Solar Hydrogen Production via a Samarium Oxide-Based Thermochemical Water Splitting Cycle
Abstract
:1. Introduction
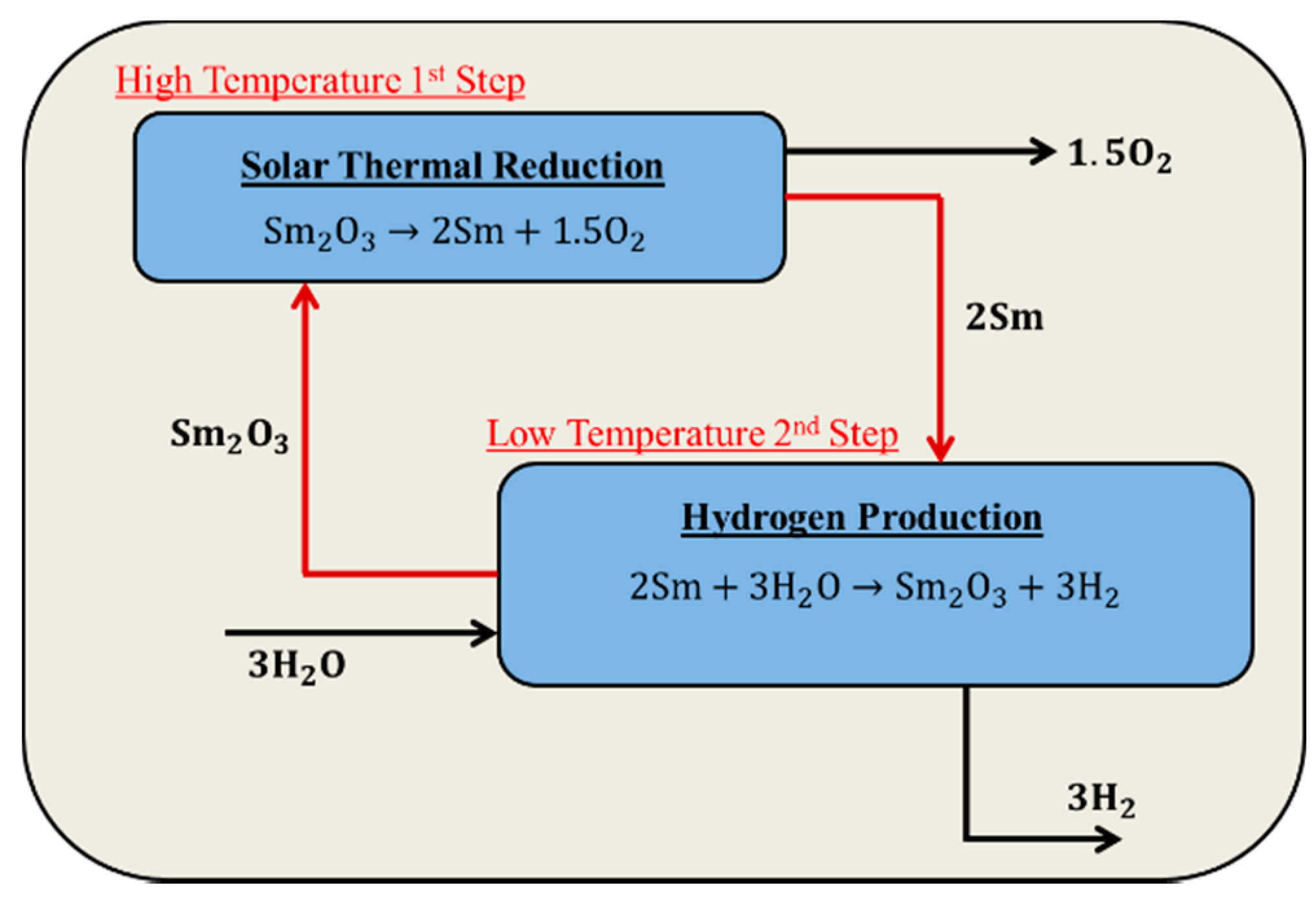
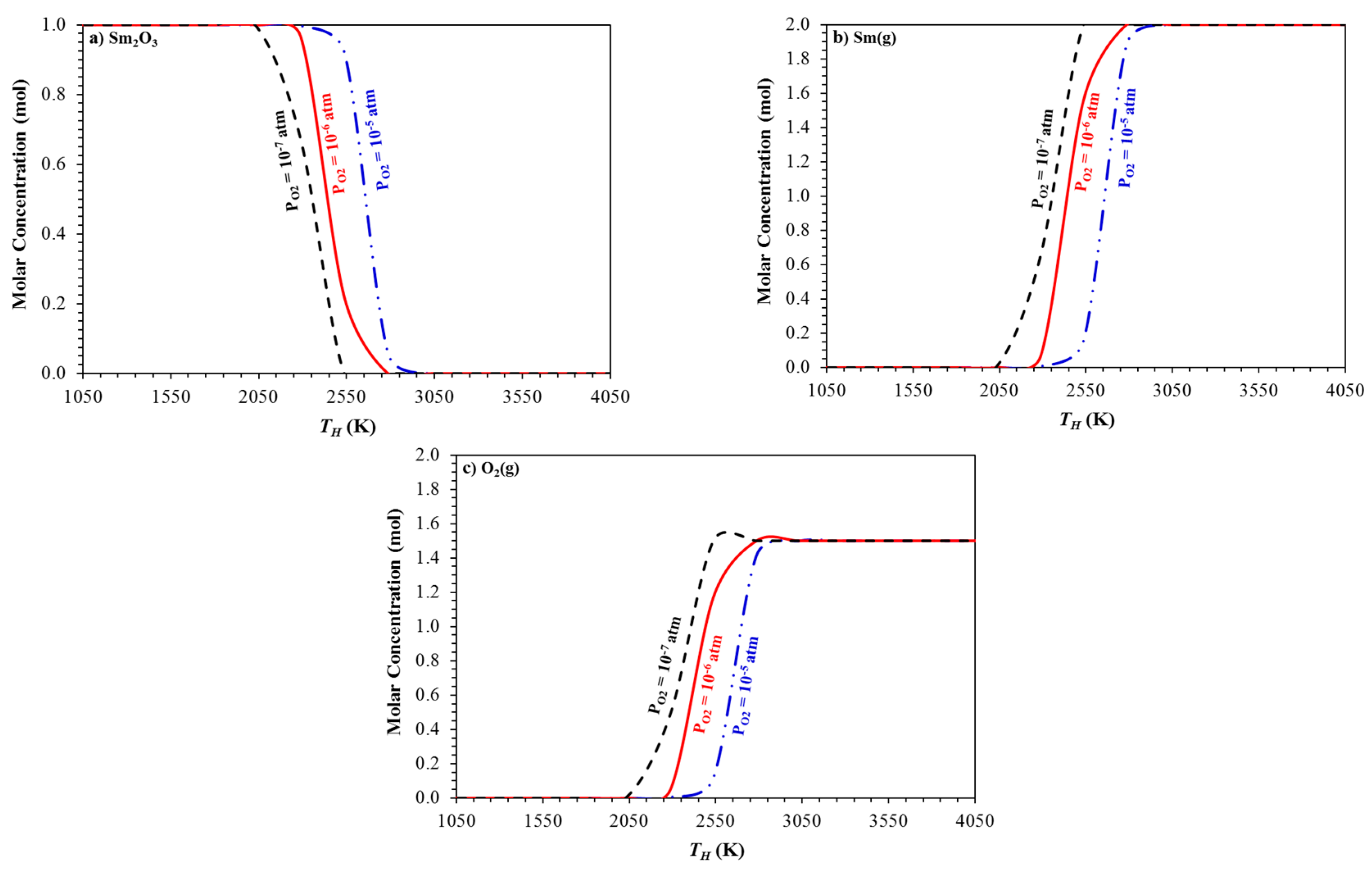
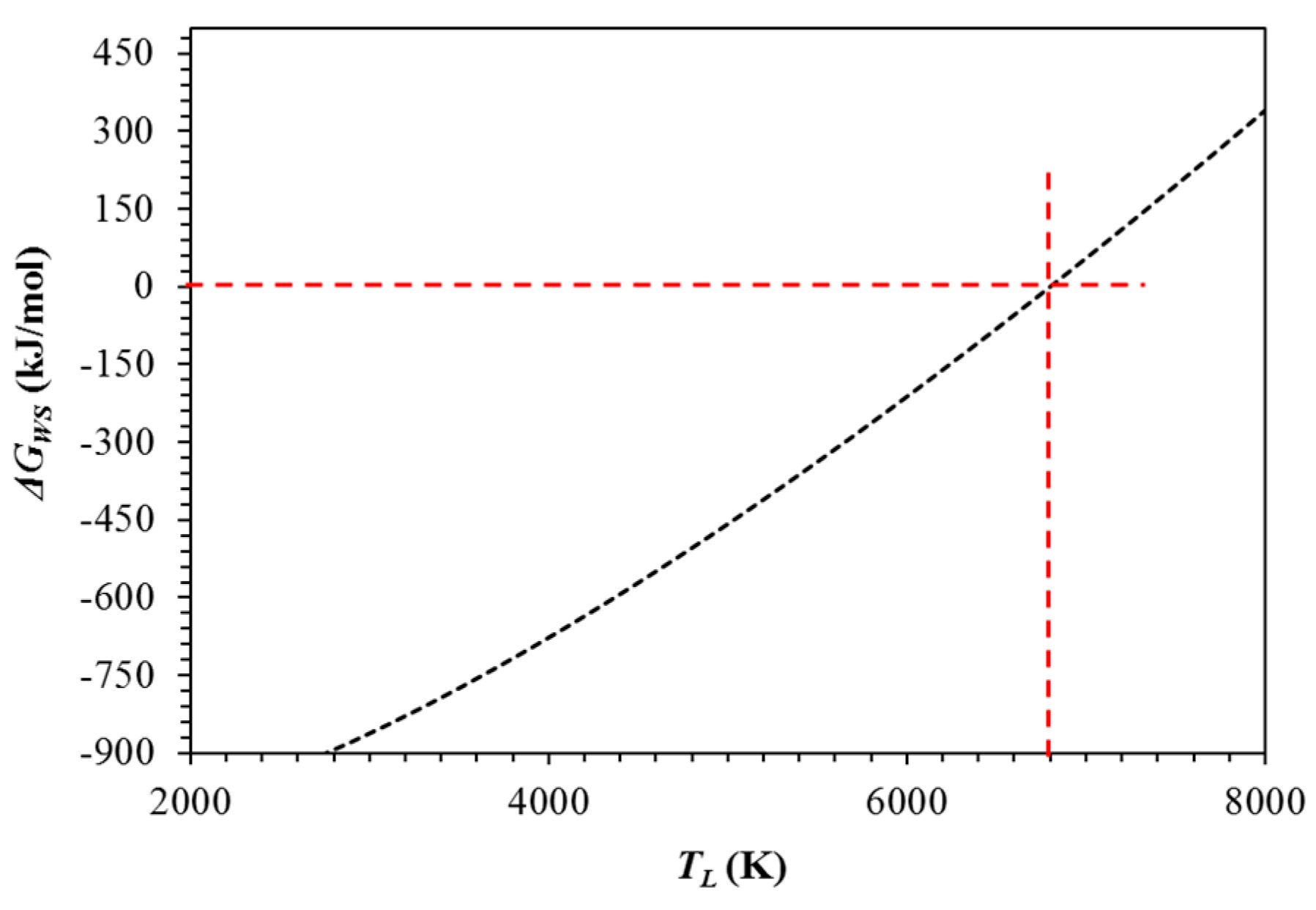
2. Chemical Thermodynamic Equilibrium
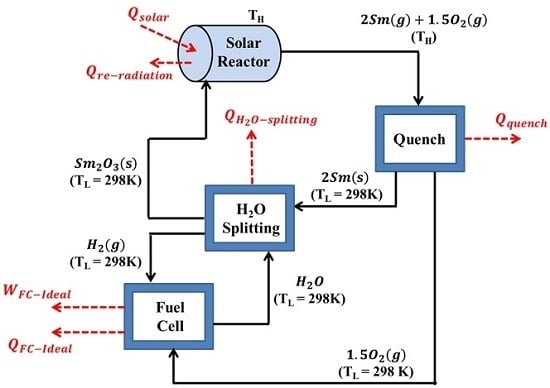
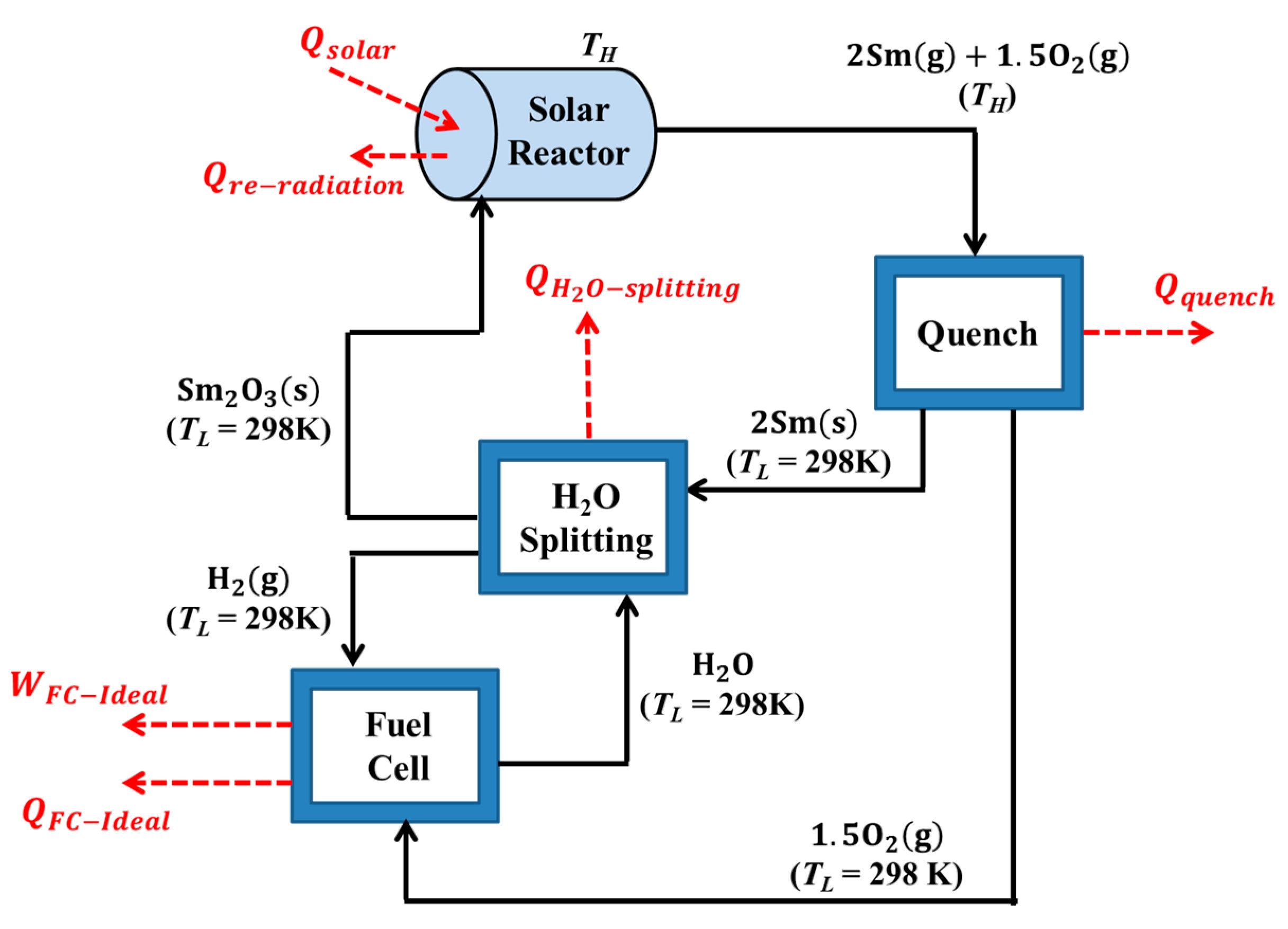
3. Energy and Exergy Analyses
- The production of H2 is carried out at 1 bar and steady state conditions, with negligible viscous losses and changes in kinetic and potential energies.
- The solar reactor is considered a perfectly insulated blackbody absorber.
- The effective emissivity and absorptivity is both equal to unity.
- There are negligible convective/conductive heat losses.
- All reactions reach 100% completion.
- All products separate naturally without expending any work.
- Heat exchangers required for recovering the sensible and latent heat are omitted.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| C | Solar flux concentration ratio (suns) |
| I | Normal beam solar insolation (W·m−2) |
| Molar flow rate (mol·s−1) | |
| Heat rejection rate to surroundings from quench unit (kW) | |
| Heat rejection rate to surroundings from ideal fuel cell (kW) | |
| Heat rejection rate to surroundings from water splitting reactor (kW) | |
| Energy rate required for heating of Sm2O3 (kW) | |
| Energy rate required for the thermal reduction of Sm2O3 (kW) | |
| Net energy input rate required for the operation of Sm-WS cycle (kW) | |
| Radiation heat loss rate from the solar reactor (kW) | |
| Total heat rate that can be recuperated (kW) | |
| Solar energy input rate (kW) | |
| Solar power input after heat recuperation (kW) | |
| TH | Thermal reduction temperature (K) |
| TL | Water splitting temperature (K) |
| Work rate output of ideal fuel cell (kW) | |
| Solar absorption efficiency | |
| Cycle efficiency | |
| Solar to fuel energy conversion efficiency | |
| Gibbs free energy change for water splitting reaction (kJ·mol−1) | |
| Stefan–Boltzmann constant, 5.670 × 10−8 (W·m−2·K−4) | |
| Rate of entropy produced across solar reactor (kW·K−1) | |
| Rate of entropy produced across quench unit (kW·K−1) | |
| Rate of entropy produced across water splitting reactor (kW·K−1) |
References
- International Energy Agency. CO2 Emissions from Fuel Combustion, 2015th ed.; International Energy Agency: Paris, France, 2015. [Google Scholar]
- Scheffe, J.R.; Steinfeld, A. Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: A review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Roeb, M.; Neises, M.; Monnerie, N.; Call, F.; Simon, H.; Sattler, C.; Schmucker, M.; Pitz-Pall, R. Materials-related aspects of thermochemical water and carbon dioxide splitting: A review. Materials 2012, 5, 2015–2054. [Google Scholar] [CrossRef]
- Smestad, G.P.; Steinfeld, A. Review: Photochemical and thermochemical production of solar fuels from H2O and CO2 using metal oxide catalysts. Ind. Eng. Chem. Res. 2012, 51, 11828–11849. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int. J. Hydrog. Energy 2002, 27, 611–619. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar thermochemical production of hydrogen–A review. Sol. Energy 2005, 78, 603–615. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Flamant, G. Design and simulation of a solar chemical reactor for the thermal reduction of metal oxides: Case study of zinc oxide dissociation. Chem. Eng. Sci. 2007, 62, 6323–6333. [Google Scholar] [CrossRef]
- Galvez, M.E.; Loutzenhiser, P.G.; Hischier, I.; Steinfeld, A. CO2 splitting via two-step solar thermochemical cycles with Zn/ZnO and FeO/Fe3O4 redox reactions II: Kinetic analysis. Energy Fuels 2009, 23, 2832–2839. [Google Scholar]
- Loutzenhiser, P.G.; Steinfeld, A. Solar syngas production from CO2 and H2O in a two-step thermochemical cycle via Zn/ZnO redox reactions: Thermodynamic cycle analysis. Int. J. Hydrog. Energy 2011, 36, 12141–12147. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Meier, A.; Steinfeld, A. Review of the two-step H2O/CO2-splitting solar thermochemical cycle based on Zn/ZnO redox reactions. Materials 2010, 3, 4922–4938. [Google Scholar] [CrossRef]
- Dardor, D.; Bhosale, R.R.; Gharbia, S.; Kumar, A.; AlMomani, F. Solar carbon production via thermochemical ZnO/Zn carbon dioxide splitting cycle. J. Emerg. Trends Eng. Appl. Sci. 2015, 6, 129–135. [Google Scholar]
- Miller, J.E.; Allendorf, M.D.; Diver, R.B.; Evans, L.R.; Siegel, N.P.; Stuecker, J.N. Metal oxide composites and structures for ultra-high temperature solar thermochemical cycles. J. Mater. Sci. 2008, 43, 4714–4728. [Google Scholar] [CrossRef]
- Ishihara, H.; Kaneko, H.; Hasegawa, N.; Tamaura, Y. Two-step water-splitting at 1273–1623K using yttria-stabilized zirconia-iron oxide solid solution via co-precipitation and solid-state reaction. Energy 2008, 33, 1788–1793. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; van den Broeke, L.J.P.; Gharbia, S.; Dardor, D.; Jilani, M.; Folady, J.; Al-Fakih, M.S.; Tarsad, M.A. Solar hydrogen production via thermochemical iron oxide–iron sulfate water splitting cycle. Int. J. Hydrog. Energy 2015, 40, 1639–1650. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.A.; Alxneit, I. Sol–gel derived CeO2–Fe2O3 nanoparticles: Synthesis, characterization and solar thermochemical application. Ceram. Int. 2016, in press. [Google Scholar] [CrossRef]
- Abanades, S. CO2 and H2O reduction by solar thermochemical looping using SnO2/SnO redox reactions: Thermogravimetric analysis. Int. J. Hydrog. Energy 2012, 37, 8223–8231. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Lemont, F.; Flamant, G. Experimental study of SnO2/SnO/Sn thermochemical systems for solar production of hydrogen. AIChE J. 2008, 54, 2759–2767. [Google Scholar] [CrossRef]
- Dardor, D.; Bhosale, R.R.; Gharbia, S.; AlNouss, A.; Kumar, A.; AlMomani, F. Solar thermochemical conversion of CO2 into C via SnO2/SnO redox cycle: Thermodynamic study. Int. J. Eng. Res. Appl. 2015, 5, 134–140. [Google Scholar]
- Bhosale, R.R.; Kumar, A.; Almomani, F. Solar thermochemical hydrogen production via terbium oxide based redox reactions. Int. J. Photoenergy 2016, 2016. [Google Scholar] [CrossRef]
- Agrafiotis, C.C.; Pagkoura, C.; Zygogianni, A.; Karagiannakis, G.; Kostoglou, M.; Konstandopoulos, A.G. Hydrogen production via solar-aided water splitting thermochemical cycles: Combustion synthesis and preliminary evaluation of spinel redox-pair materials. Int. J. Hydrog. Energy 2012, 37, 8964–8980. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Khadka, R.; Puszynski, J.; Shende, R. H2 generation from two-step thermochemical water-splitting reaction using sol-gel derived SnxFeyOz. J. Renew. Sustain. Energy 2011, 3, 063104. [Google Scholar] [CrossRef]
- Scheffe, J.; Li, J.; Weimer, A. A spinel ferrite/hercynite water-splitting redox cycle. Int. J. Hydrog. Energy 2010, 35, 3333–3340. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Shende, R.V.; Puszynski, J.A. Thermochemical water-splitting for H2 generation using sol-gel derived Mn-ferrite in a packed bed reactor. Int. J. Hydrog. Energy 2012, 37, 2924–2934. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.A.; Alxneit, I. Propylene oxide assisted sol-gel synthesis of zinc ferrite nanoparticles for solar fuel production. Ceram. Int. 2016, 42, 2431–2438. [Google Scholar] [CrossRef]
- Roeb, M.; Gathmann, N.; Neises, M.; Sattler, C.; Pitz-Paal, R. Thermodynamic analysis of two-step solar water splitting with mixed iron oxides. Int. J. Energy Res. 2009, 33, 893–902. [Google Scholar] [CrossRef]
- Bader, R.; Venstrom, L.J.; Davidson, J.H.; Lipiński, W. Thermodynamic analysis of isothermal redox cycling of ceria for solar fuel production. Energy Fuels 2013, 27, 5533–5544. [Google Scholar] [CrossRef]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Furler, P.; Scheffe, J.R.; Steinfeld, A. Syngas production by simultaneous splitting of H2O and CO2 via ceria redox reactions in a high-temperature solar reactor. Energy Environ. Sci. 2012, 5, 6098–6103. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Steinfeld, A. Thermodynamic analysis of cerium-based oxides for solar thermochemical fuel production. Energy Fuels 2012, 26, 1928–1936. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Weibel, D.; Steinfeld, A. Lanthanum–strontium–manganese perovskites as redox materials for solar thermochemical splitting of H2O and CO2. Energy Fuels 2013, 27, 4250–4257. [Google Scholar] [CrossRef]
- Demont, A.; Abanades, S. High redox activity of Sr-substituted lanthanum manganite perovskites for two-step thermochemical dissociation of CO2. RSC Adv. 2014, 4, 54885–54891. [Google Scholar] [CrossRef]
- Gálvez, M.; Jacot, R.; Scheffe, J.R.; Cooper, T.; Patzke, G.; Steinfeld, A. Physico-chemical changes in Ca, Sr and Al-doped La–Mn–O perovskites upon thermochemical splitting of CO2 via redox cycling. Phys. Chem. Chem. Phys. 2015, 17, 6629–6634. [Google Scholar] [CrossRef] [PubMed]
- Roine, A. Outokumpu HSC Chemistry for windows, version 7.1.; Outokumpu Research Oy: Pori, Finland, 2013. [Google Scholar]
- Abanades, S.; Charvin, P.; Lemont, F.; Flamant, G. Novel two-step SnO2/SnO water-splitting cycle for solar thermochemical production of hydrogen. Int. J. Hydrog. Energy 2008, 33, 6021–6030. [Google Scholar] [CrossRef]
- Diver, R.B.; Miller, J.E.; Allendorf, M.D.; Seigel, N.P.; Hogan, R.E. Solar thermochemical water-splitting ferrite-cycle heat engines. J. Sol. Energy Eng. 2008, 130, 041001. [Google Scholar] [CrossRef]

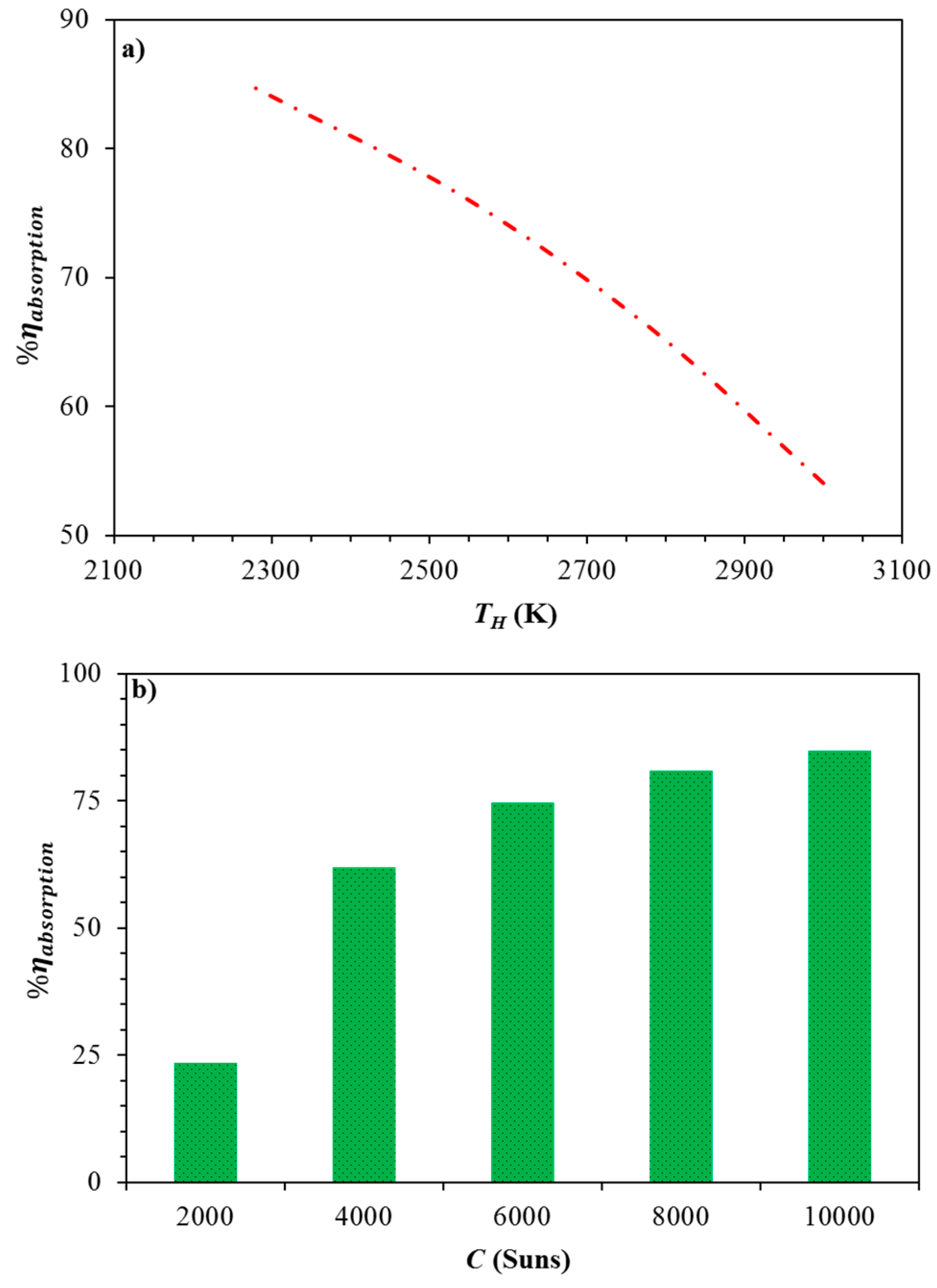
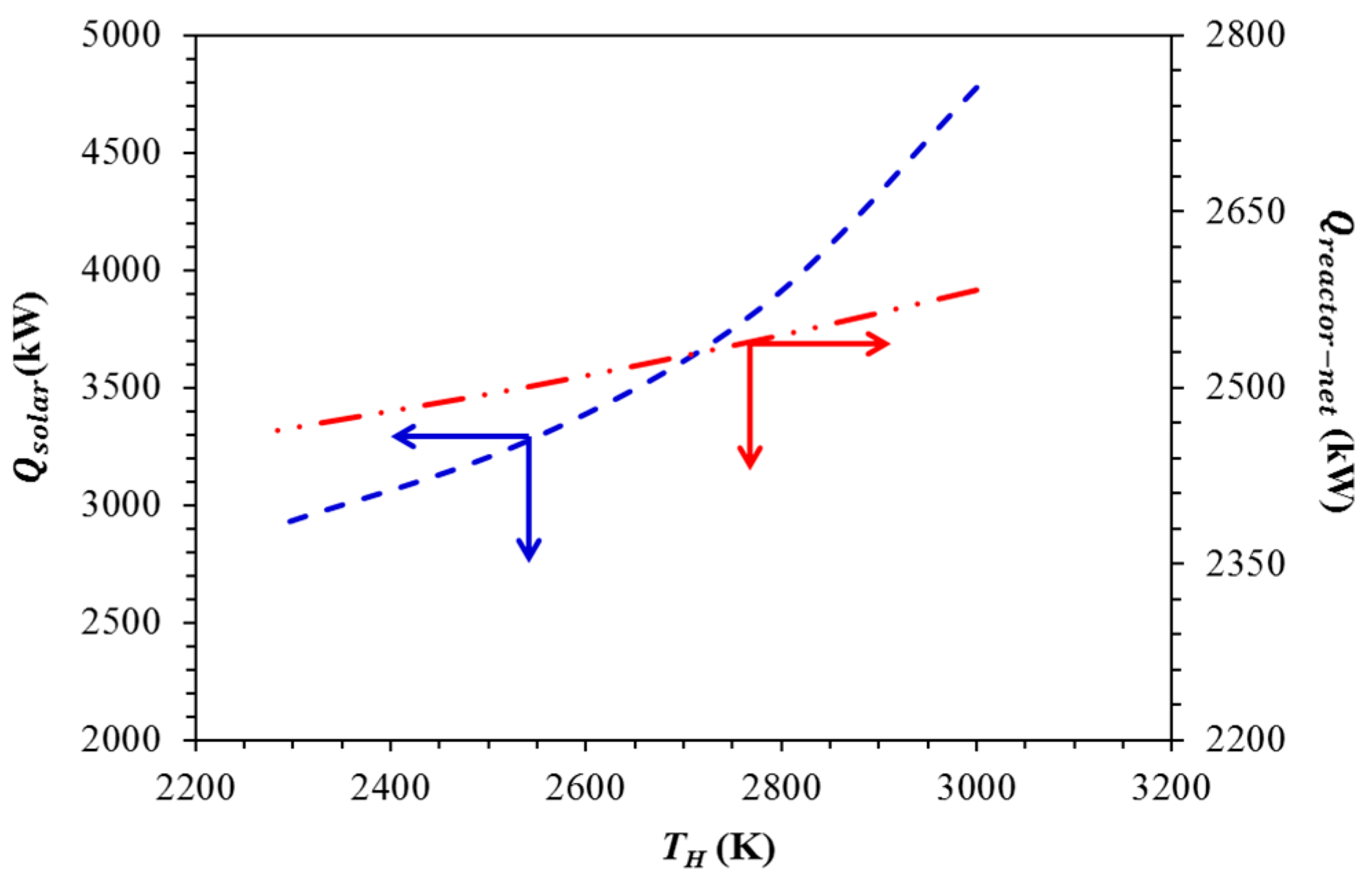
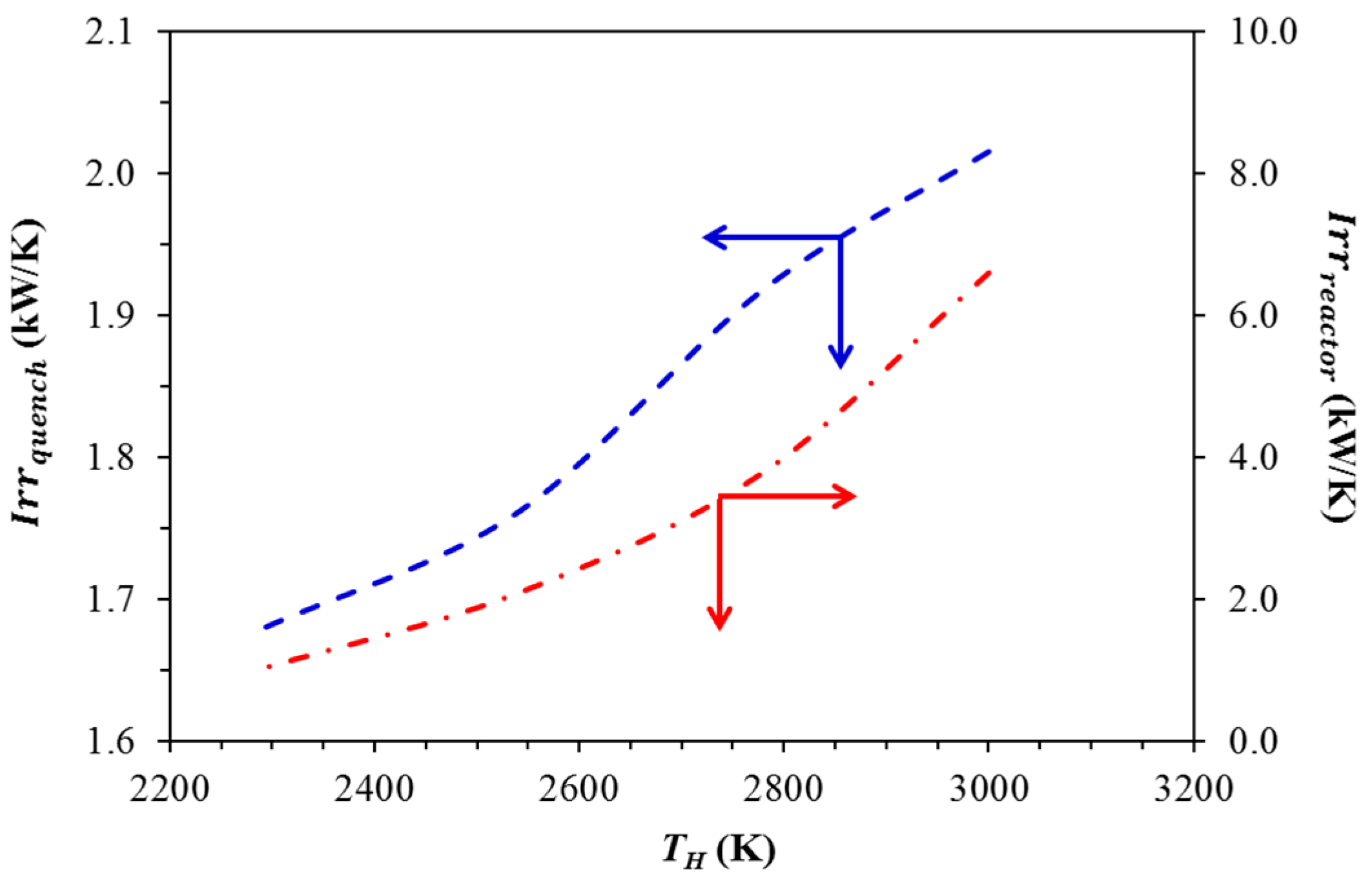

| TH (K) | Qquench (kW) | Qre−radiation (kW) |
|---|---|---|
| 3000 | 756 | 2194 |
| 2780 | 714 | 1301 |
| 2540 | 673 | 773 |
| 2280 | 636 | 446 |
| TH (K) | ηcycle (%) | ηsolar−to−fuel (%) |
|---|---|---|
| 3000 | 14.9 | 17.9 |
| 2780 | 18.5 | 22.3 |
| 2540 | 21.7 | 26.2 |
| 2280 | 24.5 | 29.5 |
| TH (K) | ηcycle (%) | ηsolar−to−fuel (%) |
|---|---|---|
| Recuperation = 0% | ||
| 2780 | 18.5 | 22.3 |
| 2540 | 21.7 | 26.2 |
| 2280 | 24.4 | 29.5 |
| Recuperation = 20% | ||
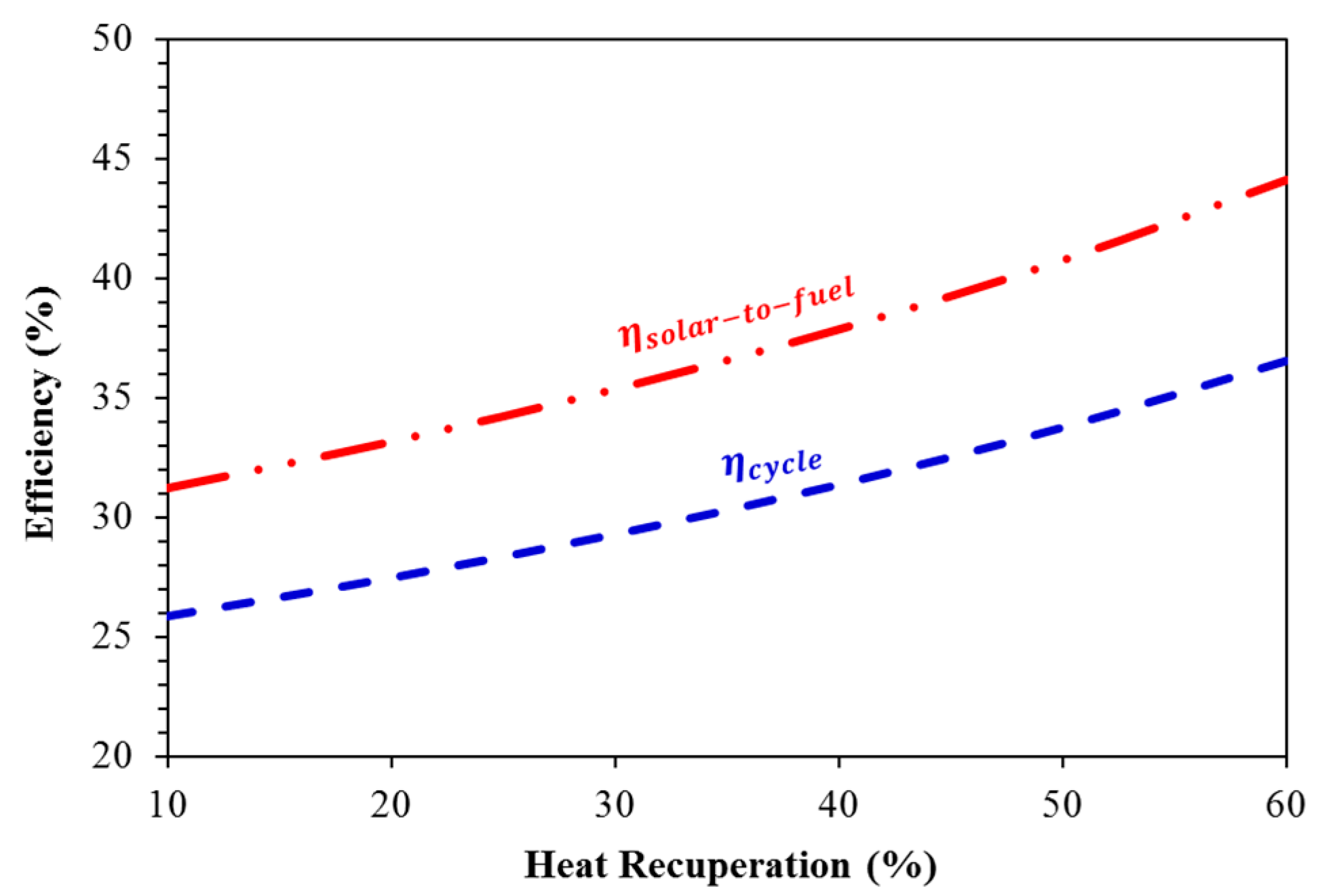
| 2780 | 20.3 | 24.5 |
| 2540 | 24.1 | 29.1 |
| 2280 | 27.5 | 33.1 |
| Recuperation = 40% | ||
| 2780 | 22.4 | 27.1 |
| 2540 | 27.2 | 32.8 |
| 2280 | 31.4 | 37.8 |
| Recuperation = 60% | ||
| 2780 | 25.1 | 30.3 |
| 2540 | 31.1 | 37.5 |
| 2280 | 36.5 | 44.1 |
| Cycle | ηsolar−to−fuel (%) |
|---|---|
| Zinc oxide cycle | 29.0 |
| Tin oxide cycle | 29.8 |
| Iron oxide cycle | 30.0 |
| Ceria cycle | 20.2 |
| Sm-WS cycle | 44.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, R.; Kumar, A.; AlMomani, F.; Ghosh, U.; Saad Anis, M.; Kakosimos, K.; Shende, R.; Rosen, M.A. Solar Hydrogen Production via a Samarium Oxide-Based Thermochemical Water Splitting Cycle. Energies 2016, 9, 316. https://doi.org/10.3390/en9050316
Bhosale R, Kumar A, AlMomani F, Ghosh U, Saad Anis M, Kakosimos K, Shende R, Rosen MA. Solar Hydrogen Production via a Samarium Oxide-Based Thermochemical Water Splitting Cycle. Energies. 2016; 9(5):316. https://doi.org/10.3390/en9050316
Chicago/Turabian StyleBhosale, Rahul, Anand Kumar, Fares AlMomani, Ujjal Ghosh, Mohammad Saad Anis, Konstantinos Kakosimos, Rajesh Shende, and Marc A. Rosen. 2016. "Solar Hydrogen Production via a Samarium Oxide-Based Thermochemical Water Splitting Cycle" Energies 9, no. 5: 316. https://doi.org/10.3390/en9050316
APA StyleBhosale, R., Kumar, A., AlMomani, F., Ghosh, U., Saad Anis, M., Kakosimos, K., Shende, R., & Rosen, M. A. (2016). Solar Hydrogen Production via a Samarium Oxide-Based Thermochemical Water Splitting Cycle. Energies, 9(5), 316. https://doi.org/10.3390/en9050316