide Field Effect Transistors for Selective Sensing of Riboflavin with Current Modulation. Sensors 2011, 11, 6645–6655. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Qi, J.; Zhang, Y.; Ren, Y.; Su, M.; Jia, B.; Wang, Y.; Wei, Q.; Du, B. Ultrasensitive photoelectrochemical aptasensing of miR-155 using efficient and stable CH3NH3PbI3 quantum dots sensitized ZnO nanosheets as light harvester. Biosens. Bioelectron. 2016, 85, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hurst, M.N.; DeLong, R.K. Two-Dimensional Fluorescence Difference Spectroscopy to Characterize Nanoparticles and their Interactions. Sci. Rep. 2016, 6, 33287. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.K.; Hurst, M.N.; Aryal, S.; Inchun, N.K. Unique Boron Carbide Nanoparticle Nanobio Interface: Effects on Protein-RNA Interactions and 3-D Spheroid Metastatic Phenotype. Anticancer Res. 2016, 36, 2097–2103. [Google Scholar] [PubMed]
- Long, S.B.; Long, M.B.; White, R.R.; Sullenger, B.A. Crystal structure of an RNA aptamer bound to thrombin. RNA 2008, 14, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Jeter, M.L.; Ly, L.V.; Fortenberry, Y.M.; Whinna, H.C.; White, R.R.; Rusconi, C.P.; Sullenger, B.A.; Church, F.C. RNA aptamer to thrombin binds anion-binding exosite-2 and alters protease inhibition by heparin-binding serpins. FEBS Lett. 2004, 568, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Bhogale, A.; Patel, N.; Sarpotdar, P.; Mariam, J.; Dongre, P.M.; Miotello, A.; Kothari, D.C. Systematic investigation on the interaction of bovine serum albumin with ZnO nanoparticles using fluorescence spectroscopy. Colloids Surf. B 2013, 102, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Gann, H.; Glaspell, G.; Garrad, R.; Wanekaya, A.; Ghosh, K.; Cillessen, L.; Scholz, A.; Parker, B.; Warner, M.; DeLong, R.K. Interaction of MnO and ZnO Nanomaterials with Biomedically Important Proteins and Cells. J. Biomed. Nanotechnol. 2010, 6, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Mechanisms and Dynamics of Fluorescence Quenching; Springer: Boston, MA, USA, 2006; pp. 331–351. ISBN 978-0-387-31278-1. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, A.; Wu, X.; Wang, J.; Brodeur, A.; Thomas, R.; Thakkar, R.; Hadi, H.; Glaspell, G.P.; Duszynski, M.; Wanekaya, A.; et al. Two-Dimensional Fluorescence Difference Spectroscopy of ZnO and Mg Composites in the Detection of Physiological Protein and RNA Interactions. Materials 2017, 10, 1430. https://doi.org/10.3390/ma10121430
Hoffman A, Wu X, Wang J, Brodeur A, Thomas R, Thakkar R, Hadi H, Glaspell GP, Duszynski M, Wanekaya A, et al. Two-Dimensional Fluorescence Difference Spectroscopy of ZnO and Mg Composites in the Detection of Physiological Protein and RNA Interactions. Materials. 2017; 10(12):1430. https://doi.org/10.3390/ma10121430
Chicago/Turabian StyleHoffman, Amanda, Xiaotong Wu, Jianjie Wang, Amanda Brodeur, Rintu Thomas, Ravindra Thakkar, Halena Hadi, Garry P. Glaspell, Molly Duszynski, Adam Wanekaya, and et al. 2017. "Two-Dimensional Fluorescence Difference Spectroscopy of ZnO and Mg Composites in the Detection of Physiological Protein and RNA Interactions" Materials 10, no. 12: 1430. https://doi.org/10.3390/ma10121430




