Waste Heat Recovery of a PEMFC System by Using Organic Rankine Cycle
Abstract
:1. Introduction
2. Models and Formulations
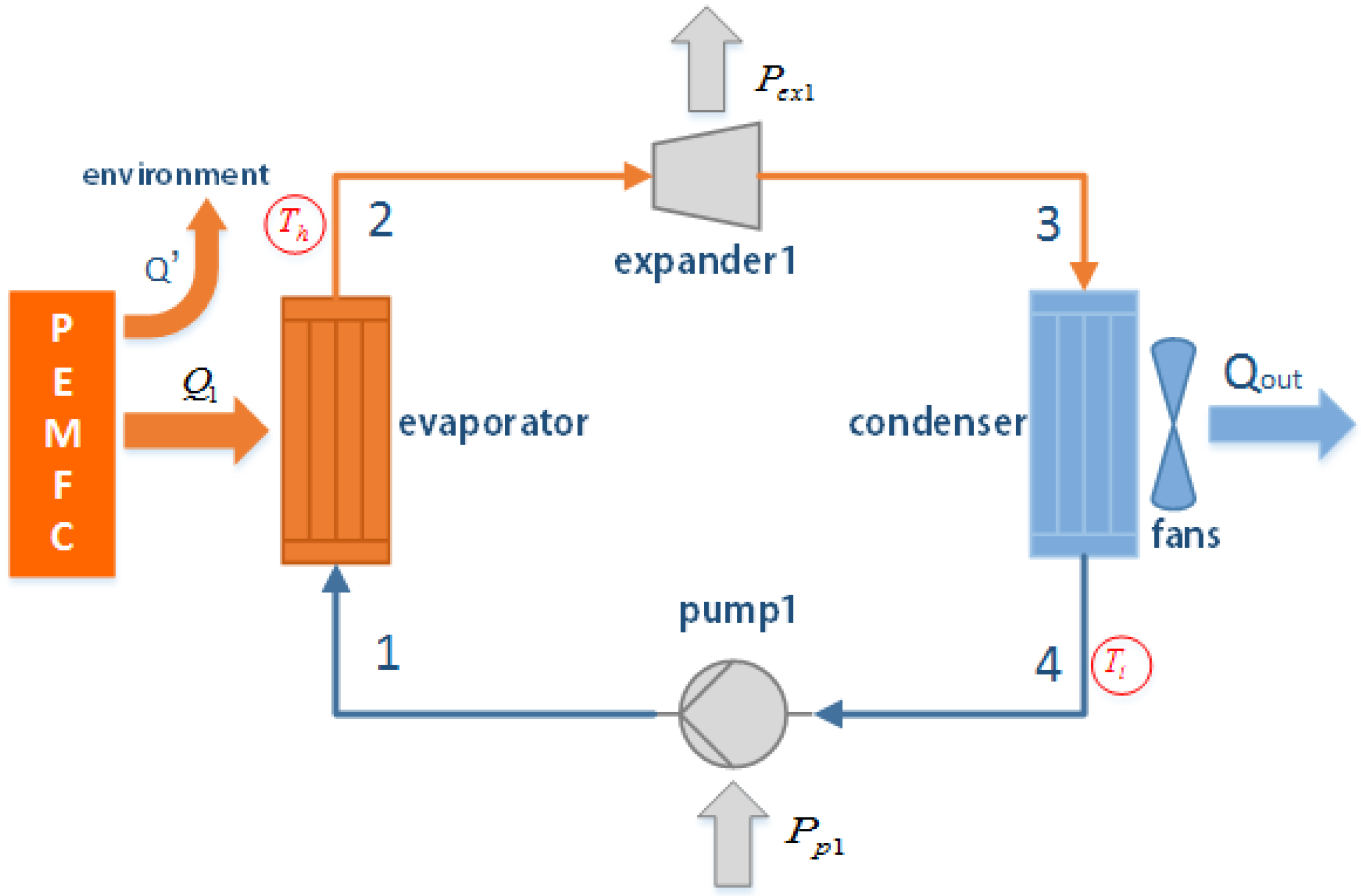
2.1. Organic Rankine Cycle (ORC) System Description
- The liquid phase working fluid in an ORC system recovers the heat () released from PEMFC and evaporates it to be overheated vapor. The redundant heat () is released to the environment by another way;
- The high pressure working fluid vapor drives the expander to generate the expansion work and relieves the pressure with the synchronously declining temperature;
- The working fluid vapor at the low pressure is cooled to be a saturated liquid phase in the condenser by the air;
- The saturated working fluid is pumped into the evaporator and completes the Rankine cycle.
2.2. HPORC System Description
- Subcooled working fluid with low pressure recovers the heat released from PEMFC and evaporates it to be overheated vapor through the evaporator of the HP;
- The overheated vapor is compressed to be of high temperature and pressure, and the superheat is enlarged;
- The high-temperature vapor expresses the heat to the working fluid of the ORC system and is cooled down to a subcooled liquid phase;
- The liquid with high pressure enters the expander and relieves the pressure.
2.3. Thermal Dynamic Model
- The critical temperature of the working fluid should be above 55 °C;
- The dry working fluid is adoptable for ORC, and the wet working fluid is better for the heat pump.
- For the pump, compressor, and expander, the isentropic efficiency ηs is set to be 0.9, the mechanical efficiency ηm is assumed to be 0.8. In fact, the efficiencies are determined by the pressure ratio and mass flow rate. Designing the components according to the operating conditions will be our work in the future. The power generation efficiency of the generator connected to the expander is assumed to be 0.9.
- The heat loss in the tube between two conjoint components of the system is ignored;
- The heat exchange efficiency is set to be 0.9, the pressure drop is assumed to be 0.1 kPa.
- The operating temperature of PEMFC is set to be 60 °C, and the temperature of the environment is kept at 20 °C;
- The heat transfer coefficient (UA ) of evaporators is set to be 1000 W/K;
- The condenser can meet the requirements of heat exchange. This is reasonable because the heat-transfer coefficient of the condenser can be controlled by the fans, as illustrated in Figure 1.
2.3.1. Model Description of ORC
2.3.2. Model Description of HPORC
2.4. Electrochemical Model of PEMFC
3. Results
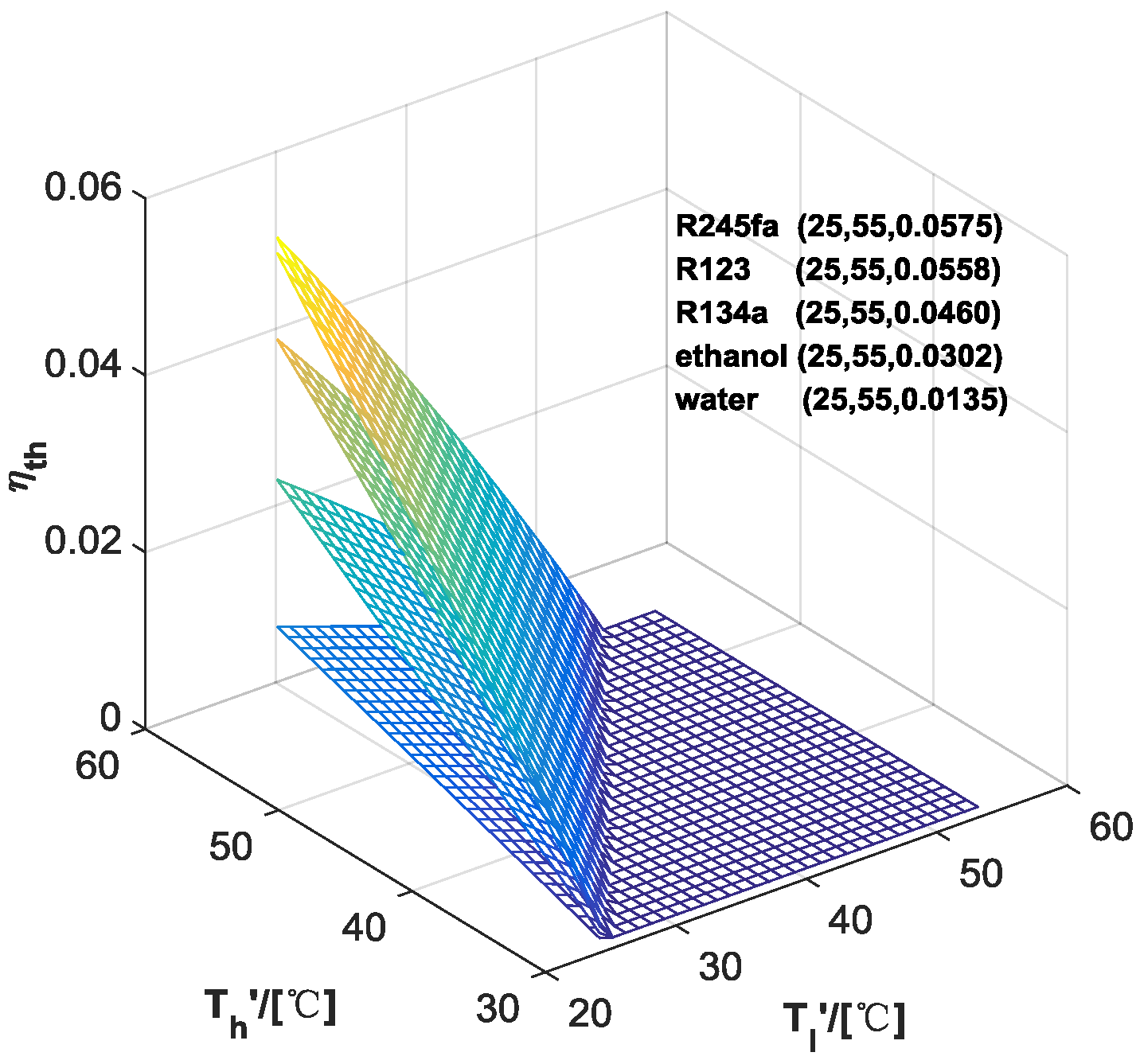
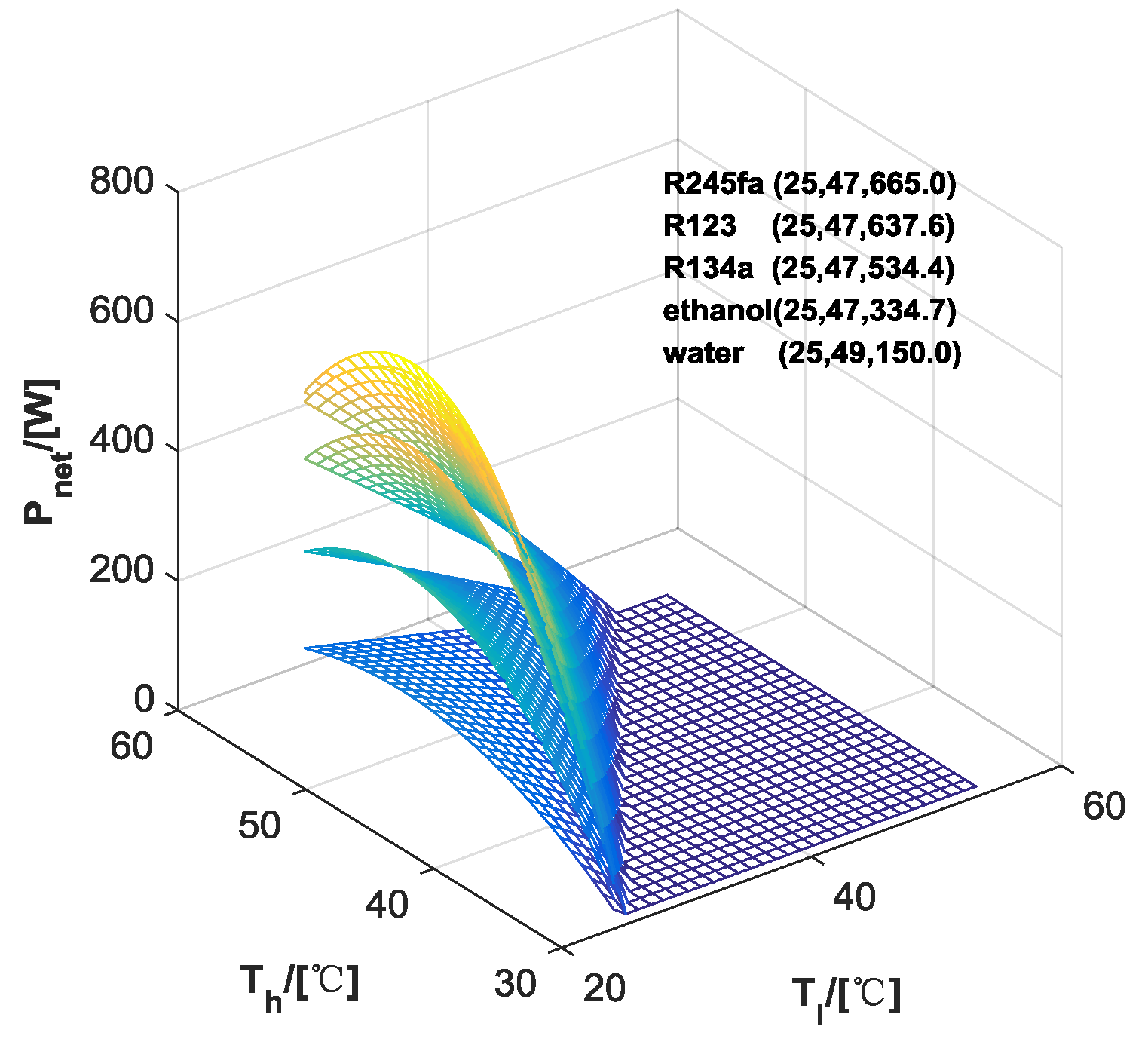
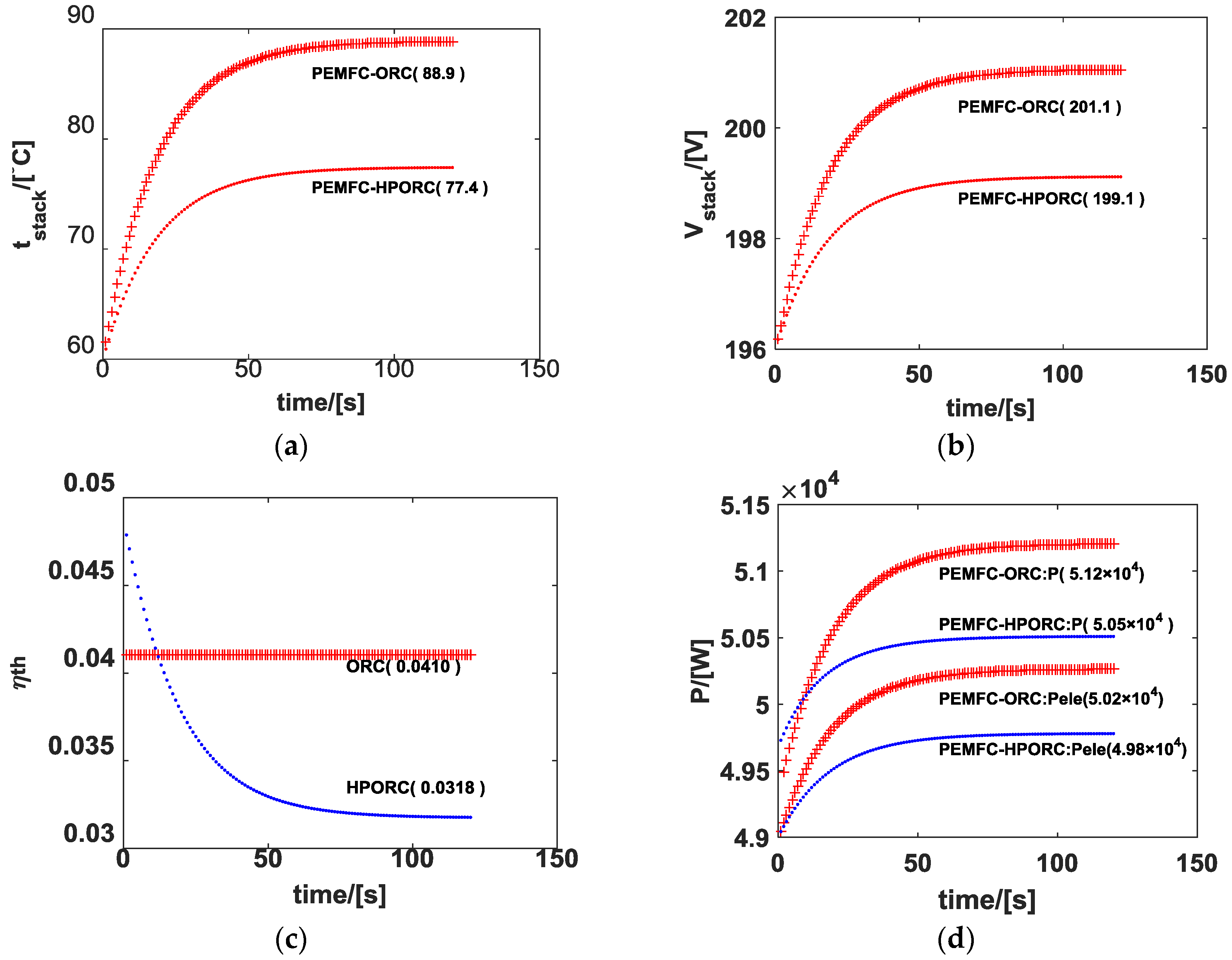
3.1. Performance of ORC
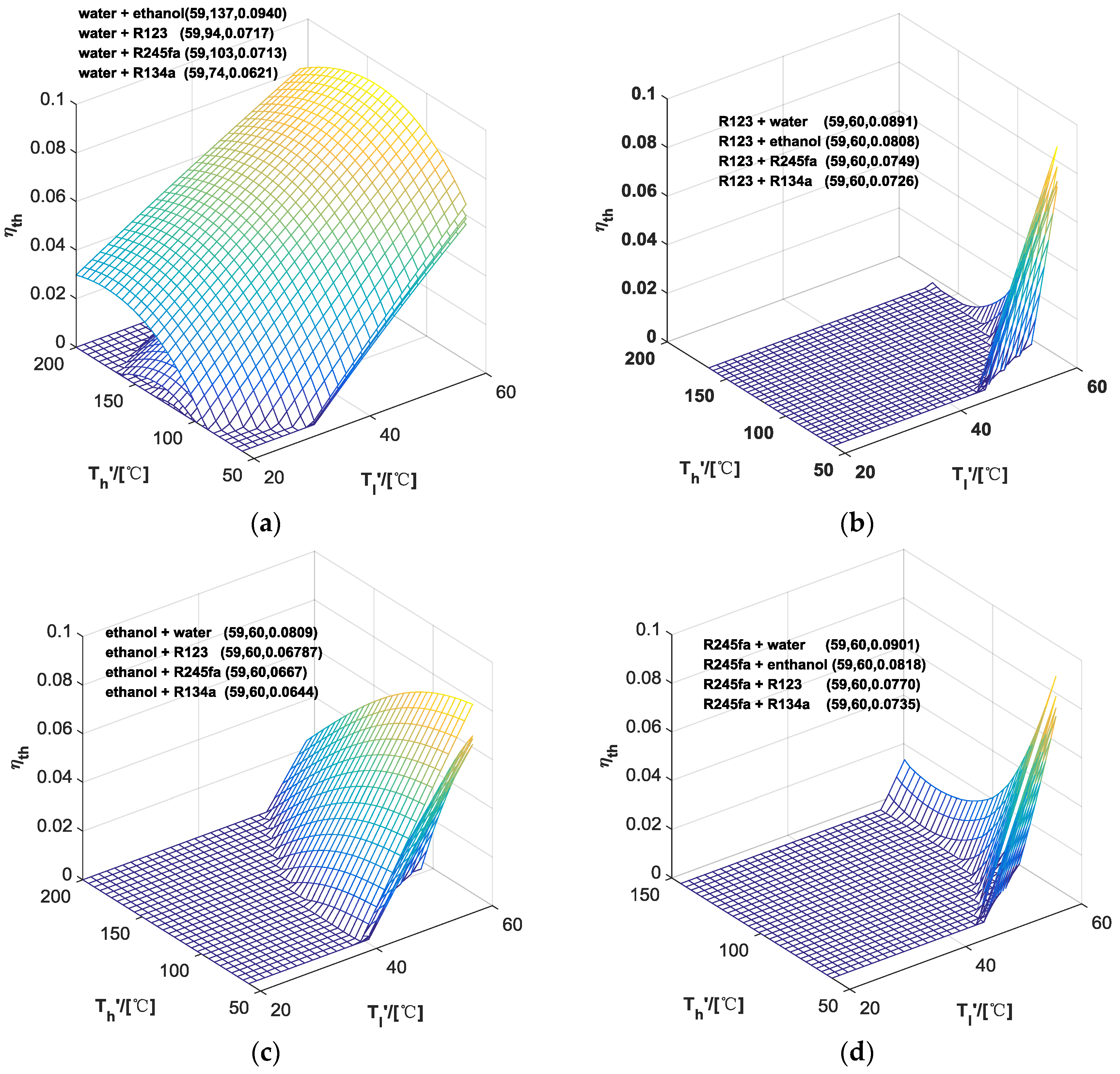
3.2. Performance of HPORC
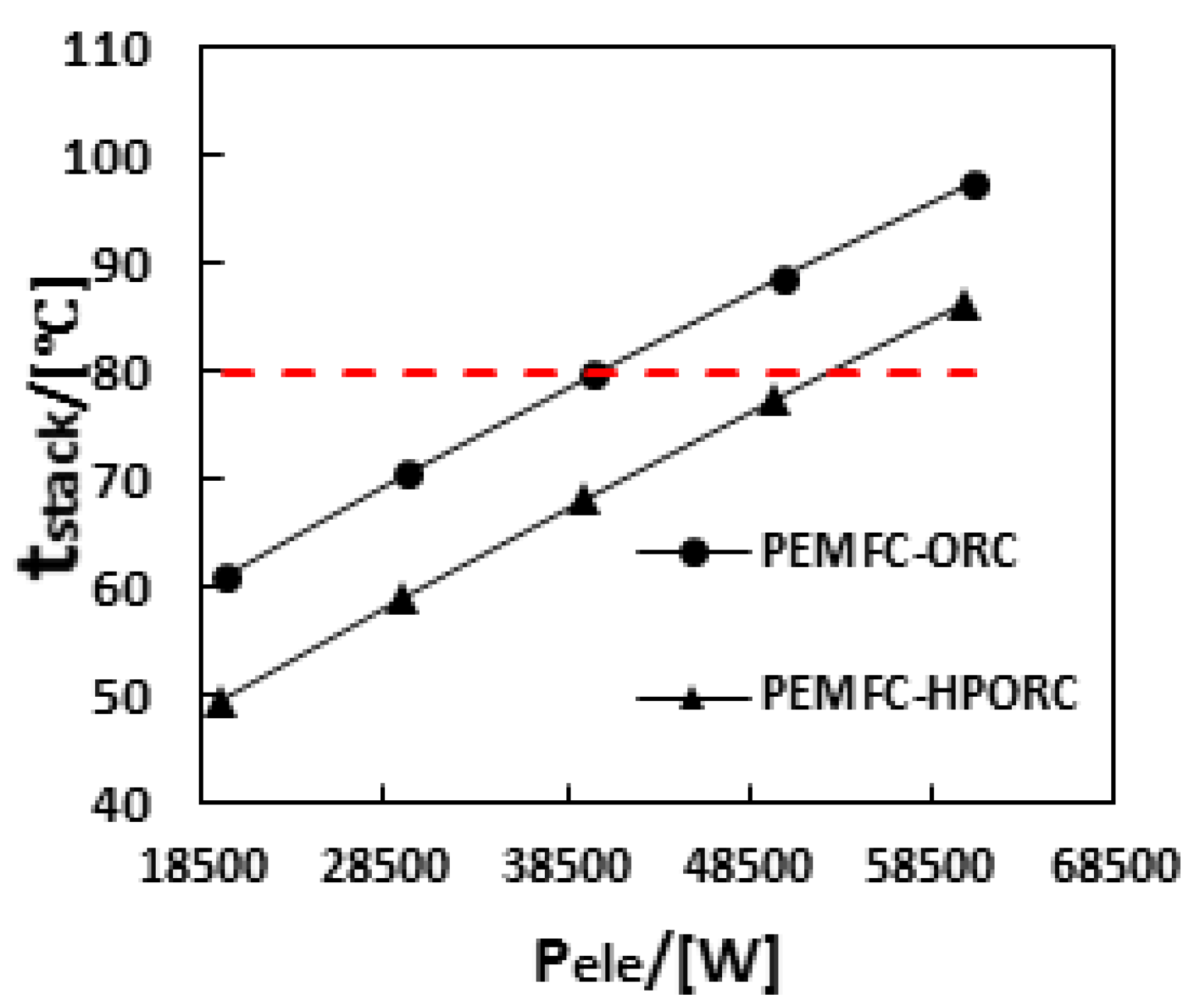
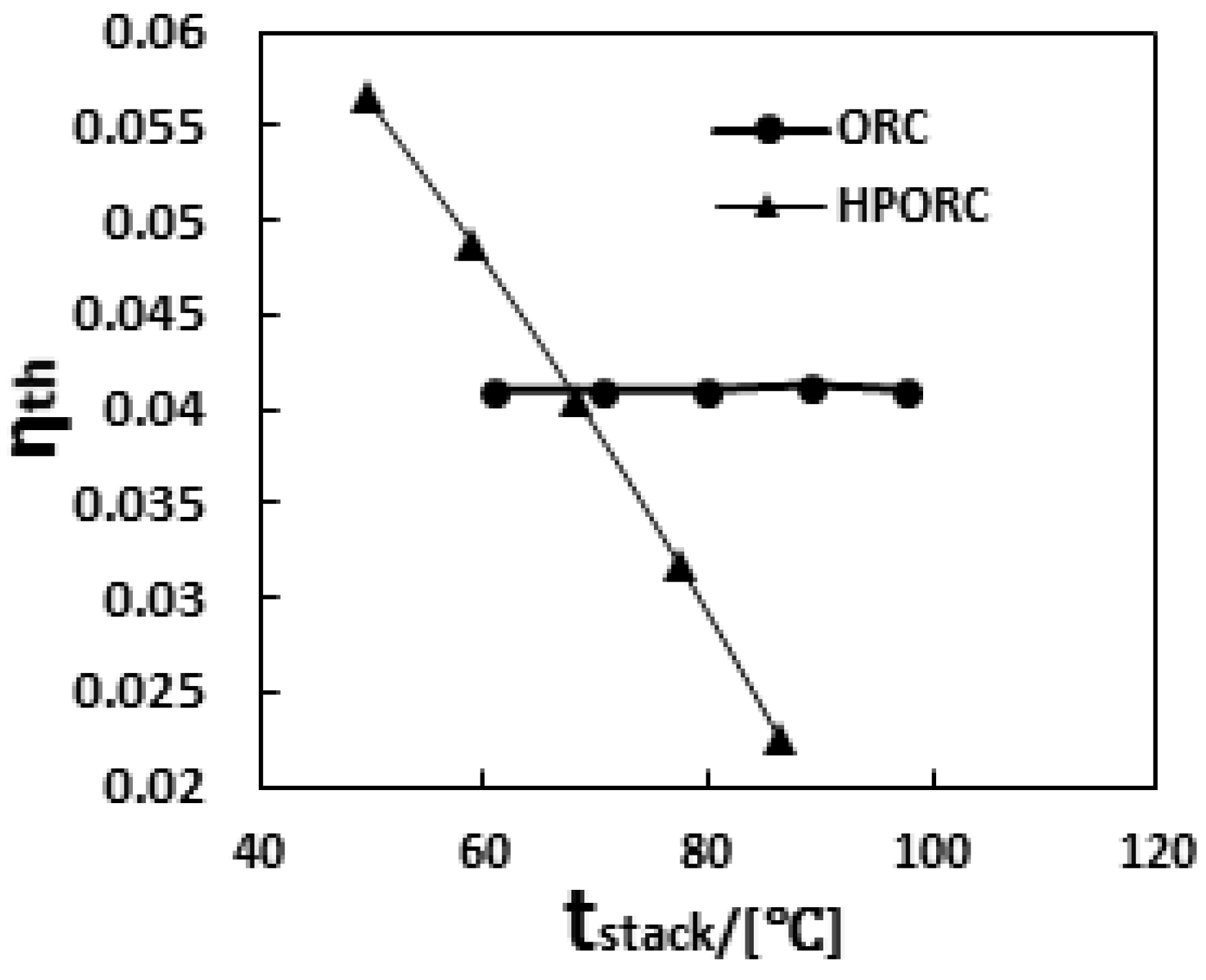
3.3. Performance of PEMFC with ORC or HPORC Acting as the Cooling System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PEMFC | Proton exchange membrane fuel cell |
| ORC | Organic Rankine cycle |
| HPORC | Heat pump combined organic Rankine cycle |
| HP | Heat pump |
| CHP | Combined heat and power |
| FCV | Fuel cell vehicle |
| SOFCF | Solid oxide fuel cell |
Nomenclature
| Symbols | |
| area (cm2) | |
| power (W) | |
| heat flow rate (W) | |
| mass flow rate (kg/s) | |
| temperature (K) | |
| heat transfer coefficient (W/K) | |
| voltage (V) | |
| efficiency | |
| Subscripts, Abbreviations | |
| act | active |
| c | compressor |
| ele | electric |
| ex | expander |
| g | generator |
| h | high |
| l | low |
| m | mass, mechanical |
| net | net power |
| o | standard state |
| p | pump |
| q | quantity of heat |
| tot | total |
| th | thermal |
References
- Kandlikar, S.G.; Lu, Z. Thermal management issues in a PEMFC stack—A brief review of current status. Appl. Therm. Eng. 2009, 29, 1276–1280. [Google Scholar] [CrossRef]
- Zakaria, I.A.; Mustaffa, M.R. Steady-state potential energy recovery modeling of an open cathode PEM fuel cell vehicle. Appl. Mech. Mater. 2014, 465, 114–119. [Google Scholar] [CrossRef]
- Colmenar-Santos, A.; Alberdi-Jiménez, L.; Nasarre-Cortés, L.; Mora-Larramona, L. Residual heat use generated by a 12 kW fuel cell in an electric vehicle heating system. Energy 2014, 68, 182–190. [Google Scholar] [CrossRef]
- Massardo, A.F.; Lubelli, F. Internal reforming solid oxide fuel cell-gas turbine combined cycles (IRSOFC-GT): Part A—Cell model and cycle thermodynamic analysis. J. Eng. Gas Turbines Power 2000, 122, 27–35. [Google Scholar] [CrossRef]
- Yu, S.; Han, J. A dynamic model of PEMFC system for the simulation of residential power generation. J. Fuel Cell Sci. Technol. 2010, 7. [Google Scholar] [CrossRef]
- Shabani, B.; Andrews, J. An experimental investigation of a PEM fuel cell to supply both heat and power in a solar-hydrogen RAPS system. Int. J. Hydrog. Energy 2011, 36, 5442–5452. [Google Scholar] [CrossRef]
- Nguyen, T.V.; White, R.E. A water and heat management model for proton exchange membrane fuel cells. J. Electrochem. Soc. 1993, 140, 2178–2186. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, Z. Performance evaluation of an energy recovery system for fuel reforming of PEM fuel cell power plants. J. Power Sources 2002, 109, 287–293. [Google Scholar] [CrossRef]
- Yu, S.; Jung, D. Thermal management strategy for a proton exchange membrane fuel cell system with a large active cell area. Renew. Energy 2008, 33, 2540–2548. [Google Scholar] [CrossRef]
- Zong, Y.; Zhou, B. Water and thermal management in a single PEM fuel cell with non-uniform stack temperature. J. Power Sources 2006, 161, 143–159. [Google Scholar] [CrossRef]
- Briguglio, N.; Ferraro, M. Evaluation of a low temperature fuel cell system for residential CHP. Int. J. Hydrog. Energy 2011, 36, 8023–8029. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H. Study of zeotropic mixtures of ORC (organic Rankine cycle) under engine various operating conditions. Energy 2013, 58, 494–510. [Google Scholar] [CrossRef]
- Ringler, J.; Seifert, M. Rankine Cycle for Waste Heat Recovery of IC Engines. SAE Int. J. Engines 2009, 2, 67–79. [Google Scholar]
- Quoilin, S.; Aumann, R. Dynamic modeling and optimal control strategy of waste heat recovery Organic Rankine Cycles. Appl. Energy 2011, 88, 2183–2190. [Google Scholar] [CrossRef]
- Chen, P.C. The dynamics analysis and controller design for the PEM fuel cell under gas flowrate constraints. Int. J. Hydrog. Energy 2011, 36, 3110–3122. [Google Scholar] [CrossRef]
- Uzunoglu, M.; Alam, M.S. Dynamic modeling, design, and simulation of a combined PEM fuel cell and ultracapacitor system for stand-alone residential applications. Energy Convers. 2006, 21, 767–775. [Google Scholar] [CrossRef]
- Liu, B.T.; Chien, K.H. Effect of working fluids on organic Rankine cycle for waste heat recovery. Energy 2004, 29, 1207–1217. [Google Scholar] [CrossRef]
- Mann, R.F.; Amphlett, J.C.; Hooper, M.A.I. Development and application of a generalized steady-state electrochemical model for a PEM fuel cell. J. Power Sources 2000, 86, 173–180. [Google Scholar] [CrossRef]


| Symbols | Explainations | Values |
|---|---|---|
| reaction area | 250 cm2 | |
| heat capacity | 17.9 kJ/K | |
| current density | 1 A/cm2 | |
| membrane thickness | 10 μm | |
| number of cells | 250 | |
| partial pressure of H2 at the reaction surface | 1.5 atm | |
| partial pressure of O2 at the reaction surface | 2 atm | |
| membrane resistance | 10 Ωcm |
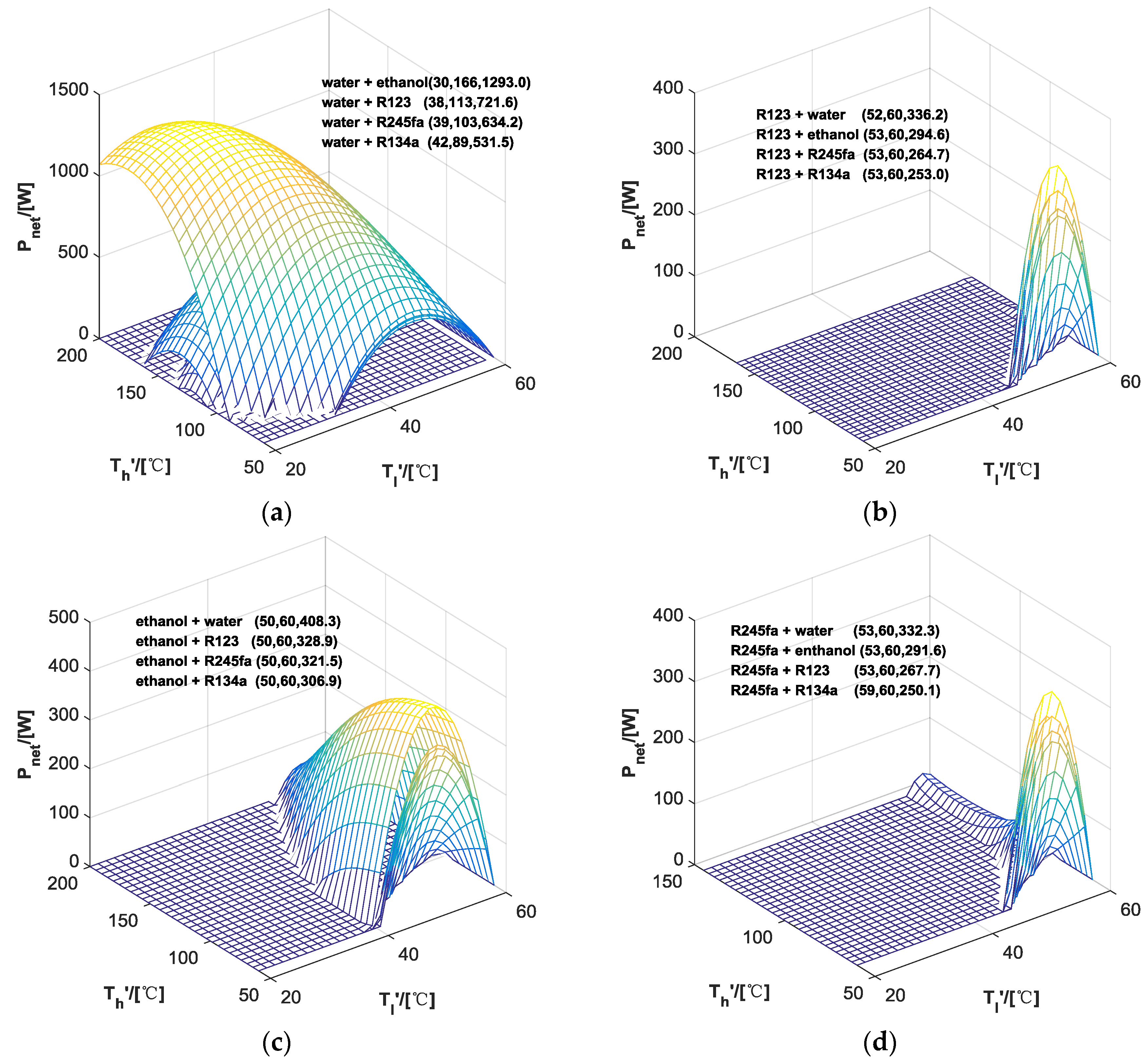
| ORC | Water | Ethanol | R123 | R245fa | R134a | |
|---|---|---|---|---|---|---|
| HP | ||||||
| - | 1.35 | 3.02 | 5.58 | 5.75 | 4.60 | |
| Water | - | 9.40 | 7.17 | 7.13 | 6.21 | |
| Ethanol | 8.09 | - | 6.79 | 6.67 | 6.44 | |
| R123 | 8.91 | 8.08 | - | 7.49 | 7.26 | |
| R245fa | 9.01 | 8.18 | 7.70 | - | 7.35 | |
| ORC | Water | Ethanol | R123 | R245fa | R134a | |
|---|---|---|---|---|---|---|
| HP | ||||||
| - | 150.0 | 334.7 | 637.6 | 665.0 | 534.4 | |
| Water | - | 1293.0 | 721.6 | 634.2 | 531.5 | |
| Ethanol | 408.3 | - | 328.9 | 321.5 | 306.9 | |
| R123 | 336.2 | 294.6 | - | 264.7 | 253 | |
| R245fa | 332.3 | 291.6 | 267.6 | - | 250.1 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Shi, R.; Peng, J.; Zhuge, W.; Zhang, Y. Waste Heat Recovery of a PEMFC System by Using Organic Rankine Cycle. Energies 2016, 9, 267. https://doi.org/10.3390/en9040267
He T, Shi R, Peng J, Zhuge W, Zhang Y. Waste Heat Recovery of a PEMFC System by Using Organic Rankine Cycle. Energies. 2016; 9(4):267. https://doi.org/10.3390/en9040267
Chicago/Turabian StyleHe, Tianqi, Rongqi Shi, Jie Peng, Weilin Zhuge, and Yangjun Zhang. 2016. "Waste Heat Recovery of a PEMFC System by Using Organic Rankine Cycle" Energies 9, no. 4: 267. https://doi.org/10.3390/en9040267





